Tracking Radiolabeled Endothelial Microvesicles Predicts Their Therapeutic Efficacy: A Proof-of-Concept Study in Peripheral Ischemia Mouse Model Using SPECT/CT Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Endothelial LEVs
2.2. Purification and Characterization of LEVs
2.2.1. Purification
2.2.2. Flow Cytometry
2.2.3. Tunable Resistive Pulse Sensing (TRPS)
2.2.4. Transmission Electron Microscopy (TEM)
2.2.5. Western Blot Analysis
2.3. Radiolabeling of LEVs
2.4. Purification of Radiolabeled LEVs from Free [99mTc]Tc-AnnV Radiotracer
2.5. Stability of Radiolabeled LEVs in Serum
2.6. In Vivo Experimentations
2.7. Mouse Model of Hind Limb Ischemia Induction and Follow-Up
2.8. Quantification of the In Vivo Biodistribution of Radiolabeled LEVs by Isotopic Imaging
2.9. Statistical Analysis
3. Results
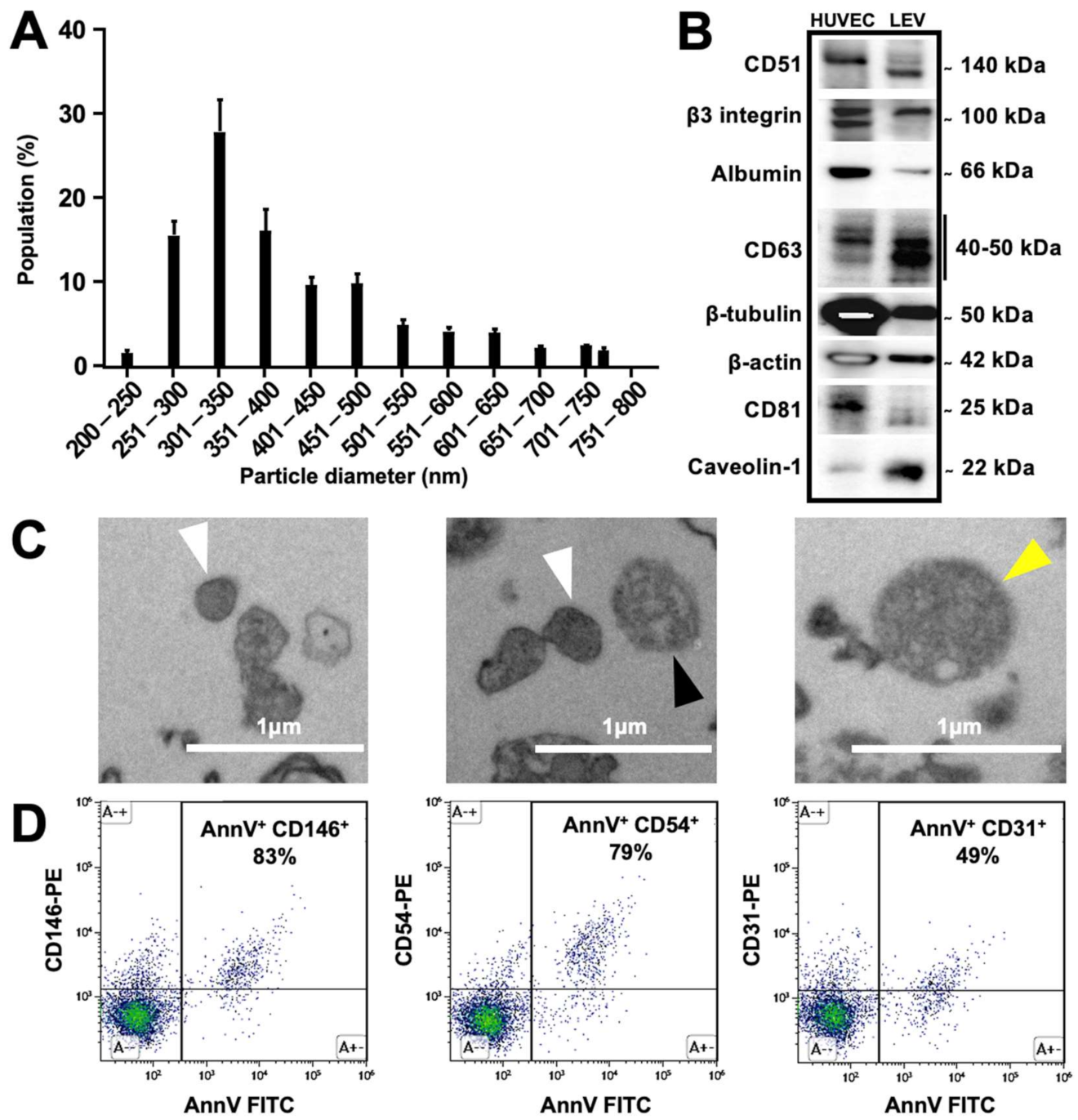
3.1. Characterization of Produced Endothelial LEVs
3.2. Endothelial LEVs Were Successfully Radiolabeled and Purified
3.3. Endothelial LEVs Preferentially Homed to the Ischemic Hind Limb
3.4. Tracking of LEV Homing Correlated with Therapeutic Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of Global Estimates of Prevalence and Risk Factors for Peripheral Artery Disease in 2000 and 2010: A Systematic Review and Analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef] [Green Version]
- Soria-Juan, B.; Escacena, N.; Capilla-González, V.; Aguilera, Y.; Llanos, L.; Tejedo, J.R.; Bedoya, F.J.; Juan, V.; De la Cuesta, A.; Ruiz-Salmerón, R.; et al. Cost-Effective, Safe, and Personalized Cell Therapy for Critical Limb Ischemia in Type 2 Diabetes Mellitus. Front. Immunol. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular Vesicles in Diagnostics and Therapy of the Ischaemic Heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Panfoli, I.; Santucci, L.; Bruschi, M.; Petretto, A.; Calzia, D.; Ramenghi, L.A.; Ghiggeri, G.; Candiano, G. Microvesicles as Promising Biological Tools for Diagnosis and Therapy. Expert Rev. Proteom. 2018, 15, 801–808. [Google Scholar] [CrossRef]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef]
- Viola, M.; de Jager, S.C.A.; Sluijter, J.P.G. Targeting Inflammation after Myocardial Infarction: A Therapeutic Opportunity for Extracellular Vesicles? Int. J. Mol. Sci. 2021, 22, 7831. [Google Scholar] [CrossRef]
- Alfì, E.; Thairi, C.; Femminò, S.; Alloatti, G.; Moccia, F.; Brizzi, M.F.; Pagliaro, P.; Penna, C. Extracellular Vesicles (EVs) in Ischemic Conditioning and Angiogenesis: Focus on Endothelial Derived EVs. Vasc. Pharmacol. 2021, 140, 106873. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.-C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/Microvesicle-Mediated Epigenetic Reprogramming of Cells. Am. J. Cancer Res. 2011, 1, 98–110. [Google Scholar]
- Van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [Green Version]
- György, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic Applications of Extracellular Vesicles: Clinical Promise and Open Questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [Green Version]
- Lässer, C.; Jang, S.C.; Lötvall, J. Subpopulations of Extracellular Vesicles and Their Therapeutic Potential. Mol. Asp. Med. 2018, 60, 1–14. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Fleury, A.; Martinez, M.C.; Le Lay, S. Extracellular Vesicles as Therapeutic Tools in Cardiovascular Diseases. Front. Immunol. 2014, 5, 370. [Google Scholar] [CrossRef] [Green Version]
- Deng, F.; Wang, S.; Zhang, L. Endothelial Microparticles Act as Novel Diagnostic and Therapeutic Biomarkers of Circulatory Hypoxia-Related Diseases: A Literature Review. J. Cell. Mol. Med. 2017, 21, 1698–1710. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Betzer, O.; Barnoy, E.; Sadan, T.; Elbaz, I.; Braverman, C.; Liu, Z.; Popovtzer, R. Advances in Imaging Strategies for in Vivo Tracking of Exosomes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1594. [Google Scholar] [CrossRef]
- Rashid, M.H.; Borin, T.F.; Ara, R.; Angara, K.; Cai, J.; Achyut, B.R.; Liu, Y.; Arbab, A.S. Differential in vivo Biodistribution of 131I-Labeled Exosomes from Diverse Cellular Origins and Its Implication for Theranostic Application. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102072. [Google Scholar] [CrossRef]
- Abello, J.; Nguyen, T.D.T.; Marasini, R.; Aryal, S.; Weiss, M.L. Biodistribution of Gadolinium- and near Infrared-Labeled Human Umbilical Cord Mesenchymal Stromal Cell-Derived Exosomes in Tumor Bearing Mice. Theranostics 2019, 9, 2325. [Google Scholar] [CrossRef]
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016, 2016, 5029619. [Google Scholar] [CrossRef] [Green Version]
- Ayers, L.; Pink, R.; Carter, D.R.F.; Nieuwland, R. Clinical Requirements for Extracellular Vesicle Assays. J. Extracell. Vesicles 2019, 8, 1593755. [Google Scholar] [CrossRef] [Green Version]
- Chuo, S.T.-Y.; Chien, J.C.-Y.; Lai, C.P.-K. Imaging Extracellular Vesicles: Current and Emerging Methods. J. Biomed. Sci. 2018, 25, 91. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; de Rosales, R.T. Radiolabelling of Extracellular Vesicles for PET and SPECT Imaging. Nanotheranostics 2021, 5, 256–274. [Google Scholar] [CrossRef]
- Tait, J.F.; Smith, C.; Blankenberg, F.G. Structural Requirements for in Vivo Detection of Cell Death with 99mTc-Annexin V. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2005, 46, 807–815. [Google Scholar]
- Garrigue, P.; Giacomino, L.; Bucci, C.; Muzio, V.; Filannino, M.A.; Sabatier, F.; Dignat-George, F.; Pisano, P.; Guillet, B. Single Photon Emission Computed Tomography Imaging of Cerebral Blood Flow, Blood-Brain Barrier Disruption, and Apoptosis Time Course after Focal Cerebral Ischemia in Rats. Int. J. Stroke 2016, 11, 117–126. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Roux, Q.; Van Deun, J.; Dedeyne, S.; Hendrix, A. The EV-TRACK Summary Add-on: Integration of Experimental Information in Databases to Ensure Comprehensive Interpretation of Biological Knowledge on Extracellular Vesicles. J. Extracell. Vesicles 2020, 9, 1699367. [Google Scholar] [CrossRef]
- Robert, S.; Lacroix, R.; Poncelet, P.; Harhouri, K.; Bouriche, T.; Judicone, C.; Wischhusen, J.; Arnaud, L.; Dignat-George, F. High-Sensitivity Flow Cytometry Provides Access to Standardized Measurement of Small-Size Microparticles-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1054–1058. [Google Scholar] [CrossRef]
- Cointe, S.; Judicone, C.; Robert, S.; Mooberry, M.J.; Poncelet, P.; Wauben, M.; Nieuwland, R.; Key, N.S.; Dignat-George, F.; Lacroix, R. Standardization of Microparticle Enumeration across Different Flow Cytometry Platforms: Results of a Multicenter Collaborative Workshop. J. Thromb. Haemost. 2017, 15, 187–193. [Google Scholar] [CrossRef]
- Maas, S.L.N.; De Vrij, J.; Broekman, M.L.D. Quantification and Size-Profiling of Extracellular Vesicles Using Tunable Resistive Pulse Sensing. J. Vis. Exp. 2014, e51623. [Google Scholar] [CrossRef]
- Percie du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Moyon, A.; Garrigue, P.; Balasse, L.; Fernandez, S.; Brige, P.; Nollet, M.; Hache, G.; Blot-Chabaud, M.; Dignat-George, F.; Guillet, B. Early Prediction of Revascularisation by Angiomotin-Targeting Positron Emission Tomography. Theranostics 2018, 8, 4985. [Google Scholar] [CrossRef]
- Suffee, N.; Le Visage, C.; Hlawaty, H.; Aid-Launais, R.; Vanneaux, V.; Larghero, J.; Haddad, O.; Oudar, O.; Charnaux, N.; Sutton, A. Pro-Angiogenic Effect of RANTES-Loaded Polysaccharide-Based Microparticles for a Mouse Ischemia Therapy. Sci. Rep. 2017, 7, 13294. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.A.; Bengel, F.M.; Blasberg, R.G. The AQARA Principle: Proposing Standard Requirements for Radionuclide-Based Images in Medical Journals. J. Nucl. Med. 2020, 61, 1–2. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Combes, V.; Simon, A.C.; Grau, G.E.; Arnoux, D.; Camoin, L.; Sabatier, F.; Mutin, M.; Sanmarco, M.; Sampol, J.; Dignat-George, F. In Vitro Generation of Endothelial Microparticles and Possible Prothrombotic Activity in Patients with Lupus Anticoagulant. J. Clin. Investig. 1999, 104, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Lázaro-Ibáñez, E.; Faruqu, F.N.; Saleh, A.F.; Silva, A.M.; Tzu-Wen Wang, J.; Rak, J.; Al-Jamal, K.T.; Dekker, N. Selection of Fluorescent, Bioluminescent, and Radioactive Tracers to Accurately Reflect Extracellular Vesicle Biodistribution in vivo. ACS Nano 2021, 15, 3212–3227. [Google Scholar] [CrossRef]
- Bennis, Y.; Sarlon-Bartoli, G.; Guillet, B.; Hubert, L.; Pellegrini, L.; Velly, L.; Blot-Chabaud, M.; Dignat-George, F.; Sabatier, F.; Pisano, P. Priming of Late Endothelial Progenitor Cells with Erythropoietin before Transplantation Requires the CD131 Receptor Subunit and Enhances Their Angiogenic Potential. J. Thromb. Haemost. 2012, 10, 1914–1928. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [Green Version]
- Hache, G.; Garrigue, P.; Bennis, Y.; Stalin, J.; Moyon, A.; Cerami, A.; Brines, M.; Blot-Chabaud, M.; Sabatier, F.; Dignat-George, F.; et al. ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability. Shock 2016, 46, 390–397. [Google Scholar] [CrossRef]
- Garrigue, P.; Hache, G.; Bennis, Y.; Brige, P.; Stalin, J.; Pellegrini, L.; Velly, L.; Orlandi, F.; Castaldi, E.; Dignat-George, F.; et al. EPO Pretreatment of ECFCs Enhances Functional Recovery after Transplantation in a Rat Model of Cerebral Ischemia through an Increase of Their Homing Abilities: A SPECT/CT Study. J. Nucl. Med. 2016, 57, 1798–1804. [Google Scholar] [CrossRef] [Green Version]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.-W. Matrix Mechanics and Water Permeation Regulate Extracellular Vesicle Transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef]
- Margolis, L.; Sadovsky, Y. The Biology of Extracellular Vesicles: The Known Unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Federico, F.; Andrea, R.; Cristina, G.; Massimo, C.; Marta, T.; Claudia, C.; Andrea, R.; Gabriele, T.; Saveria, F.; Vittoria, G.M.; et al. Extracellular Vesicles from Adipose Stem Cells Prevent Muscle Damage and Inflammation in a Mouse Model of Hind Limb Ischemia. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 239–254. [Google Scholar] [CrossRef]
- Ranghino, A.; Cantaluppi, V.; Grange, C.; Vitillo, L.; Fop, F.; Biancone, L.; Deregibus, M.C.; Tetta, C.; Segoloni, G.P.; Camussi, G. Endothelial Progenitor Cell-Derived Microvesicles Improve Neovascularization in a Murine Model of Hindlimb Ischemia. Int. J. Immunopathol. Pharmacol. 2012, 25, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Cavallari, C.; Ranghino, A.; Tapparo, M.; Cedrino, M.; Figliolini, F.; Grange, C.; Giannachi, V.; Garneri, P.; Deregibus, M.C.; Collino, F.; et al. Serum-Derived Extracellular Vesicles (EVs) Impact on Vascular Remodeling and Prevent Muscle Damage in Acute Hind Limb Ischemia. Sci. Rep. 2017, 7, 8180. [Google Scholar] [CrossRef]
- Lopatina, T.; Favaro, E.; Grange, C.; Cedrino, M.; Ranghino, A.; Occhipinti, S.; Fallo, S.; Buffolo, F.; Gaykalova, D.A.; Zanone, M.M.; et al. PDGF Enhances the Protective Effect of Adipose Stem Cell-Derived Extracellular Vesicles in a Model of Acute Hindlimb Ischemia. Sci. Rep. 2018, 8, 17458. [Google Scholar] [CrossRef]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.-L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in Vascular Homeostasis and Diseases. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Oh, J.M.; Oh, E.J.; Hong, C.M.; Chung, H.Y.; Lee, J.; Ahn, B.-C. Identification of Angiogenic Cargo in Extracellular Vesicles Secreted from Human Adipose Tissue-Derived Stem Cells and Induction of Angiogenesis In Vitro and In Vivo. Pharmaceutics 2021, 13, 495. [Google Scholar] [CrossRef]


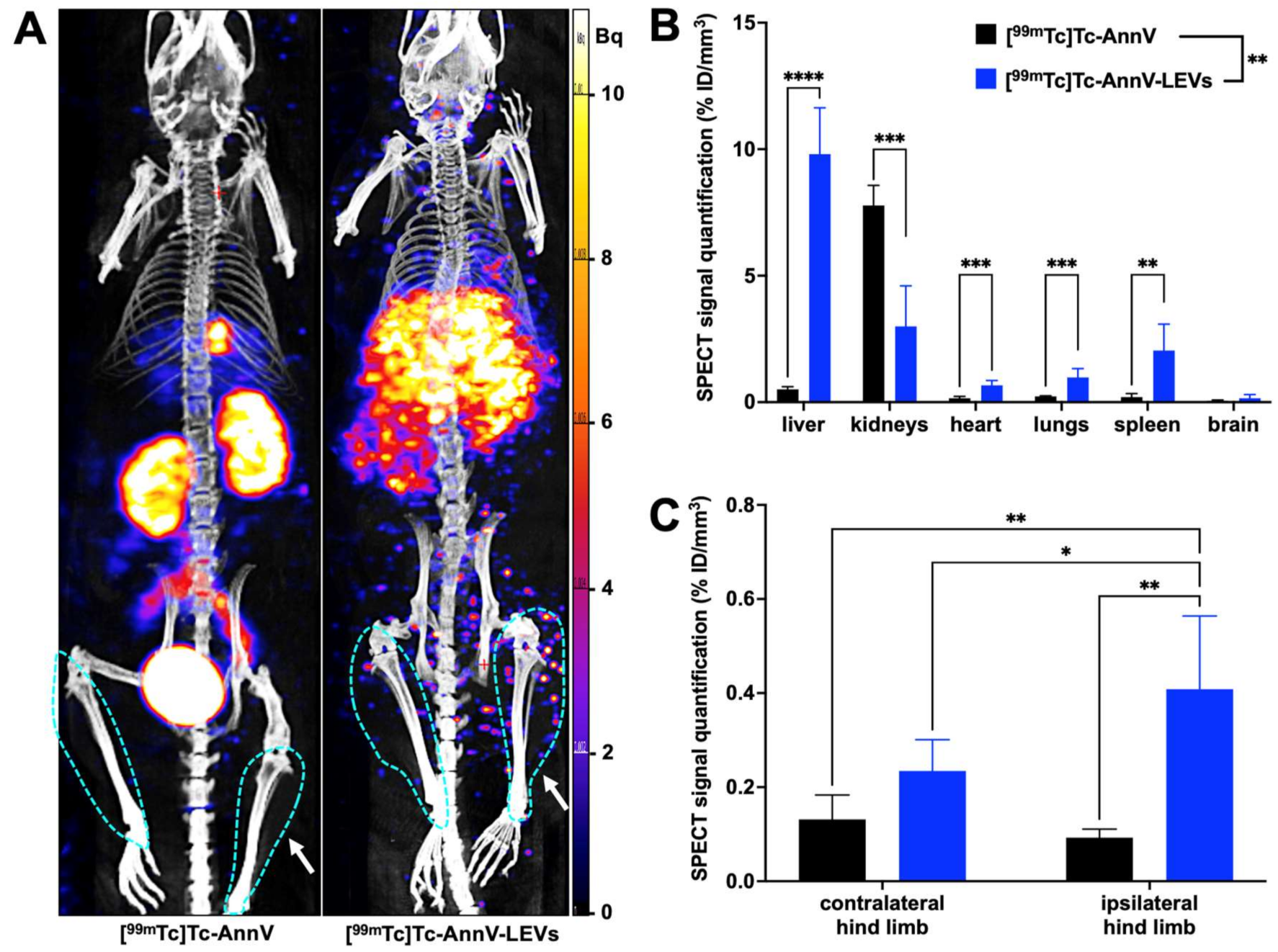

| %ID/mm3 | Liver | Kidneys | Heart | Lungs | Spleen | Brain | Ipsi Hind Limb | Contra Hind Limb |
|---|---|---|---|---|---|---|---|---|
| free [99mTc]Tc-AnnV (n = 3) | 0.51 ± 0.10 | 7.78 ± 0.79 | 0.16 ± 0.07 | 0.23 ± 0.02 | 0.20 ± 0.14 | 0.05 ± 0.04 | 0.09 ± 0.02 € | 0.13 ± 0.05 € |
| [99mTc]Tc-AnnV-LEVs (n = 10) | 9.81 ± 1.83 | 2.99 ± 1.60 | 0.67 ± 0.19 | 0.97 ± 0.36 | 2.04 ± 1.05 | 0.16 ± 0.14 | 0.41 ± 0.09 ¥ | 0.23 ± 0.06 ¥ |
| Post-hoctest p value | **** <0.0001 | *** 0.0010 | *** 0.0003 | *** 0.0005 | ** 0.0019 | ns 0.2957 | ** 0.0013 | ns 0.4958 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraud, R.; Moyon, A.; Simoncini, S.; Duchez, A.-C.; Nail, V.; Chareyre, C.; Bouhlel, A.; Balasse, L.; Fernandez, S.; Vallier, L.; et al. Tracking Radiolabeled Endothelial Microvesicles Predicts Their Therapeutic Efficacy: A Proof-of-Concept Study in Peripheral Ischemia Mouse Model Using SPECT/CT Imaging. Pharmaceutics 2022, 14, 121. https://doi.org/10.3390/pharmaceutics14010121
Giraud R, Moyon A, Simoncini S, Duchez A-C, Nail V, Chareyre C, Bouhlel A, Balasse L, Fernandez S, Vallier L, et al. Tracking Radiolabeled Endothelial Microvesicles Predicts Their Therapeutic Efficacy: A Proof-of-Concept Study in Peripheral Ischemia Mouse Model Using SPECT/CT Imaging. Pharmaceutics. 2022; 14(1):121. https://doi.org/10.3390/pharmaceutics14010121
Chicago/Turabian StyleGiraud, Romain, Anaïs Moyon, Stéphanie Simoncini, Anne-Claire Duchez, Vincent Nail, Corinne Chareyre, Ahlem Bouhlel, Laure Balasse, Samantha Fernandez, Loris Vallier, and et al. 2022. "Tracking Radiolabeled Endothelial Microvesicles Predicts Their Therapeutic Efficacy: A Proof-of-Concept Study in Peripheral Ischemia Mouse Model Using SPECT/CT Imaging" Pharmaceutics 14, no. 1: 121. https://doi.org/10.3390/pharmaceutics14010121






