Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Identification, and Susceptibility Tests
2.2. Whole Genome Sequencing and Bioinformatic Analysis
2.3. Preparation and Characterization of the Tobramycin Liposomes (TL) and Tobramycin-N-Acetylcysteine Liposomes (TNL) Formulations
2.4. Tobramycin Encapsulation Efficiency (EE%) of the TL and TNL Formulations
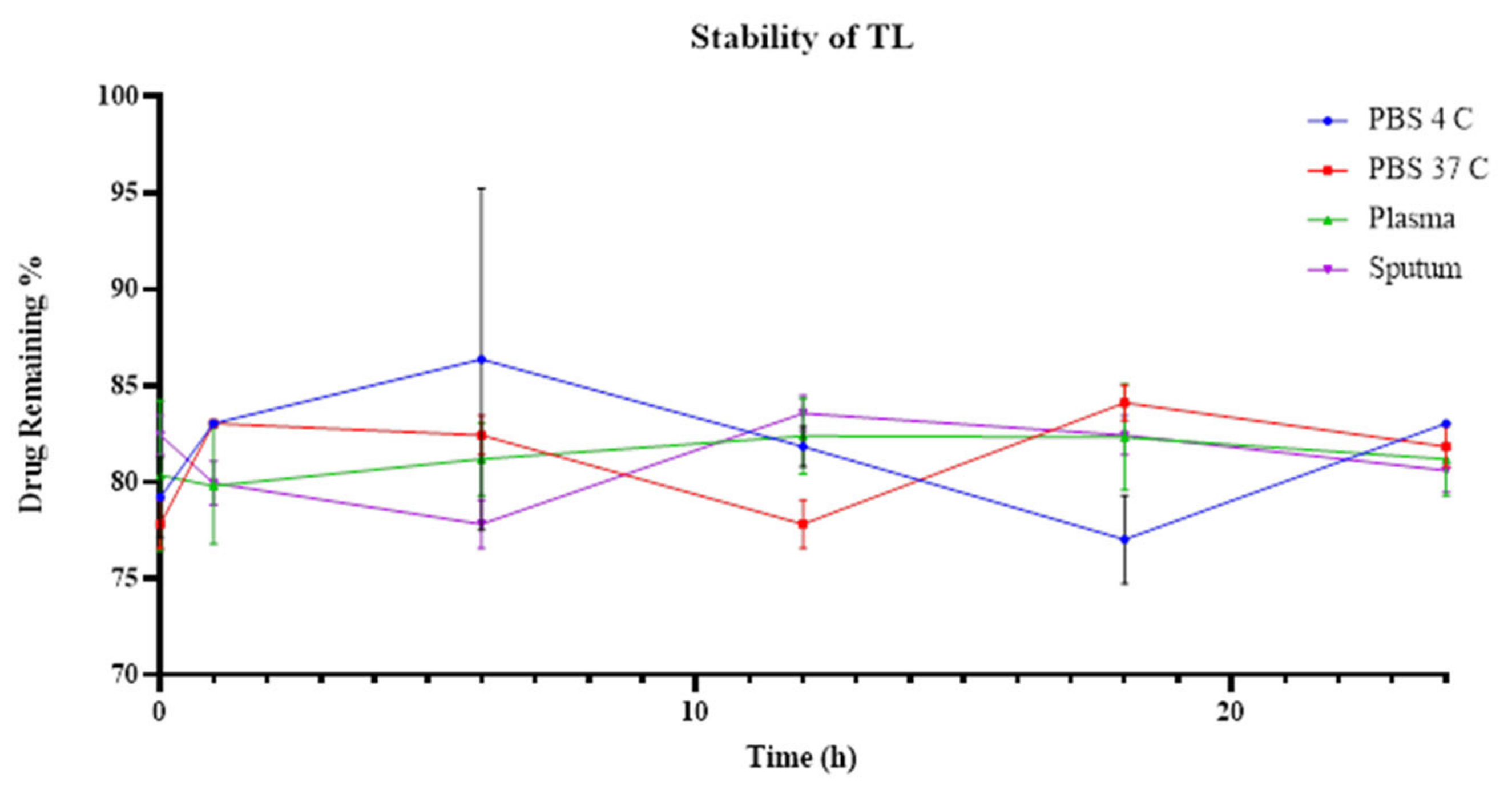
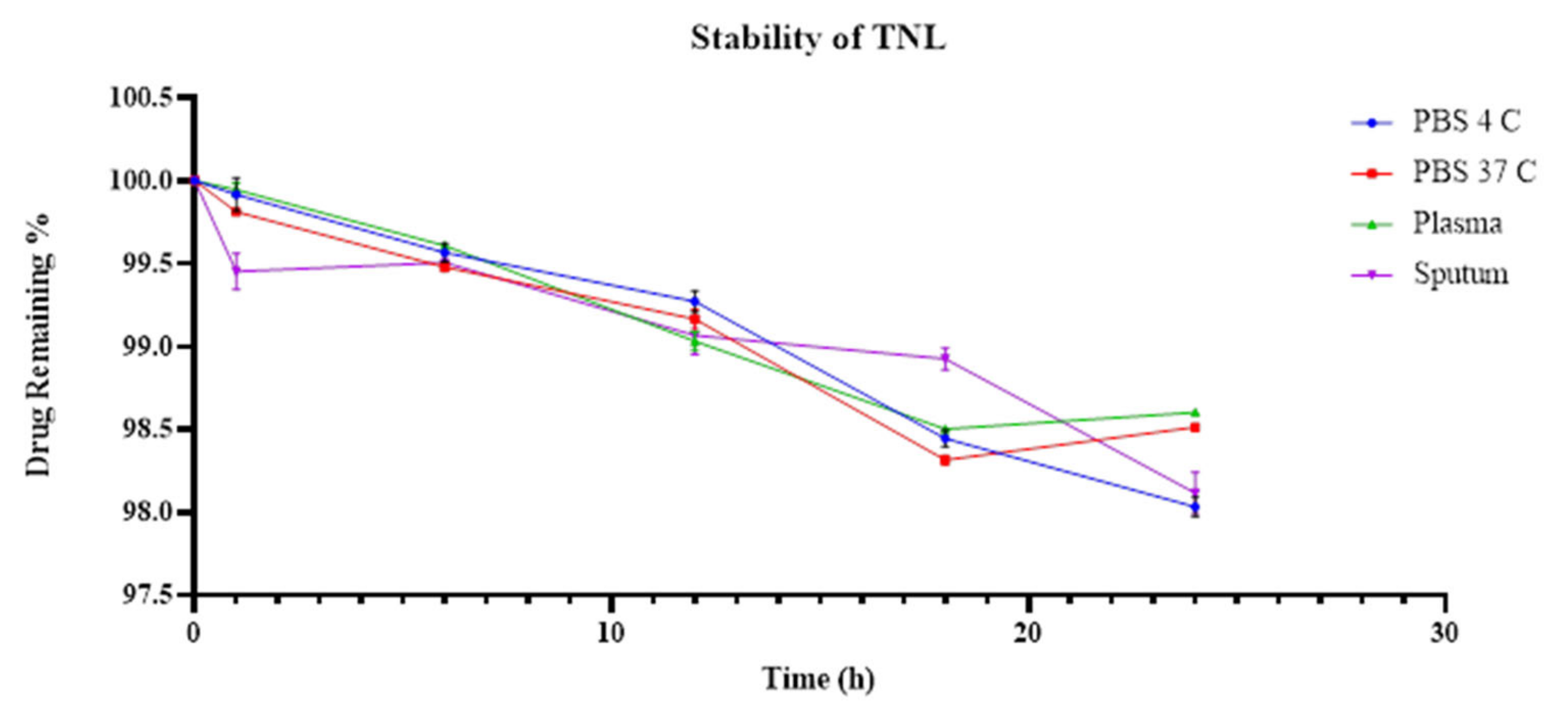
2.5. The Stability of the TL and TNL Formulations in Biological and Storage Conditions
2.6. Antibacterial Activity of TL and TNL Formulations
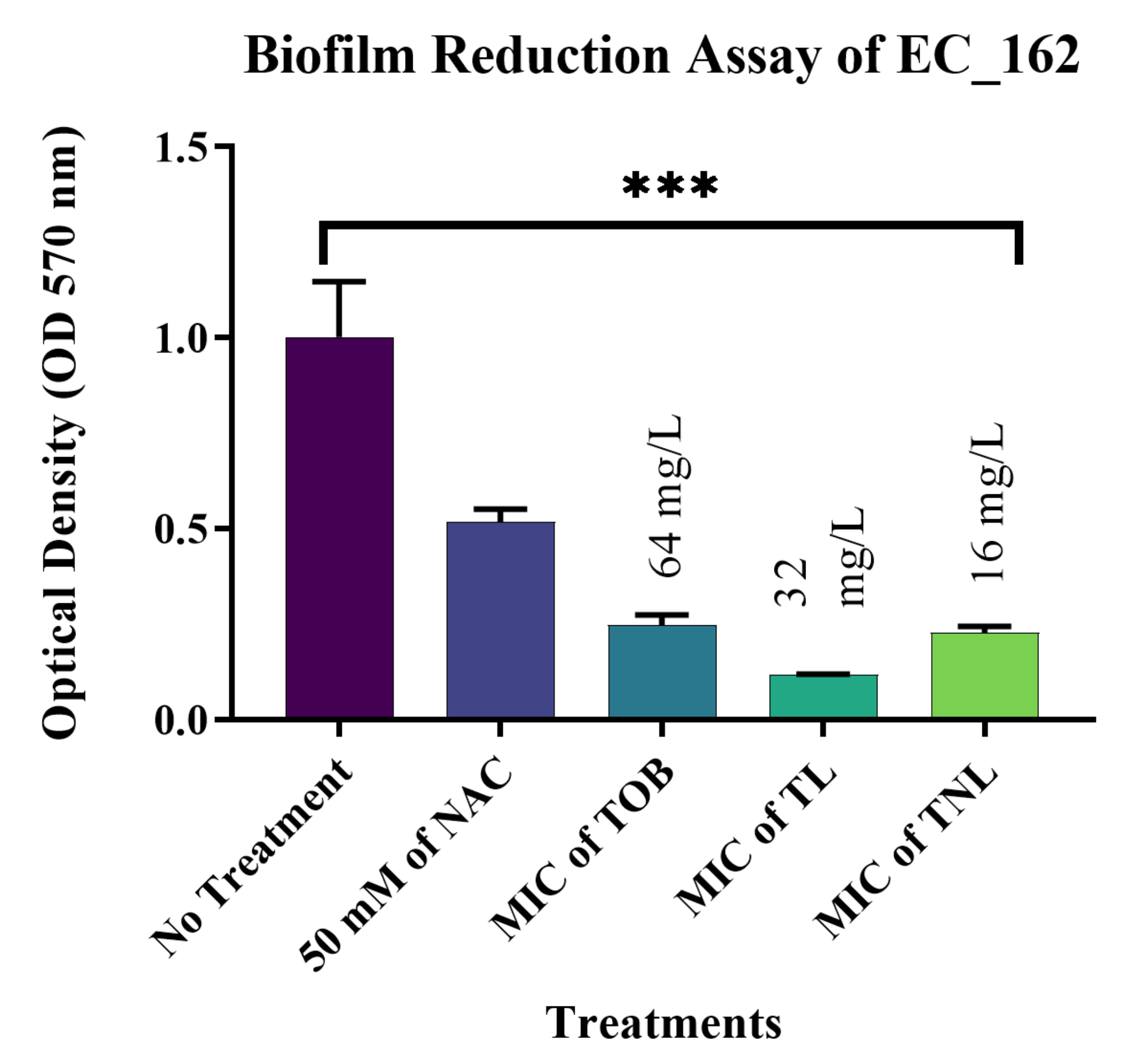
2.7. Biofilm Reduction of TL and TNL Formulations
2.8. Statistical Analysis
3. Results
3.1. Susceptibility Profiles of the E. coli, K. pneumoniae, and A. baumannii Clinical Isolates
3.2. Whole Genome Sequencing and Bioinformatic Analysis
3.3. TL and TNL Formulation Characterization
3.4. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Tobramycin against the Clinical Bacterial Isolates
3.5. TL and TNL Formulations Stability within Biological and Storage Conditions
3.6. The Antibacterial Activity of the TL and TNL Formulations against the Genetically Resistant Clinical Bacterial Isolates
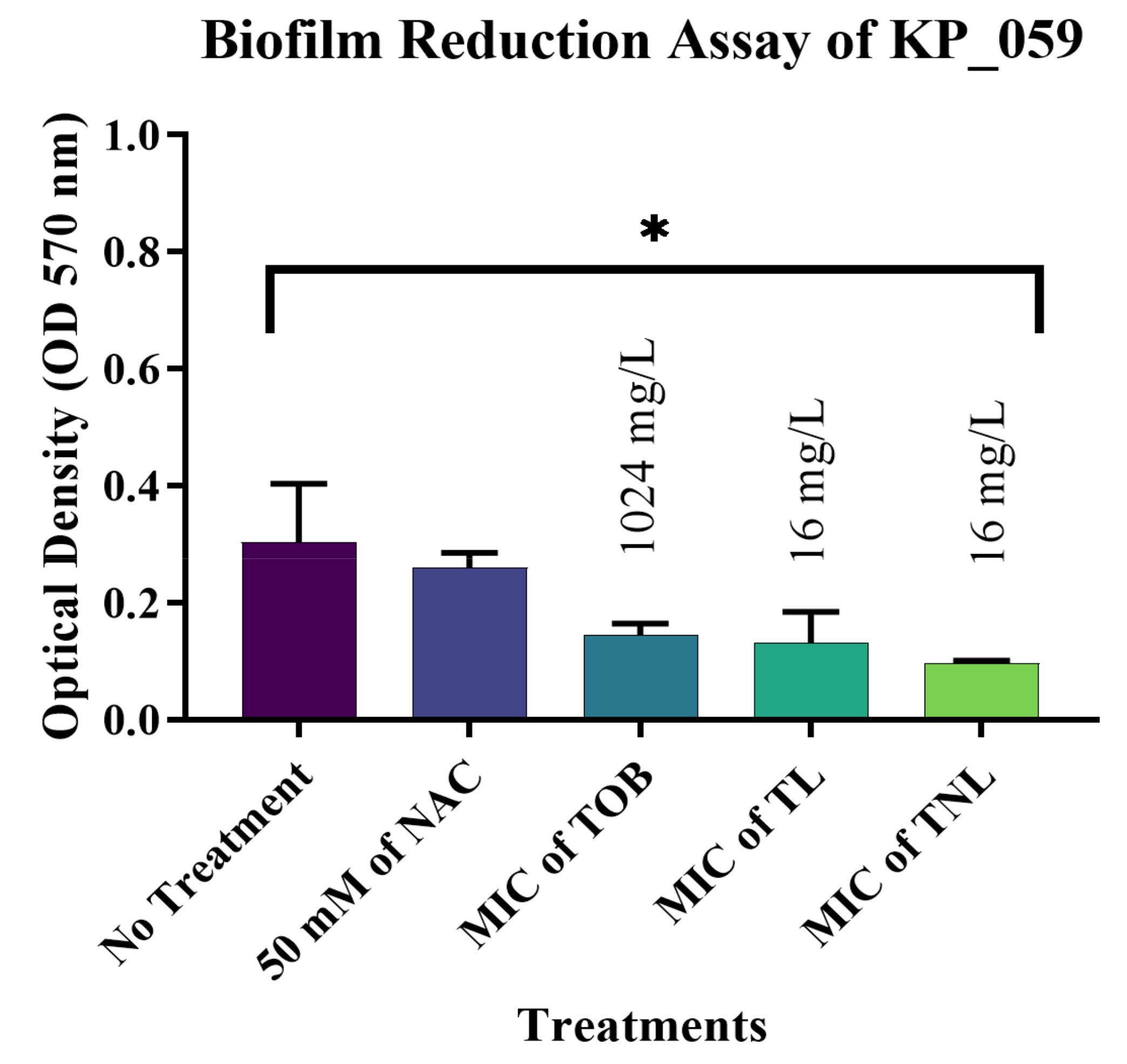
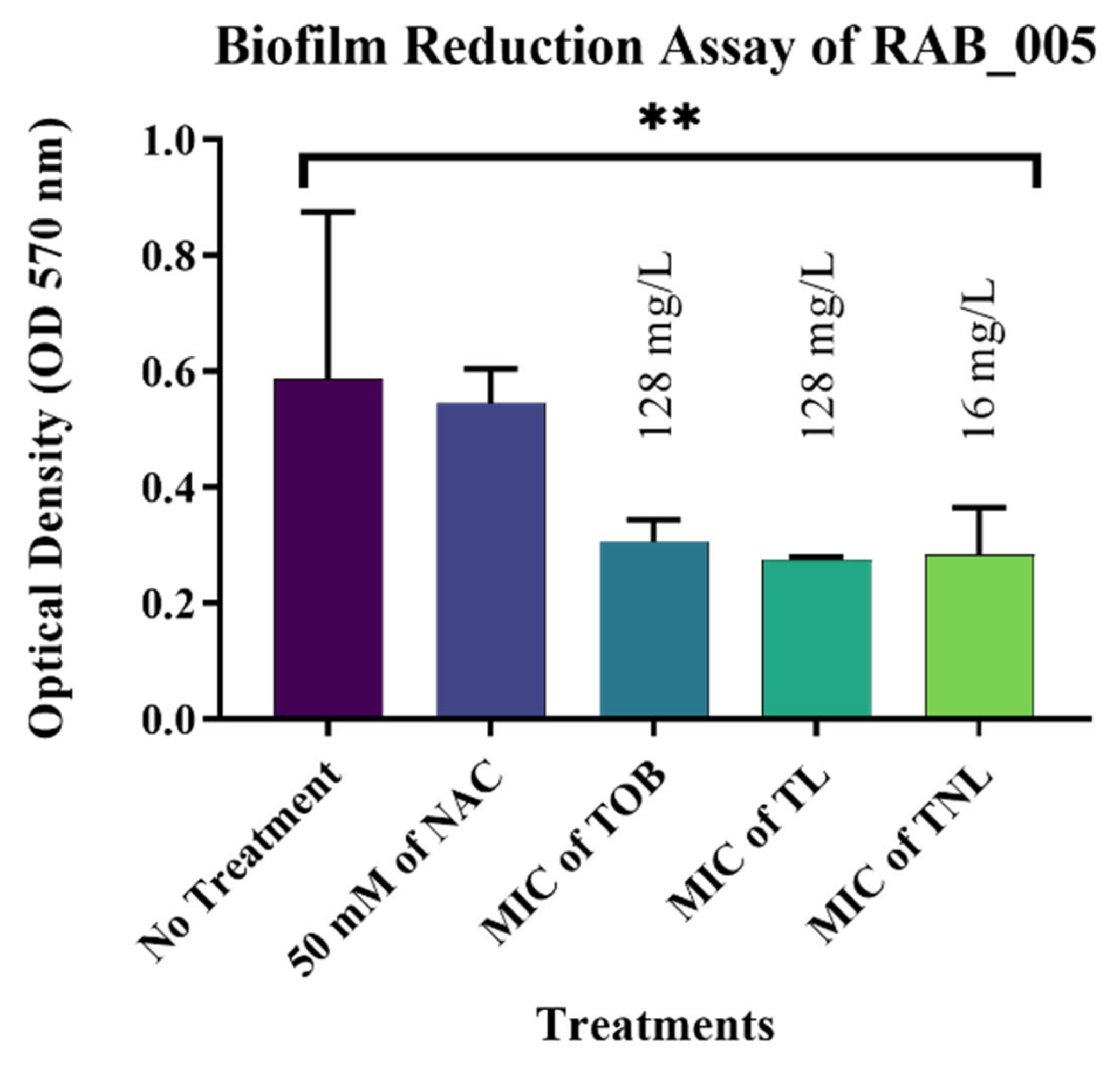
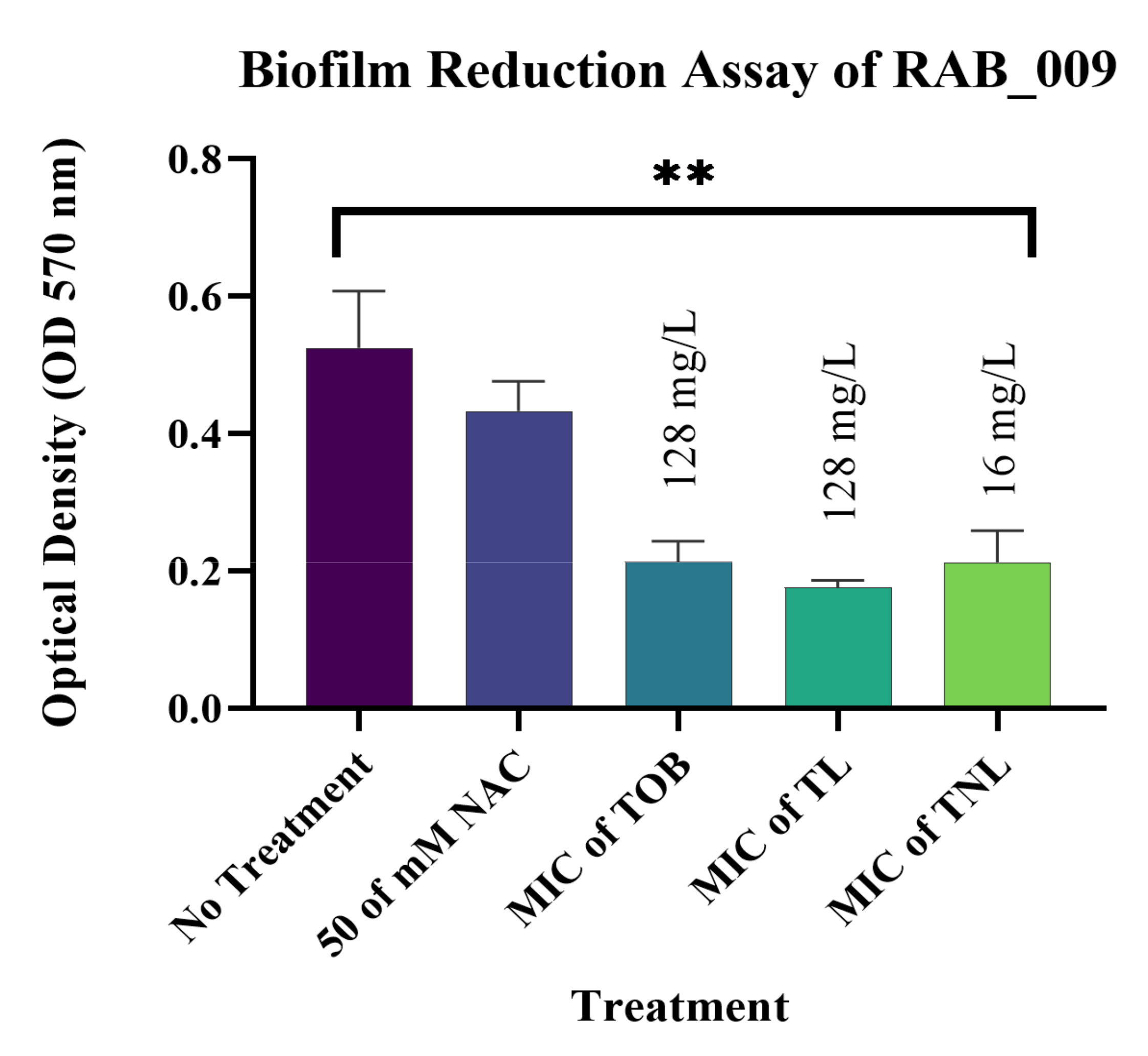
3.7. Biofilm Reduction Activity of TL and TNL Formulations against Clinical Bacterial Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falagas, E.M.; Bliziotis, A.I.; Kasiakou, S.K.; Samonis, G.; Athanassopoulou, P.; Michalopoulos, A. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect. Dis. 2005, 5, 24–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Johani, S.M.; Akhter, J.; Balkhy, H.; El-Saed, A.; Younan, M.; Memish, Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann. Saudi Med. 2010, 30, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/index.html (accessed on 10 November 2021).
- Ruppé, É.; Woerther, P.-L.; Barbier, F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann. Intensiv. Care 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. MedChemComm 2016, 7, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leggett, J.E. Aminoglycosides. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1233–1238.e1. [Google Scholar]
- Champney, W.S. Antibiotics targeting bacterial ribosomal subunit biogenesis. J. Antimicrob. Chemother. 2020, 75, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Frazier, A.D.; Champney, W.S. Impairment of ribosomal subunit synthesis in aminoglycoside-treated ribonuclease mutants of Escherichia coli. Arch. Microbiol. 2012, 194, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Ojdana, D.; Sieńko, A.; Sacha, P.; Majewski, P.; Wieczorek, P.; Wieczorek, A.; Tryniszewska, E. Genetic basis of enzymatic resistance of E. coli to aminoglycosides. Adv. Med. Sci. 2018, 63, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Minshew, B.H.; Holmes, R.K.; Sanford, J.P.; Baxter, C.R. Transferrable Resistance to Tobramycin in Klebsiella pneumoniae and Enterobacter cloacae Associated with Enzymatic Acetylation of Tobramycin. Antimicrob. Agents Chemother. 1974, 6, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Casanova, T.; Garigliany, M. N-acetylcysteine: An old drug with variable Anti-influenza properties. J. Controv. Biomed. Res. 2016, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, A.-C.; Hermansson, M.; Elwing, H. N-Acetyl-l-Cysteine Affects Growth, Extracellular Polysaccharide Production, and Bacterial Biofilm Formation on Solid Surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef] [Green Version]
- DiNicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar]
- Li, X.; Kim, J.; Wu, J.; I Ahamed, A.; Wang, Y.; Martins-Green, M. N-Acetyl-cysteine and Mechanisms Involved in Resolution of Chronic Wound Biofilm. J. Diabetes Res. 2020, 2020, 9589507. [Google Scholar] [CrossRef] [Green Version]
- Blasi, F.; Page, C.P.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Kranzer, K.; Elamin, W.; Cox, H.; Seddon, J.; Ford, N.; Drobniewski, F. A systematic review and meta-analysis of the efficacy and safety ofN-acetylcysteine in preventing aminoglycoside-induced ototoxicity: Implications for the treatment of multidrug-resistant TB. Thorax 2015, 70, 1070–1077. [Google Scholar] [CrossRef] [Green Version]
- Tepel, M. N-Acetylcysteine in the prevention of ototoxicity. Kidney Int. 2007, 72, 231–232. [Google Scholar] [CrossRef] [Green Version]
- Somdas, M.A.; Korkmaz, F.; Gurgen, S.G.; Sagit, M.; Akcadag, A. N-acetylcysteine Prevents Gentamicin Ototoxicity in a Rat Model. J. Int. Adv. Otol. 2015, 11, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.; Efrati, S.; Eviatar, E.; Abramsohn, R.; Yarovoy, I.; Gersch, E.; Averbukh, Z.; Weissgarten, J. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007, 72, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coca, A.; Martinez, A.; Soriano, E.; Blade, J.; Segura, F.; Ribas-Mundo, M. Tobramycin nephrotoxicity. A prospective clinical study. Postgrad. Med. J. 1979, 55, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlmeter, G. Gentamicin and Tobramycin Clinical Pharmacokinetics and Nephrotoxicity Aspects on Assay Techniques. Scand. J. Infect. Dis. 1979, 11, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-Induced Nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Gomez, A.G.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Halwani, M.; Azghani, A.O.; Lafrenie, R.; Omri, A. Mechanism of Enhanced Activity of Liposome-Entrapped Aminoglycosides against Resistant Strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2016–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef]
- Fresta, M.; Spadaro, A.; Cerniglia, G.; Ropero, I.M.; Puglisi, G.; Furneri, P.M. Intracellular accumulation of ofloxacin-loaded liposomes in human synovial fibroblasts. Antimicrob. Agents Chemother. 1995, 39, 1372–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachetelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Sénéchal, S.; Lagacé, J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta Biomembr. 2000, 1463, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle Technologies for Cancer Therapy. In Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2009; Volume 197, pp. 55–86. [Google Scholar]
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-M.; Chen, C.-H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.-F.; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.-Q.; Kupferwasser, L.I.; Zack, P.M.; Bayer, A.S. Comparative Efficacies of Liposomal Amikacin (MiKasome) plus Oxacillin versus Conventional Amikacin plus Oxacillin in Experimental Endocarditis Induced by Staphylococcus aureus: Microbiological and Echocardiographic Analyses. Antimicrob. Agents Chemother. 1999, 43, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Schiffelers, R.; Storm, G.; Bakker-Woudenberg, I. Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies. J. Antimicrob. Chemother. 2001, 48, 333–344. [Google Scholar] [CrossRef] [Green Version]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Abricate, Github. Available online: https://github.com/tseemann/abricate (accessed on 10 November 2021).
- Doster, E.; Lakin, S.M.; Dean, C.J.; Wolfe, C.; Young, J.G.; Boucher, C.; E Belk, K.; Noyes, N.R.; Morley, P.S. MEGARes 2.0: A database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res. 2020, 48, D561–D569. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef] [PubMed]
- Alhariri, M.; A Majrashi, M.; Bahkali, A.H.; Almajed, F.S.; O Azghani, A.; A Khiyami, M.; Alyamani, E.J.; Aljohani, S.M.; Halwani, M. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int. J. Nanomed. 2017, ume12, 6949–6961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- XcaliburTM Software. Available online: https://www.thermofisher.com/order/catalog/product/OPTON-30965 (accessed on 10 November 2021).
- Ramos, L.D.P.; Santos, C.E.D.R.; Mello, D.C.R.; Theodoro, L.N.; De Oliveira, F.E.; Brito, G.N.B.; Junqueira, J.C.; Jorge, A.O.C.; Oliveira, L. Klebsiella pneumoniae Planktonic and Biofilm Reduction by Different Plant Extracts: In Vitro Study. Sci. World J. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graphpad. Prism. Available online: https://www.graphpad.com (accessed on 10 November 2021).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKinnon, M.C.; Sargeant, J.M.; Pearl, D.L.; Reid-Smith, R.J.; Carson, C.A.; Parmley, E.J.; McEwen, S.A. Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 200. [Google Scholar] [CrossRef]
- Nji, E.; Kazibwe, J.; Hambridge, T.; Joko, C.A.; Larbi, A.A.; Damptey, L.A.O.; Nkansa-Gyamfi, N.A.; Lundborg, C.S.; Lien, L.T.Q. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci. Rep. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Genet. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Bertran, S.; Sauca, G.; Julià, A.; Vila, X.; Gómez, E.; de Anta, M.T.J.; Vila, J. Isolation of an amikacin-resistant Escherichia coli strain after tobramycin treatment of previous recurrent episodes of respiratory tract infections caused by Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2005, 11, 71–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Zhang, J.; Zhou, J.; Xu, C.; Fan, Z.; Pan, X.; Li, S.; Liang, Y.; Chen, S.; Xu, J.; et al. Synergistic bactericidal activities of tobramycin with ciprofloxacin and azithromycin against Klebsiella pneumoniae. J. Antibiot. 2021, 74, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Gounden, R.; Bamford, C.; Van Zyl-Smit, R.; Cohen, K.; Maartens, G. Safety and effectiveness of colistin compared with tobramycin for multi-drug resistant Acinetobacter baumannii infections. BMC Infect. Dis. 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G.; Balice, M.; Libori, M.; Tiracchia, V.; Salvia, A.; Varaldo, P. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control 2016, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Asaad, A.M.; Ansari, S.; Ajlan, S.E.; Awad, S.M. Epidemiology of Biofilm Producing Acinetobacter baumannii Nosocomial Isolates from a Tertiary Care Hospital in Egypt: A Cross-Sectional Study. Infect. Drug Resist. 2021, 14, 709–717. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Quah, S.Y.; Wu, S.; Lui, J.N.; Sum, C.P.; Tan, K.S. N-Acetylcysteine Inhibits Growth and Eradicates Biofilm of Enterococcus faecalis. J. Endod. 2012, 38, 81–85. [Google Scholar] [CrossRef]
- Drago, L.; De Vecchi, E.; Mattina, R.; Romanò, C.L. Activity of N-acetyl-L-cysteine against Biofilm of Staphylococcus aureus and Pseudomonas aeruginosa on Orthopedic Prosthetic Materials. Int. J. Artif. Organs 2013, 36, 39–46. [Google Scholar] [CrossRef]
- Pollini, S.; Di Pilato, V.; Landini, G.; Di Maggio, T.; Cannatelli, A.; Sottotetti, S.; Cariani, L.; Aliberti, S.; Blasi, F.; Sergio, F.; et al. In vitro activity of N-acetylcysteine against Stenotrophomonas maltophilia and Burkholderia cepacia complex grown in planktonic phase and biofilm. PLoS ONE 2018, 13, e0203941. [Google Scholar] [CrossRef] [Green Version]
- Mitsopoulos, P.; Omri, A.; Alipour, M.; Vermeulen, N.; Smith, M.G.; Suntres, Z.E. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int. J. Pharm. 2008, 363, 106–111. [Google Scholar] [CrossRef]
- Arias, J.M.; Picot, R.A.C.; Tuttolomondo, M.E.; Ben Altabef, A.; Díaz, S.B. Interaction of N-acetylcysteine with DPPC liposomes at different pH: A physicochemical study. New J. Chem. 2020, 44, 14837–14848. [Google Scholar] [CrossRef]
- Suntres, Z.E. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J. Toxicol. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Alipour, M.; Buonocore, C.; Omri, A.; Szabo, M.; Pucaj, K.; Suntres, Z.E. Therapeutic effect of liposomal-N-acetylcysteine against acetaminophen-induced hepatotoxicity. J. Drug Target. 2013, 21, 466–473. [Google Scholar] [CrossRef]
- Mitsopoulos, P.; Suntres, Z.E. Protective Effects of Liposomal N-Acetylcysteine against Paraquat-Induced Cytotoxicity and Gene Expression. J. Toxicol. 2011, 2011, 808967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsopoulos, P. The Role of Free-and Liposomal-N-Acetycysteine in Paraquat-Induced Cytotoxicity. Master’s Thesis, Lakehead University, Thunder Bay, ON, Canada, 2012. [Google Scholar]
- Rosenberg, E.Y.; Ma, D.; Nikaido, H. AcrD of Escherichia coli Is an Aminoglycoside Efflux Pump. J. Bacteriol. 2000, 182, 1754–1756. [Google Scholar] [CrossRef] [Green Version]
- Vidal, O.; Longin, R.; Prigent-Combaret, C.; Dorel, C.; Hooreman, M.; Lejeune, P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: Involvement of a new ompR allele that increases curli expression. J. Bacteriol. 1998, 180, 2442–2449. [Google Scholar] [CrossRef] [Green Version]
- Swasthi, H.M.; Bhasne, K.; Mahapatra, S.; Mukhopadhyay, S. Human Fibrinogen Inhibits Amyloid Assembly of Biofilm-Forming CsgA. Biochemistry 2018, 57, 6270–6273. [Google Scholar] [CrossRef]
- Uhlich, G.A.; Gunther, N.W.; Bayles, D.O.; Mosier, D.A. The CsgA and Lpp Proteins of an Escherichia coli O157:H7 Strain Affect HEp-2 Cell Invasion, Motility, and Biofilm Formation. Infect. Immun. 2009, 77, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Richmond, G.E.; Evans, L.P.; Anderson, M.J.; Wand, M.; Bonney, L.C.; Ivens, A.; Chua, K.L.; Webber, M.; Sutton, J.M.; Peterson, M.L.; et al. The Acinetobacter baumannii Two-Component System AdeRS Regulates Genes Required for Multidrug Efflux, Biofilm Formation, and Virulence in a Strain-Specific Manner. mBio 2016, 7, e00430-16. [Google Scholar] [CrossRef] [Green Version]
- Messiaen, A.-S.; Forier, K.; Nelis, H.; Braeckmans, K.; Coenye, T. Transport of Nanoparticles and Tobramycin-loaded Liposomes in Burkholderia cepacia Complex Biofilms. PLoS ONE 2013, 8, e79220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanin, A.; Omri, A. Liposomal N-acetylcysteine Modulates the Pathogenesis of P. aeruginosa Isolated from the Lungs of Cystic Fibrosis Patient. J. Nanomed. Nanotechnol. 2014, 5, 219–230. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Halwani, M.; Hebert, S.; Suntres, Z.E.; Lafrenie, R.; Azghani, A.O.; Omri, A. Bismuth–thiol incorporation enhances biological activities of liposomal tobramycin against bacterial biofilm and quorum sensing molecules production by Pseudomonas aeruginosa. Int. J. Pharm. 2009, 373, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Marier, J.F.; Brazier, J.L.; Lavigne, J.; Ducharme, M.P. Liposomal tobramycin against pulmonary infections of Pseudomonas aeruginosa: A pharmacokinetic and efficacy study following single and multiple intratracheal administrations in rats. J. Antimicrob. Chemother. 2003, 52, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, E.; Georgopoulos, A.; Kagkadis, K.A.; Demetzos, C. Preparation and Characterization of Lyophilized Liposomes with Incorporated Quercetin. J. Liposome Res. 2006, 16, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.-Q.; Zhu, J.-F.; Wang, X.-B.; Ba, K. Improving the Stability of Liposomal Curcumin by Adjusting the Inner Aqueous Chamber pH of Liposomes. ACS Omega 2020, 5, 1120–1126. [Google Scholar] [CrossRef]
- Beaulac, C.; Sachetelli, S.; Lagace, J. In-vitro bactericidal efficacy of sub-MIC concentrations of liposome-encapsulated antibiotic against gram-negative and gram-positive bacteria. J. Antimicrob. Chemother. 1998, 41, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galimand, M.; Sabtcheva, S.; Courvalin, P.; Lambert, T. Worldwide Disseminated armA Aminoglycoside Resistance Methylase Gene Is Borne by Composite Transposon Tn1548. Antimicrob. Agents Chemother. 2005, 49, 2949–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogaerts, P.; Galimand, M.; Bauraing, C.; Deplano, A.; Vanhoof, R.; De Mendonca, R.; Rodriguez-Villalobos, H.; Struelens, M.; Glupczynski, Y. Emergence of ArmA and RmtB aminoglycoside resistance 16S rRNA methylases in Belgium. J. Antimicrob. Chemother. 2007, 59, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Tada, T.; Shrestha, B.; Kirikae, T.; Ohara, H.; Rijal, B.P.; Pokhrel, B.M.; Sherchand, J.B. Emergence of Aminoglycoside Resistance Due to armA methylase in Multi-drug Resistant Acinetobacter baumannii Isolates in a University Hospital in Nepal. J. Nepal Health Res. Counc. 2016, 14, 72–76. [Google Scholar] [PubMed]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.F.M.; Baca, P.; Arias-Moliz, M.T.; Rodriguez-Archilla, A.; Ferrer-Luque, C.M. Antimicrobial activity of alexidine alone and associated with N-acetylcysteine against Enterococcus faecalis biofilm. Int. J. Oral. Sci. 2013, 5, 146–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Bozzolasco, M.; Gualco, L.; Debbia, E.A.; Schito, G.C.; Schito, A.M. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int. J. Antimicrob. Agents 2003, 22, 95–100. [Google Scholar] [CrossRef]
- Serramitjana, E.S.; Jorba, M.; Fusté, E.; Pedraz, J.L.; Vinuesa, T.; Viñas, M. Free and Nanoencapsulated Tobramycin: Effects on Planktonic and Biofilm Forms of Pseudomonas. Microorganisms 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Category | Escherichia coli | Acinetobacter baumannii | Klebsiella pneumoniae |
|---|---|---|---|
| Outer membrane proteins (OMPs) | ompA | ompA | ompA, omp37 |
| Efflux pumps | acrA, acrB, acrD, acre, acrF, acrS, mdtA-C, mdtE-K, mdtM-P | adeA-C, adeI, adeK, adeL, adeR, adeT1, adeT2, abeM, abeS | acrA, acrB, acrD |
| Biofilm formation | csgB, csgD, csgF, csgG | pgaA-D, csuA-D, bap, csgB, csgD, csgF, csgG | - |
| Formula | Peak | PDI |
|---|---|---|
| TL | 347.33 ± 62.27 | 0.85 |
| TNL | 229.47 ± 47.57 | 0.68 |
| Liposomal Formulations | Entrapped Concentration (mg/L) | EE% |
|---|---|---|
| TL | 71 | 7.1 |
| TNL | 127.6 | 12.8 |
| Isolate ID | Tobramycin | TL | TNL | |||
|---|---|---|---|---|---|---|
| (mg/L) | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| E. coli clinical isolates | ||||||
| EC_057 | 32 | 64 | 32 | 64 | 16 | 32 |
| EC_068 | 32 | 64 | 32 | 64 | 16 | 32 |
| EC_077 | 64 | 128 | 32 | 64 | 16 | 32 |
| EC_083 | 32 | 64 | 32 | 64 | 16 | 32 |
| EC_089 | 64 | 128 | 8 | 16 | 16 | 32 |
| EC_162 | 64 | 128 | 32 | 64 | 16 | 32 |
| EC_219 | 64 | 128 | 32 | 64 | 32 | 64 |
| K. pneumoniae clinical isolates | ||||||
| KP_002 | 1024 | 2048 | 8 | 16 | 16 | 32 |
| KP_017 | 512 | 1024 | 32 | 64 | 32 | 64 |
| KP_019 | 32 | 64 | 16 | 32 | 32 | 128 |
| KP_026 | 1024 | 2048 | 8 | 16 | 16 | 32 |
| KP_050 | 1024 | 2048 | 8 | 16 | 16 | 32 |
| KP_057 | 1024 | 2048 | 16 | 32 | 16 | 32 |
| KP_059 | 1024 | 2048 | 16 | 32 | 16 | 32 |
| KP_086 | 256 | 512 | 16 | 32 | 32 | 64 |
| KP_095 | 1024 | 2048 | 16 | 32 | 32 | 64 |
| A. baumannii clinical isolates | ||||||
| RAB_005 | 128 | 256 | 128 | 256 | 16 | 32 |
| RAB_009 | 128 | 256 | 128 | 256 | 16 | 32 |
| RAB_014 | 128 | 256 | 128 | 256 | 16 | 32 |
| RAB_030 | 128 | 256 | 128 | 256 | 16 | 32 |
| RAB_055 | 128 | 256 | 128 | 256 | 16 | 32 |
| S. aureus ATCC 29213 * | 4 | 8 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarfaj, R.E.; Alkhulaifi, M.M.; Al-Fahad, A.J.; Aljihani, S.; Yassin, A.E.B.; Alghoribi, M.F.; Halwani, M.A. Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics 2022, 14, 130. https://doi.org/10.3390/pharmaceutics14010130
Alarfaj RE, Alkhulaifi MM, Al-Fahad AJ, Aljihani S, Yassin AEB, Alghoribi MF, Halwani MA. Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics. 2022; 14(1):130. https://doi.org/10.3390/pharmaceutics14010130
Chicago/Turabian StyleAlarfaj, Reem E., Manal M. Alkhulaifi, Ahmed J. Al-Fahad, Shokran Aljihani, Alaa Eldeen B. Yassin, Majed F. Alghoribi, and Majed A. Halwani. 2022. "Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii" Pharmaceutics 14, no. 1: 130. https://doi.org/10.3390/pharmaceutics14010130
APA StyleAlarfaj, R. E., Alkhulaifi, M. M., Al-Fahad, A. J., Aljihani, S., Yassin, A. E. B., Alghoribi, M. F., & Halwani, M. A. (2022). Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics, 14(1), 130. https://doi.org/10.3390/pharmaceutics14010130








