pH-Responsive Liposomes of Dioleoyl Phosphatidylethanolamine and Cholesteryl Hemisuccinate for the Enhanced Anticancer Efficacy of Cisplatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cisplatin Loaded pH-Responsive Liposomes
2.3. Physicochemical Characterization of Liposomes
2.3.1. Particle Size, Polydispersity Index (PDI) and Zeta Potential
2.3.2. Entrapment Efficiency
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
2.3.5. Differential Scanning Calorimetric (DSC) Analysis
2.3.6. In Vitro Release and Kinetic Modeling
2.4. Cell Lines and Cell Culture
2.4.1. Cell Lines
2.4.2. Cytotoxicity Study
2.4.3. Cell Uptake Study
Fluorescence Microscopy
2.5. Stability Studies
2.6. Acute Toxicity Study
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of CDDP@PLs
3.1.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential
3.1.2. Entrapment Efficiency (E.E)
3.1.3. Transmission Electron Microscopy (TEM)
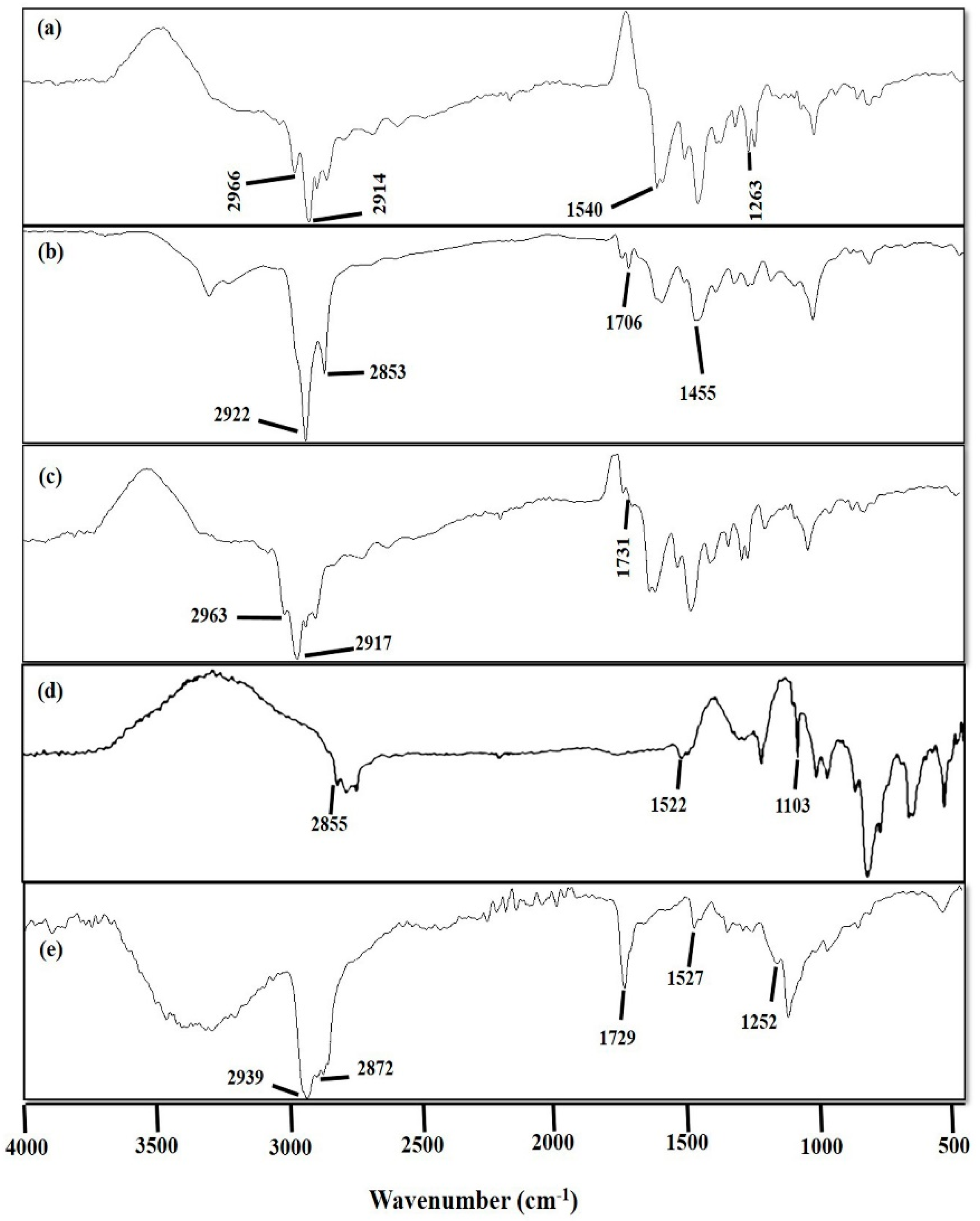
3.1.4. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
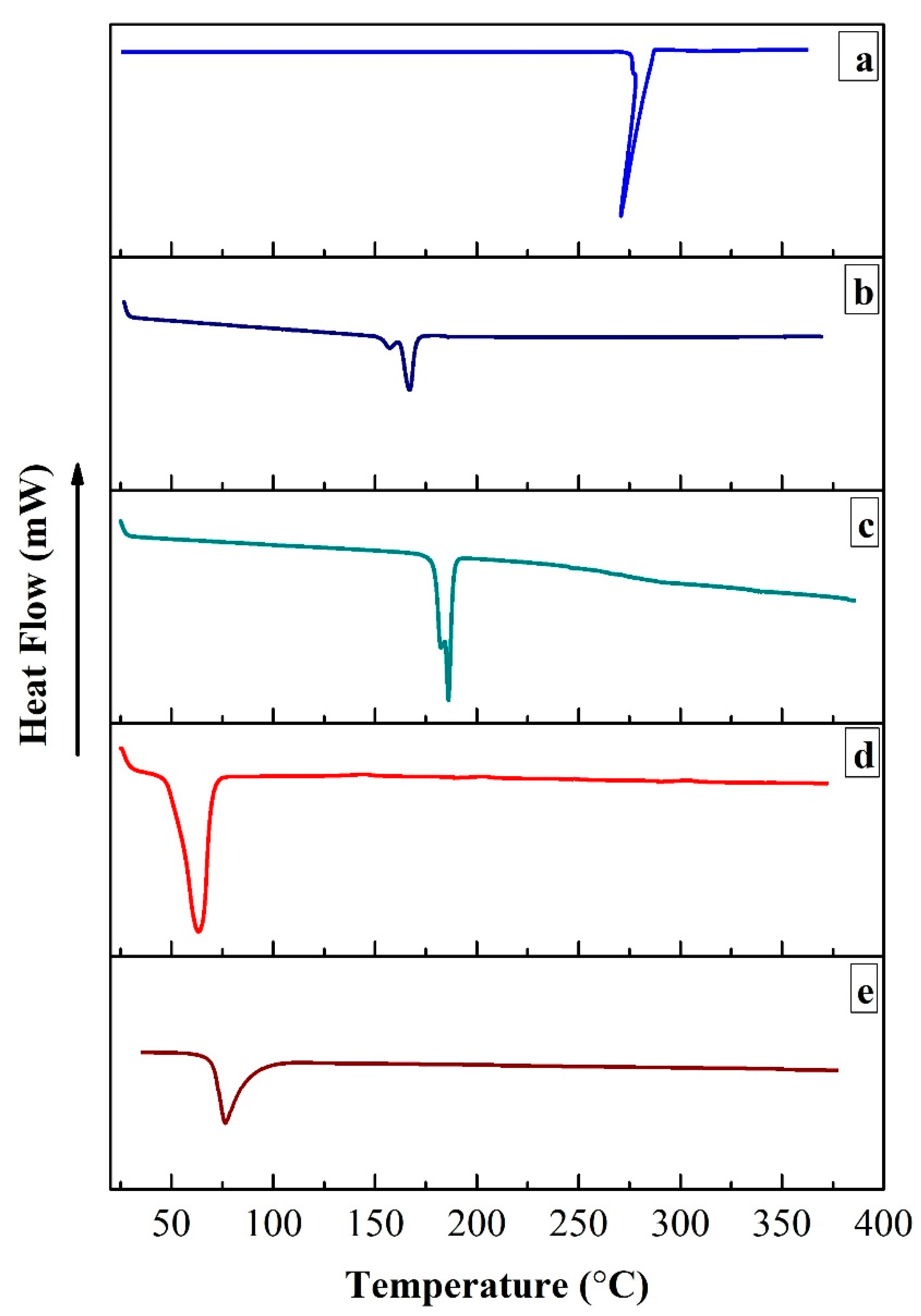
3.1.5. Differential Scanning Calorimetric (DSC) Analysis
3.1.6. In Vitro Release and Kinetic Modeling
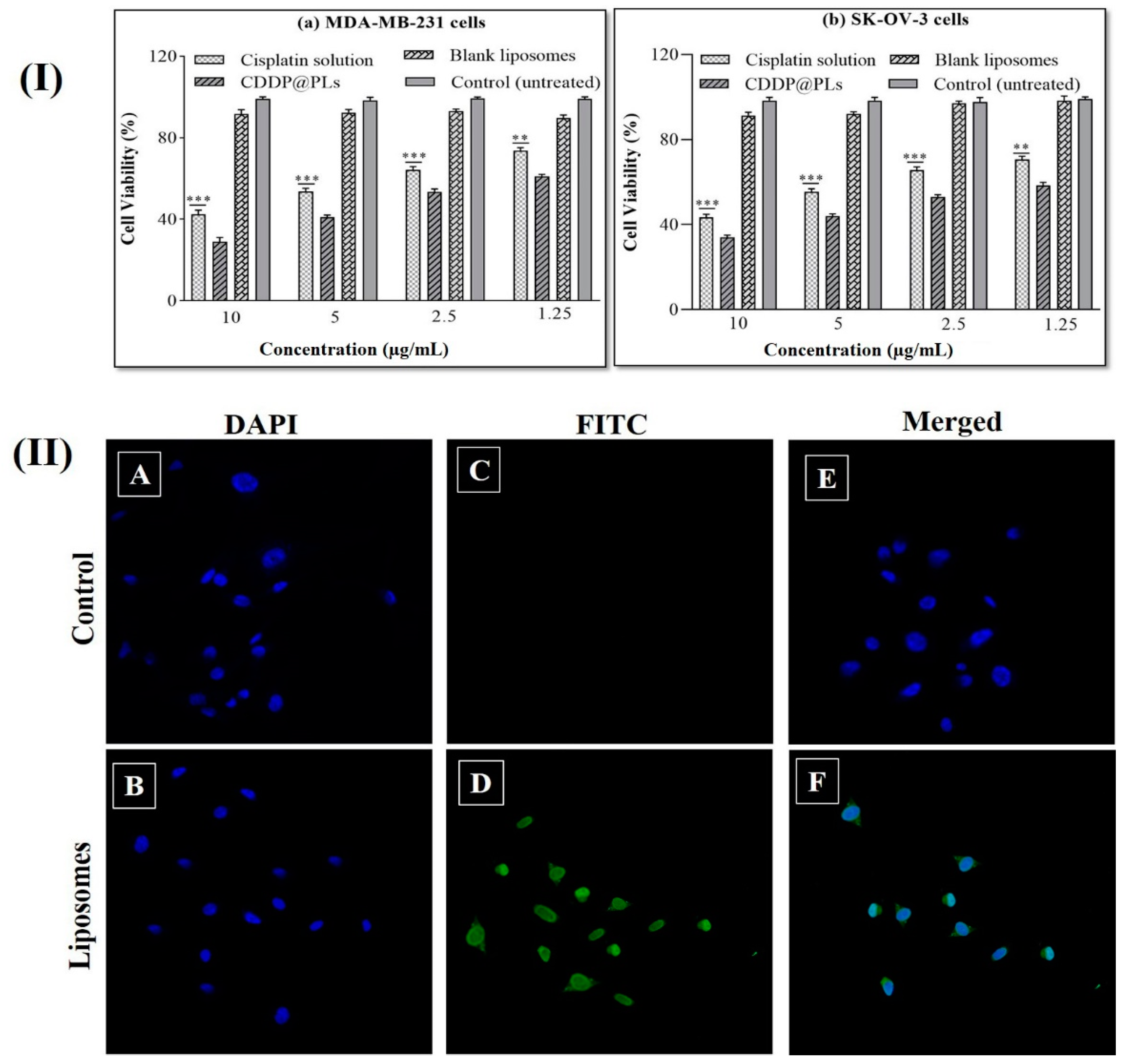
3.2. Cytotoxicity Study
3.3. Cell Uptake Studies
3.4. Stability Study
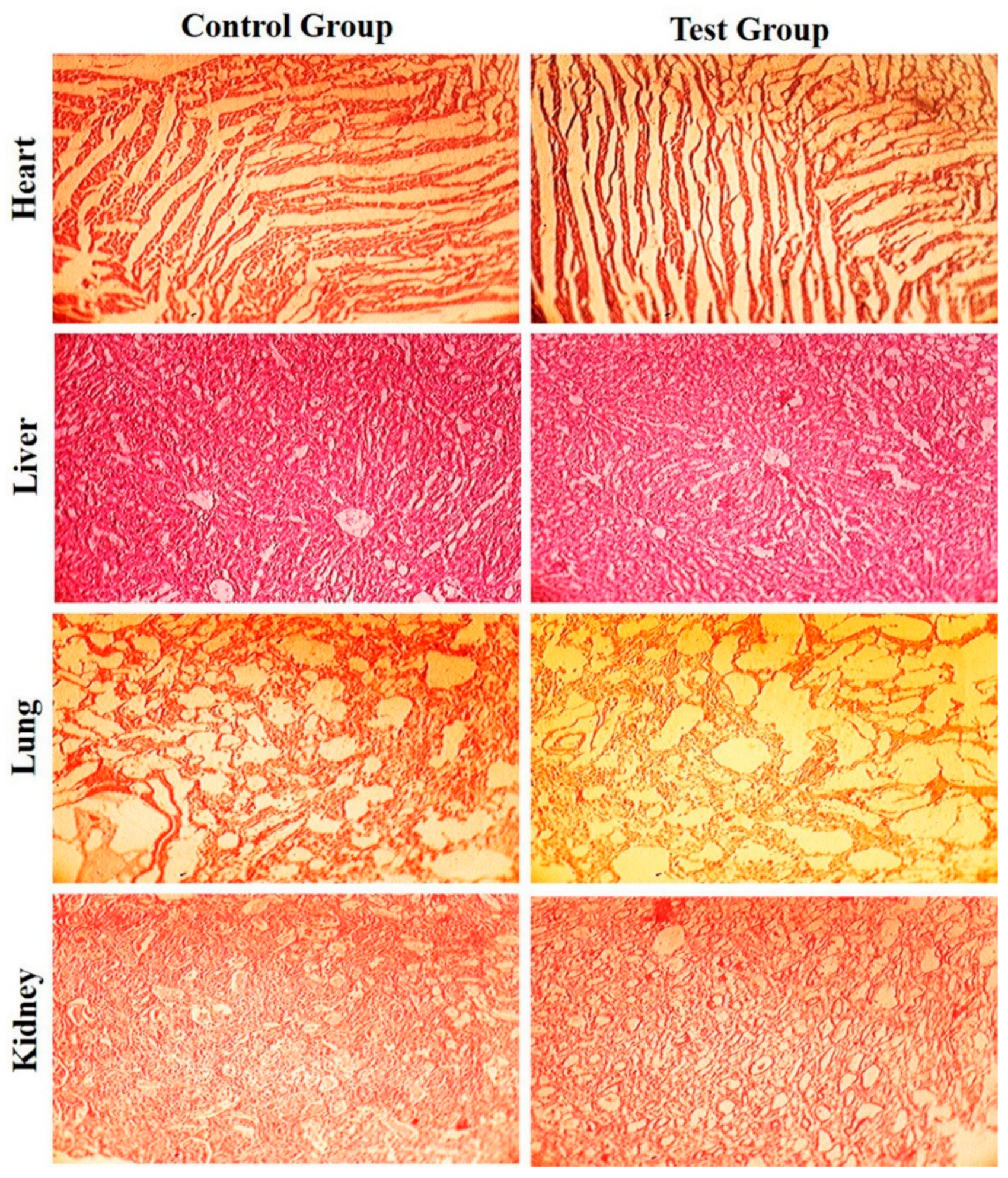
3.5. Acute Toxicity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahim, M.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, C.; Huang, Y.; Zhang, F.; Lin, G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surfaces B Biointerfaces 2017, 151, 19–25. [Google Scholar] [CrossRef]
- Tian, L.; Bae, Y.H. Cancer nanomedicines targeting tumor extracellular pH. Colloids Surfaces B Biointerfaces 2012, 99, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Pispas, S.; Demetzos, C. Stimuli-responsive nanocarriers for drug delivery. In Nanomaterials for Clinical Applications; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 99–121. [Google Scholar]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 07–15. [Google Scholar] [CrossRef]
- Cosaert, J.; Quoix, E. Platinum drugs in the treatment of non-small-cell lung cancer. Br. J. Cancer 2002, 87, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2011, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabik, C.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Kong, S.D.; Sartor, M.; Hu, C.-M.J.; Zhang, W.; Zhang, L.; Jin, S. Magnetic field activated lipid–polymer hybrid nanoparticles for stimuli-responsive drug release. Acta Biomater. 2013, 9, 5447–5452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2012, 5, 96–107. [Google Scholar] [CrossRef]
- Leite, E.A.; Souza, C.M.; Carvalho-Júnior, Á.D.; Coelho, L.G.; Lana, Â.M.; Cassali, G.D.; Oliveira, M.C. Encapsulation of cisplatin in long-circulating and pH-sensitive liposomes improves its antitumor effect and reduces acute toxicity. Int. J. Nanomed. 2012, 7, 5259–5269. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Madni, A.; Tahir, N.; Jan, N.; Shah, H.; Khan, S.; Ullah, R.; Bari, A.; Khan, M.S. Mild Hyperthermia Responsive Liposomes for Enhanced In Vitro and In Vivo Anticancer Efficacy of Doxorubicin against Hepatocellular Carcinoma. Pharmaceutics 2021, 13, 1310. [Google Scholar] [CrossRef]
- Duan, Y.; Wei, L.; Petryk, J.; Ruddy, T.D. Formulation, characterization and tissue distribution of a novel pH-sensitive long-circulating liposome-based theranostic suitable for molecular imaging and drug delivery. Int. J. Nanomed. 2016, 11, 5697–5708. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically Cross-Linked Chitosan Nanoparticles for Sustained Delivery of Docetaxel: Fabrication, Post-Formulation and Acute Oral Toxicity Evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef] [Green Version]
- Bhalerao, S.S.; Harshal, A.R. Preparation, Optimization, Characterization, and Stability Studies of Salicylic Acid Liposomes. Drug Dev. Ind. Pharm. 2003, 29, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Madni, A.; Rahim, M.A.; Khan, N.U.; Jamshaid, T.; Khan, A.; Jabar, A.; Khan, S.; Shah, H. In vitro anti-leukemic assessment and sustained release behaviour of cytarabine loaded biodegradable polymer based nanoparticles. Life Sci. 2021, 267, 118971. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int. J. Food Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Z.; Lv, S.; Song, W.; Hong, H.; Jing, X.; Zhang, Y.; Chen, X. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials 2014, 35, 3851–3864. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Rahim, M.A.; Mahmood, M.A.; Jabar, A.; Rehman, M.; Shah, H.; Khan, A.; Tahir, N.; Shah, A. Enhancement of Dissolution and Skin Permeability of Pentazocine by Proniosomes and Niosomal Gel. AAPS PharmSciTech 2018, 19, 1544–1553. [Google Scholar] [CrossRef]
- Yu, R.; Jin, H.; Jin, C.; Huang, X.; Lin, J.; Teng, Y. Inhibition of the CSF-1 receptor sensitizes ovarian cancer cells to cisplatin. Cell Biochem. Funct. 2018, 36, 80–87. [Google Scholar] [CrossRef]
- Khan, S.; Aamir, M.N.; Madni, A.; Jan, N.; Khan, A.; Jabar, A.; Shah, H.; Rahim, M.A.; Ali, A. Lipid poly (ɛ-caprolactone) hybrid nanoparticles of 5-fluorouracil for sustained release and enhanced anticancer efficacy. Life Sci. 2021, 284, 119909. [Google Scholar] [CrossRef]
- Muppidi, K.; Pumerantz, A.S.; Wang, J.; Betageri, G. Development and Stability Studies of Novel Liposomal Vancomycin Formulations. ISRN 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Madni, A.; Rahim, M.A.; Shah, H.; Jabar, A.; Khan, M.M.; Khan, A.; Jan, N.; Mahmood, M.A. Enhanced in vitro release and permeability of glibenclamide by proliposomes: Development, characterization and histopathological evaluation. J. Drug Deliv. Sci. Technol. 2021, 63, 102450. [Google Scholar] [CrossRef]
- Khan, K.U.; Akhtar, N.; Minhas, M.U. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS PharmSciTech 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Jain, S.; Deore, S.V.; Ghadi, R.; Chaudhari, D.; Kuche, K.; Katiyar, S.S. Tumor microenvironment responsive VEGF-antibody functionalized pH sensitive liposomes of docetaxel for augmented breast cancer therapy. Mater. Sci. Eng. C 2021, 121, 111832. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Vaibhav, K.; Pandey, R.S.; Jain, U.K.; Katare, O.P.; Katyal, A.; Madan, J. Improved cisplatin delivery in cervical cancer cells by utilizing folate-grafted non-aggregated gelatin nanoparticles. Biomed. Pharmacother. 2015, 69, 1–10. [Google Scholar] [CrossRef]
- Munaweera, I.; Shi, Y.; Koneru, B.; Patel, A.; Dang, M.H.; Di Pasqua, A.J.; Balkus, K.J., Jr. Nitric oxide-and cisplatin-releasing silica nanoparticles for use against non-small cell lung cancer. J. Inorg. Biochem. 2015, 153, 23–31. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Y.; Cao, Z.; Xu, Y.; Lu, C.; Zhao, M.; Gou, J.; Yin, T.; Zhang, Y.; He, H.; et al. Aprepitant Intravenous Emulsion Based on Ion Pairing/Phospholipid Complex for Improving Physical and Chemical Stability During Thermal Sterilization. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lai, Y.; Shi, S.; Hao, S.; Wei, J.; Chen, X. Copper Sulfide Nanoparticles with Phospholipid-PEG Coating for In Vivo Near-Infrared Photothermal Cancer Therapy. Chem.-Asian J. 2015, 10, 370–376. [Google Scholar] [CrossRef]
- Rehman, M.; Ihsan, A.; Madni, A.; Bajwa, S.Z.; Shi, D.; Webster, T.J.; Khan, W.S. Solid lipid nanoparticles for thermoresponsive targeting: Evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int. J. Nanomed. 2017, 12, 8325–8336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Yellepeddi, V.; Vangara, K.K.; Palakurthi, S. Poly(amido)amine (PAMAM) dendrimer–cisplatin complexes for chemotherapy of cisplatin-resistant ovarian cancer cells. J. Nanoparticle Res. 2013, 15, 1–15. [Google Scholar] [CrossRef]
- Sacchetti, F.; D’Arca, D.; Genovese, F.; Pacifico, S.; Maretti, E.; Hanuskova, M.; Iannuccelli, V.; Costi, M.P.; Leo, E. Conveying a newly designed hydrophilic anti-human thymidylate synthase peptide to cisplatin resistant cancer cells: Are pH-sensitive liposomes more effective than conventional ones? Drug Dev. Ind. Pharm. 2017, 43, 465–473. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the lipid layer of lipid–PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Romero, A.-M.; Maestrelli, F.; Mura, P.A.; Rabasco, A.M.; González-Rodríguez, M.L. Novel Findings about Double-Loaded Curcumin-in-HPβcyclodextrin-in Liposomes: Effects on the Lipid Bilayer and Drug Release. Pharmaceutics 2018, 10, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadon, R.S.; Sharma, M. Docetaxel-loaded lipid-polymer hybrid nanoparticles for breast cancer therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kwak, S.S.; Jang, M.H.; Kang, M.H.; Sung, S.W.; Kim, C.H.; Kim, S.R.; Yeom, D.W.; Kang, M.J.; Choi, Y.W. Docetaxel-loaded RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes: Drug release, cytotoxicity, and antitumor efficacy. Int. J. Pharm. 2017, 523, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Lu, Y.; Tao, C.; Huang, J.; Zhang, H.; Yu, Y.; Zou, H.; Gao, J.; Zhong, Y. Complexes containing cationic and anionic pH-sensitive liposomes: Comparative study of factors influencing plasmid DNA gene delivery to tumors. Int. J. Nanomed. 2013, 8, 1573–1593. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Shah, H.; Khan, K.U.; Thakur, R.R.S. A difunctional Pluronic® 127-based in situ formed injectable thermogels as prolonged and controlled curcumin depot, fabrication, in vitro characterization and in vivo safety evaluation. J. Biomater. Sci. Polym. Ed. 2021, 32, 281–319. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.U.; Minhas, M.U.; Sohail, M.; Badshah, S.F.; Abdullah, O.; Khan, S.; Munir, A.; Suhail, M. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev. Ind. Pharm. 2021, 47, 465–476. [Google Scholar] [CrossRef] [PubMed]


| Code | Lipid Mixture Ratio (DOPE: CHEMS: DSPE:PEG2000) | Lipid Mixture Weight (mg) DOPE: CHEMS: DSPE-PEG2000 | Cisplatin (mg) | Particle Size (nm) | PDI | Zeta Potential (mV) | (%) E.E |
|---|---|---|---|---|---|---|---|
| PL1 | 45:50:05 | 33.48:24.33:14.02 | 5.0 | 206.4 ± 2.26 | 0.417 ± 0.008 | −24.6 ± 1.72 | 69.47 ± 1.23 |
| PL2 | 50:45:05 | 37.20:21.90:14.02 | 5.0 | 194.3 ± 2.21 | 0.422 ± 0.010 | −22.8 ± 2.01 | 65.52 ± 2.14 |
| PL3 | 55:40:05 | 40.92:19.46:14.02 | 5.0 | 191.2 ± 1.67 | 0.386 ± 0.009 | −22.5 ± 0.38 | 61.23 ± 1.98 |
| PL4 | 60:35:05 | 44.64:17.03:14.02 | 5.0 | 171.9 ± 2.26 | 0.371 ± 0.011 | −20.2 ± 2.69 | 52.19 ± 1.45 |
| PL5 | 65:30:05 | 48.36:14.60:14.02 | 5.0 | 153.2 ± 3.08 | 0.261 ± 0.007 | −17.8 ± 1.26 | 47.25 ± 1.21 |
| Formulation Code | pH | Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | N | ||
| PL1 | 7.4 | 0.0072 | 0.1690 | 0.8103 | 0.9898 | 0.279 |
| 6.5 | 0.0837 | 0.4866 | 0.8409 | 0.9690 | 0.309 | |
| 5.5 | 0.5912 | 0.9015 | 0.9823 | 0.9949 | 0.426 | |
| PL2 | 7.4 | 0.0728 | 0.2674 | 0.8440 | 0.9910 | 0.279 |
| 6.5 | 0.0778 | 0.4938 | 0.8405 | 0.9731 | 0.307 | |
| 5.5 | 0.6863 | 0.9278 | 0.9915 | 0.9940 | 0.464 | |
| PL3 | 7.4 | 0.0734 | 0.1488 | 0.7930 | 0.9952 | 0.271 |
| 6.5 | 0.1466 | 0.5724 | 0.8670 | 0.9823 | 0.316 | |
| 5.5 | 0.6707 | 0.9242 | 0.9904 | 0.9943 | 0.456 | |
| PL4 | 7.4 | 0.0297 | 0.2282 | 0.8335 | 0.9968 | 0.289 |
| 6.5 | 0.3215 | 0.6595 | 0.9233 | 0.9910 | 0.349 | |
| 5.5 | 0.5821 | 0.8919 | 0.9803 | 0.9946 | 0.421 | |
| PL5 | 7.4 | 0.0759 | 0.2496 | 0.8439 | 0.9936 | 0.294 |
| 6.5 | 0.3830 | 0.6810 | 0.9375 | 0.9900 | 0.364 | |
| 5.5 | 0.4436 | 0.8238 | 0.9582 | 0.9988 | 0.377 | |
| Formulation | Storage Condition | Time | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| PL1 | Initial | 206.4 ± 2.26 | 0.417 ± 0.008 | −24.6 ± 1.72 | |
| 2–8 °C | After 90 days | 209.5 ± 1.78 | 0.423 ± 0.004 | −23.2 ± 1.07 | |
| 25 °C | 214.7 ± 2.45 | 0.441 ± 0.005 | −22.8 ± 0.86 | ||
| 37 °C | 219.8 ± 1.96 | 0.446 ± 0.003 | −22.3 ± 1.01 | ||
| PL2 | Initial | 194.3 ± 2.21 | 0.422 ± 0.010 | −22.8 ± 2.01 | |
| 2–8 °C | After 90 days | 197.1 ± 1.41 | 0.429 ± 0.011 | −22.3 ± 0.61 | |
| 25 °C | 199.2 ± 0.97 | 0.434 ± 0.008 | −21.7 ± 0.70 | ||
| 37 °C | 204.7 ± 0.92 | 0.440 ± 0.005 | −20.9 ± 1.40 | ||
| PL3 | Initial | 191.2 ± 1.67 | 0.386 ± 0.009 | −22.5 ± 0.38 | |
| 2–8 °C | After 90 days | 196.3 ± 0.52 | 0.388 ± 0.004 | −22.2 ± 1.87 | |
| 25 °C | 198.8 ± 1.41 | 0.391 ± 0.006 | −21.7 ± 0.56 | ||
| 37 °C | 201.4 ± 1.35 | 0.398 ± 0.013 | −21.3 ± 1.02 | ||
| PL4 | Initial | 171.9 ± 2.26 | 0.371 ± 0.011 | −20.2 ± 2.69 | |
| 2–8 °C | After 90 days | 173.6 ± 2.08 | 0.372 ± 0.007 | −19.5 ± 1.63 | |
| 25 °C | 174.7 ± 1.15 | 0.376 ± 0.009 | −19.3 ± 2.07 | ||
| 37 °C | 182.9 ± 1.14 | 0.81 ± 0.013 | −18.8 ± 0.91 | ||
| PL5 | Initial | 153.2 ± 3.08 | 0.261 ± 0.007 | −17.8 ± 1.26 | |
| 2–8 °C | After 90 days | 156.7 ± 1.85 | 0.264 ± 0.003 | −17.3 ± 1.21 | |
| 25 °C | 159.2 ± 2.92 | 0.267 ± 0.006 | −16.5 ± 1.56 | ||
| 37 °C | 163.4 ± 1.60 | 0.273 ± 0.010 | −16.2 ± 2.17 | ||
| Biochemical Parameters | Control Group | Test Group |
| Bilirubin (mg/dL) | 0.58 ± 0.09 | 0.61 ± 0.13 |
| Urea (mg/dL) | 35.45 ± 1.98 | 34.21 ± 2.34 |
| Creatinine (mg/dL) | 0.19 ± 0.12 | 0.23 ± 0.16 |
| Uric acid (mg/dL) | 2.07 ± 0.45 | 2.10 ± 0.39 |
| Cholesterol (mg/dL) | 59.87 ± 3.44 | 61.22 ± 2.87 |
| Triglycerides (mg/dL) | 74.32 ± 1.34 | 72.10 ± 1.65 |
| ALT(IU/L) | 69.55 ± 1.23 | 70.98 ± 0.97 |
| Alkaline Phosphatase (IU/L) | 486.43 ± 4.59 | 493.29 ± 4.11 |
| Hematological Parameters | Control Group | Test Group |
| Red blood cells | 5.48 ± 0.67 | 5.21 ± 0.84 |
| White blood cells | 7.67 ± 0.98 | 7.58 ± 0.77 |
| Platelets | 4.24 ± 0.59 | 4.21 ± 0.68 |
| Lymphocytes | 61.42 ± 1.33 | 60.34 ± 1.19 |
| Monocytes | 1.53 ± 1.90 | 1.49 ± 1.76 |
| Neutrophils | 44.32 ± 0.88 | 45.90 ± 0.93 |
| Mean corpuscular volume (MCV) | 63.56 ± 0.61 | 62.87 ± 0.74 |
| Mean corpuscular hemoglobin | 21.33 ± 0.56 | 22.04 ± 0.43 |
| Hemoglobin (g/dL) | 12.1 ± 0.35 | 12.5 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, H.; Madni, A.; Khan, M.M.; Ahmad, F.-u.-D.; Jan, N.; Khan, S.; Rahim, M.A.; Khan, S.; Ali, M.M.; Kazi, M. pH-Responsive Liposomes of Dioleoyl Phosphatidylethanolamine and Cholesteryl Hemisuccinate for the Enhanced Anticancer Efficacy of Cisplatin. Pharmaceutics 2022, 14, 129. https://doi.org/10.3390/pharmaceutics14010129
Shah H, Madni A, Khan MM, Ahmad F-u-D, Jan N, Khan S, Rahim MA, Khan S, Ali MM, Kazi M. pH-Responsive Liposomes of Dioleoyl Phosphatidylethanolamine and Cholesteryl Hemisuccinate for the Enhanced Anticancer Efficacy of Cisplatin. Pharmaceutics. 2022; 14(1):129. https://doi.org/10.3390/pharmaceutics14010129
Chicago/Turabian StyleShah, Hassan, Asadullah Madni, Muhammad Muzamil Khan, Fiaz-ud-Din Ahmad, Nasrullah Jan, Safiullah Khan, Muhammad Abdur Rahim, Shahzeb Khan, Meser M. Ali, and Mohsin Kazi. 2022. "pH-Responsive Liposomes of Dioleoyl Phosphatidylethanolamine and Cholesteryl Hemisuccinate for the Enhanced Anticancer Efficacy of Cisplatin" Pharmaceutics 14, no. 1: 129. https://doi.org/10.3390/pharmaceutics14010129
APA StyleShah, H., Madni, A., Khan, M. M., Ahmad, F.-u.-D., Jan, N., Khan, S., Rahim, M. A., Khan, S., Ali, M. M., & Kazi, M. (2022). pH-Responsive Liposomes of Dioleoyl Phosphatidylethanolamine and Cholesteryl Hemisuccinate for the Enhanced Anticancer Efficacy of Cisplatin. Pharmaceutics, 14(1), 129. https://doi.org/10.3390/pharmaceutics14010129











