Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment
Abstract
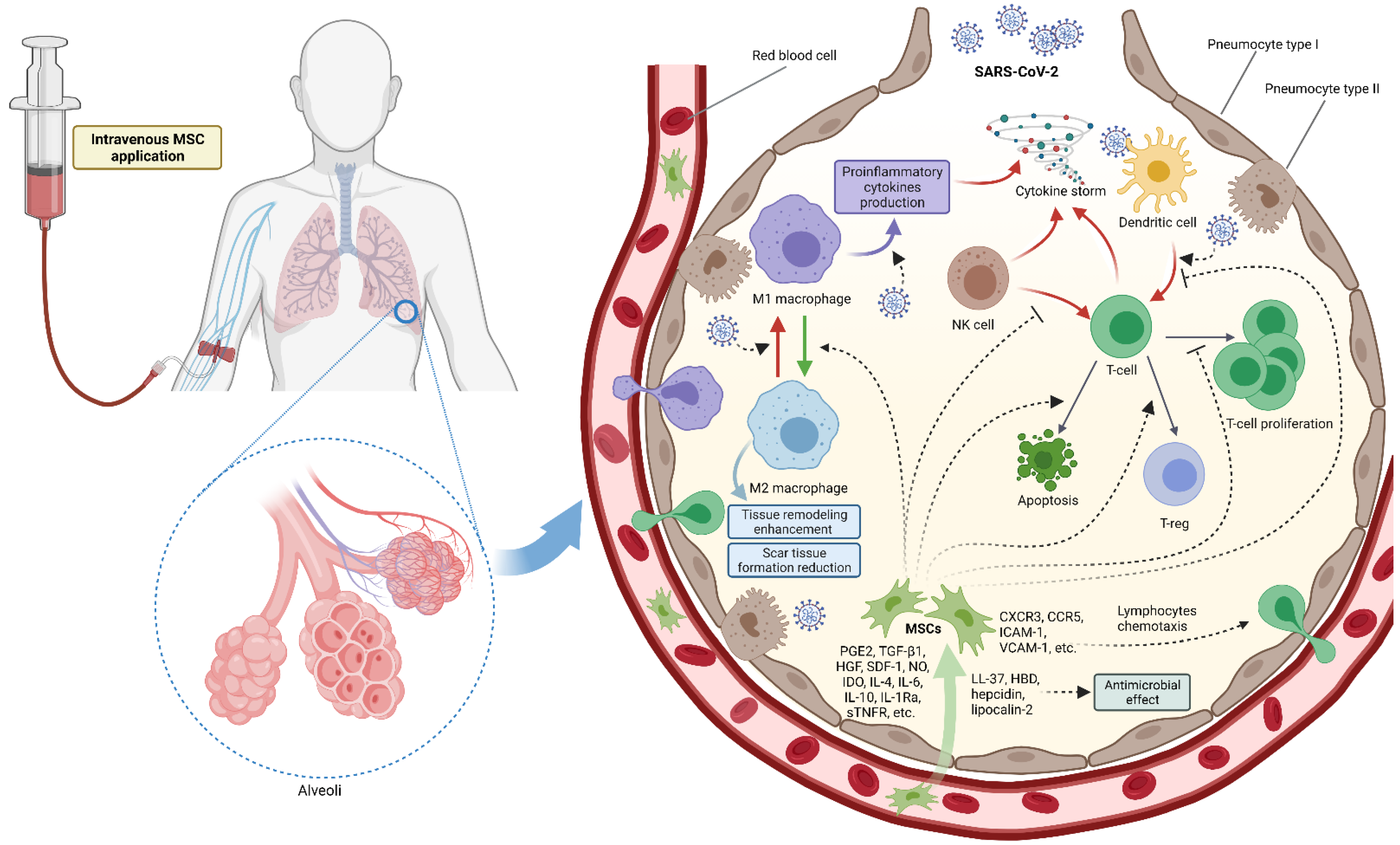
:1. Introduction
2. COVID-19
2.1. Pathophysiology
2.2. Cytokine Storm
2.3. Current Treatment Options
3. Mesenchymal Stromal Cells Treatment
3.1. MSC Mechanism of Action
3.2. MSC Markers
3.3. Systemic MSC Treatment
4. Review of Available Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, W.-Z.; Lin, Y.-H.; Su, L.-J.; Wu, M.-S.; Jeng, H.-Y.; Chang, H.-C.; Huang, Y.-H.; Ling, T.-Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, B.; Tian, Y.; Jiao, H.; Zheng, W.; Wang, J.; Guan, F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell. Immunol. 2011, 272, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Cuellar, A.P.; Kang, J.-H.; Jeung, E.-B.; Choi, K.-C. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol. Ther. (Seoul) 2019, 27, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M.; Robba, C.; Rocco, P.R.M.; Pelosi, P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol. Toxicol. 2020, 36, 83–102. [Google Scholar] [CrossRef]
- Thompson, M.; Mei, S.H.J.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.W.; et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 19, 100249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonus BioGroup Ltd. MesenCure. Available online: https://www.bonusbiogroup.com/index.php/products/mesencure-coronavirus-therapy (accessed on 30 August 2021).
- Alene, M.; Yismaw, L.; Assemie, M.A.; Ketema, D.B.; Mengist, B.; Kassie, B.; Birhan, T.Y. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249090. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 30 August 2021).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 3, 1444–1448. [Google Scholar]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020, 157, 104833. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Caruso Neves, C.; Rocco, P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Ge, H.; Wang, X.; Yuan, X.; Xiao, G.; Wang, C.; Deng, T.; Yuan, Q.; Xiao, X. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Niu, S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020, 96, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.M.E.; et al. Angiotensin-converting enzyme 2 and the pathophysiology of coronavirus disease 2019. J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J. Il Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Gracia-Hernandez, M.; Sotomayor, E.M.; Villagra, A. Targeting Macrophages as a Therapeutic Option in Coronavirus Disease 2019. Front. Pharmacol. 2020, 11, 577571. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, Y.; Zhang, Z.; Liu, Y.; Le, K.; Cui, M.; Yu, Y. Efficacy and safety of current therapeutic options for COVID-19-lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol. Res. 2020, 157, 104872. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; An, M.H.; Kim, W.J.; Hwang, T.-H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 2020, 17, e1003501. [Google Scholar] [CrossRef]
- van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.I.I.; Boot, P.C.; Arbous, S.M. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit. Care 2020, 24, 696. [Google Scholar] [CrossRef]
- Sterne, J.A.C.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021, 222, 108634. [Google Scholar] [CrossRef]
- Tlayjeh, H.; Mhish, O.H.; Enani, M.A.; Alruwaili, A. Association of corticosteroids use and outcomes in COVID-19 patients: A systematic review and meta-analysis. J. Infect. Public Health 2020, 13, 1652–1663. [Google Scholar] [CrossRef]
- Tfi, M.R.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar]
- Zhang, J.J.Y.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected with COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. 2020, 71, 2199–2206. [Google Scholar] [CrossRef]
- Al-Abdouh, A.; Bizanti, A.; Barbarawi, M.; Jabri, A.; Kumar, A.; Fashanu, O.E.; Khan, S.U.; Zhao, D.; Antar, A.A.R.; Michos, E.D. Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Contemp. Clin. Trials 2021, 101, 106272. [Google Scholar] [CrossRef] [PubMed]
- Salian, V.S.; Wright, J.A.; Vedell, P.T.; Nair, S.; Li, C.; Kandimalla, M.; Tang, X.; Carmona Porquera, E.M.; Kalari, K.R.; Kandimalla, K.K. COVID-19 Transmission, Current Treatment, and Future Therapeutic Strategies. Mol. Pharm. 2021, 18, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Piscoya, A.; Ng-Sueng, L.F.; del Riego, A.P.; Cerna-Viacava, R.; Pasupuleti, V.; Roman, Y.M.; Thota, P.; White, C.M.; Hernandez, A.V. Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0243705. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Devasenapathy, N.; Ye, Z.; Loeb, M.; Fang, F.; Najafabadi, B.T.; Xiao, Y.; Couban, R.; Bégin, P.; Guyatt, G. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: A systematic review and meta-analysis. Cmaj 2020, 192, E745–E755. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Soni, K.D.; Khanna, P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M. (A Little) Clarity on Convalescent Plasma for Covid-19. N. Engl. J. Med. 2021, 384, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef]
- Rnjak, D.; Ravlić, S.; Šola, A.-M.; Halassy, B.; Šemnički, J.; Šuperba, M.; Hećimović, A.; Kurolt, I.-C.; Kurtović, T.; Mačak Šafranko, Ž.; et al. COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus. Clin. Biol. 2021, 28, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Singer, N.G.; Caplan, A.I. Mesenchymal Stem Cells: Mechanisms of Inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 628767. [Google Scholar] [CrossRef] [Green Version]
- de Souza, L.E.B.; Malta, T.M.; Kashima Haddad, S.; Covas, D.T. Mesenchymal Stem Cells and Pericytes: To What Extent Are They Related? Stem Cells Dev. 2016, 25, 1843–1852. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkers, T.; Kaipe, H.; Nava, S.; Molldén, P.; Gustafsson, B.; Axelsson, R.; Ringdén, O. Treatment of Severe Chronic Graft-Versus-Host Disease with Decidual Stromal Cells and Tracing with 111 Indium Radiolabeling. Stem Cells Dev. 2015, 24, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: Knowns and unknowns. Intensive Care Med. Exp. 2019, 7, 41. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Bieback, K.; Kluter, H. Mesenchymal Stromal Cells from Umbilical Cord Blood. Curr. Stem Cell Res. Ther. 2007, 2, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells fro. J. Cell. Biochem. 2011, 112, 1206–1218. [Google Scholar] [CrossRef]
- Devine, S.M.; Hoffman, R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr. Opin. Hematol. 2000, 7, 358–363. [Google Scholar] [CrossRef]
- Conget, P.A.; Minguell, J.J. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J. Cell. Physiol. 1999, 181, 67–73. [Google Scholar] [CrossRef]
- Krause, D.S.; Theise, N.D.; Collector, M.I.; Henegariu, O.; Hwang, S.; Gardner, R.; Neutzel, S.; Sharkis, S.J. Multi-Organ, Multi-Lineage Engraftment by a Single Bone Marrow-Derived Stem Cell. Cell 2001, 105, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Fibbe, W.E.; Noort, W.A. Mesenchymal Stem Cells and Hematopoietic Stem Cell Transplantation. Ann. N. Y. Acad. Sci. 2003, 996, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.A.; Hong, S.H.; Gang, E.J.; Ahn, C.; Hwang, S.H.; Yang, I.H.; Han, H.; Kim, H. Differential Gene Expression Profiling of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells by DNA Microarray. Stem Cells 2005, 23, 584–593. [Google Scholar] [CrossRef]
- Martins, A.A.; Paiva, A.; Morgado, J.M.; Gomes, A.; Pais, M.L. Quantification and Immunophenotypic Characterization of Bone Marrow and Umbilical Cord Blood Mesenchymal Stem Cells by Multicolor Flow Cytometry. Transplant. Proc. 2009, 41, 943–946. [Google Scholar] [CrossRef]
- Lu, L.-L.; Liu, Y.-J.; Yang, S.-G.; Zhao, Q.-J.; Wang, X.; Gong, W.; Han, Z.-B.; Xu, Z.-S.; Lu, Y.-X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Moravcikova, E.; Meyer, E.M.; Corselli, M.; Donnenberg, V.S.; Donnenberg, A.D. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytom. Part A 2018, 93, 894–904. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, J.H. Tissue Factor: A Key Molecule in Hemostatic and Nonhemostatic Systems. Int. J. Hematol. 2004, 79, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Česen Mazič, M.; Girandon, L.; Kneževič, M.; Avčin, S.L.; Jazbec, J. Treatment of Severe Steroid-Refractory Acute-Graft-vs.-Host Disease with Mesenchymal Stem Cells–Single Center Experience. Front. Bioeng. Biotechnol. 2018, 6, 93. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Mallea, J.; Zubair, A.C. Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. NPJ Regen. Med. 2020, 5, 17. [Google Scholar] [CrossRef]
- Chan, M.C.W.; Kuok, D.I.T.; Leung, C.Y.H.; Hui, K.P.Y.; Valkenburg, S.A.; Lau, E.H.Y.; Nicholls, J.M.; Fang, X.; Guan, Y.; Lee, J.W.; et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 3621–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, J.; Shi, W.; Chen, C.; Shao, Y.; Zhu, L.; Lu, W.; Han, X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res. Ther. 2016, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ding, J.; Ren, S.; Wang, W.; Yang, Y.; Li, S.; Meng, M.; Wu, T.; Liu, D.; Tian, S.; et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020, 11, 207. [Google Scholar] [CrossRef]
- Tang, L.; Jiang, Y.; Zhu, M.; Chen, L.; Zhou, X.; Zhou, C.; Ye, P.; Chen, X.; Wang, B.; Xu, Z.; et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 2020, 14, 664–673. [Google Scholar] [CrossRef]
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine (Baltimore) 2020, 99, e21429. [Google Scholar] [CrossRef]
- Peng, H.; Gong, T.; Huang, X.; Sun, X.; Luo, H.; Wang, W.; Luo, J.; Luo, B.; Chen, Y.; Wang, X.; et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: A clinical case report. Stem Cell Res. Ther. 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Nie, Y.; Wu, H.; Cheng, L.; Qiu, Y.; Fu, J.; Jiang, X. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J. Infect. Dev. Ctries. 2020, 14, 1138–1145. [Google Scholar] [CrossRef]
- Zengin, R.; Beyaz, O.; Koc, E.S.; Akinci, I.O.; Kocagoz, S.; Sagcan, G.; Ovali, E.; Cuhadaroglu, C. Mesenchymal stem cell treatment in a critically ill COVID-19 patient: A case report. Stem Cell Investig. 2020, 7, 17. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Liu, K.; Li, X.; Chen, D.; Bai, D.; Luo, J.; Liu, Y.; Zhang, Y.; Li, L.; et al. Human Umbilical Cord Mesenchymal Stem Cells for Adjuvant Treatment of a Critically Ill COVID-19 Patient: A Case Report. Infect. Drug Resist. 2020, 13, 3295–3300. [Google Scholar] [CrossRef]
- Soler Rich, R.; Rius Tarruella, J.; Melgosa Camarero, M.T. Expanded mesenchymal stem cells: A novel therapeutic approach for SARS-CoV-2 pneumonia (COVID-19). Concepts regarding a first case in Spain. Med. Clin. (Engl. Ed.) 2020, 155, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xie, Z.-Y.; Zhu, D.-H.; Li, L.-J. Human menstrual blood-derived stem cells as immunoregulatory therapy in COVID-19: A case report and review of the literature. World J. Clin. Cases 2021, 9, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Senegaglia, A.C.; Rebelatto, C.L.K.; Franck, C.L.; Lima, J.S.; Boldrini-Leite, L.M.; Daga, D.R.; Leitão, C.A.; Shigunov, P.; de Azambuja, A.P.; Bana, E.; et al. Combined Use of Tocilizumab and Mesenchymal Stromal Cells in the Treatment of Severe Covid-19: Case Report. Cell Transplant. 2021, 30, 096368972110210. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Stojanović, S.; Stipić; Strbad, M.; Girandon, L.; Barlič, A.; Frankić, M.; Ivić, I.; Marasović, D.; Krstulović; et al. Compassionate mesenchymal stem cell treatment in a severe COVID-19 patient: A case report. Croat. Med. J. 2021, 62, 288–296. [Google Scholar] [CrossRef]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T.; Shi, L.; Fu, J.; Jiang, T.; et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target. Ther. 2020, 5, 172. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, W.; Chen, L.; Xu, Z.; Zhang, Q.; Zhu, M.; Ye, P.; Li, H.; Yu, L.; Zhou, X.; et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin. Transl. Med. 2021, 11, e297. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 361. [Google Scholar] [CrossRef]
- Hashemian, S.-M.R.; Aliannejad, R.; Zarrabi, M.; Soleimani, M.; Vosough, M.; Hosseini, S.-E.; Hossieni, H.; Keshel, S.H.; Naderpour, Z.; Hajizadeh-Saffar, E.; et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Res. Ther. 2021, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Vaezi, A.A.; Aliannejad, R.; Sohrabpour, A.A.; Kiaei, S.Z.F.; Shadnoush, M.; Siavashi, V.; Aghaghazvini, L.; Khoundabi, B.; Abdoli, S.; et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: A phase 1 clinical trial. Stem Cell Res. Ther. 2021, 12, 410. [Google Scholar] [CrossRef]
- Sánchez-Guijo, F.; García-Arranz, M.; López-Parra, M.; Monedero, P.; Mata-Martínez, C.; Santos, A.; Sagredo, V.; Álvarez-Avello, J.-M.; Guerrero, J.E.; Pérez-Calvo, C.; et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 2020, 25, 100454. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, J.; Wu, J.; Xu, Y.; Chen, B.; Jiang, L.; Xiang, H.; Peng, Z.; Wang, X. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020, 53, e12947. [Google Scholar] [CrossRef]
| Author | Origin of MSCs Delivered | MSC Dosing | Method of Delivery |
|---|---|---|---|
| Zhang et al. [83] | umbilical cord | 106/kg single dose | iv |
| Tang et al. (2 cases) [84] | menstrual blood | 106/kg in 3 doses | iv |
| Liang et al. [85] | umbilical cord | 5 × 107/kg in 3 doses | iv |
| Peng et al. [86] | umbilical cord | 106/kg in 3 doses | iv |
| Tao et al. [87] | umbilical cord | 1.5 × 106/kg in 5 doses | iv |
| Zengin et al. [88] | umbilical cord | 0.7 × 106/kg in 2 doses iv | iv |
| 0.3 × 106/kg in 2 doses it | it | ||
| Zhu et al. [89] | umbilical cord | 106/kg single dose | iv |
| Rich et al. [90] | bone marrow | 106/kg single dose | iv |
| Lu et al. [91] | menstrual blood | 3000, 2000, 3000 units per dose | iv |
| Senegaglia et al. [92] | umbilical cord | 5 × 105/kg in 3 doses | iv |
| Primorac et al. [93] | bone marrow | 106/kg in 3 doses | iv |
| Author | Number of Participants (MSC-Control/Placebo) | Origin of MSCs Delivered | MSC Dosing |
|---|---|---|---|
| Meng et al. [94] | 18 (9-9) | umbilical cord | 3 × 107 in 3 doses |
| Shi et al. [95] | 100 (65-35) | umbilical cord | 4 × 107 in 3 doses |
| Xu et al. [96] | 44 (26-18) | menstrual blood | 3 × 107 in 3 doses |
| Lanzoni et al. [97] | 24 (12-12) | umbilical cord | 100 ± 20 × 106 in 2 doses |
| Shu et al. [98] | 41 (12-29) | umbilical cord | 2 × 106/kg |
| Hashemian et al. [99] | 11 | umbilical cord (6 patients) or placental (5 patients) | 200 × 106 in 2 doses |
| Saleh et al. [100] | 5 | Wharton’s jelly | 150 × 106 in 3 doses |
| Sanchez-Gujio et al. [101] | 13 | adipose tissue | 0.96 × 106/kg in 1–3 doses |
| Feng et al. [102] | 16 | umbilical cord | 108 in 4 doses |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primorac, D.; Čemerin, M.; Matišić, V.; Molnar, V.; Strbad, M.; Girandon, L.; Zenić, L.; Knežević, M.; Minger, S.; Polančec, D. Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment. Pharmaceutics 2021, 13, 1481. https://doi.org/10.3390/pharmaceutics13091481
Primorac D, Čemerin M, Matišić V, Molnar V, Strbad M, Girandon L, Zenić L, Knežević M, Minger S, Polančec D. Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment. Pharmaceutics. 2021; 13(9):1481. https://doi.org/10.3390/pharmaceutics13091481
Chicago/Turabian StylePrimorac, Dragan, Martin Čemerin, Vid Matišić, Vilim Molnar, Marko Strbad, Lenart Girandon, Lucija Zenić, Miomir Knežević, Stephen Minger, and Denis Polančec. 2021. "Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment" Pharmaceutics 13, no. 9: 1481. https://doi.org/10.3390/pharmaceutics13091481







