Optimisation of the Chicken Chorioallantoic Membrane Assay in Uveal Melanoma Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell and Spheroid Culture
2.2. Chick Chorioallantoic Membrane Assay
2.3. Tumour Spheroid Implantation Procedure
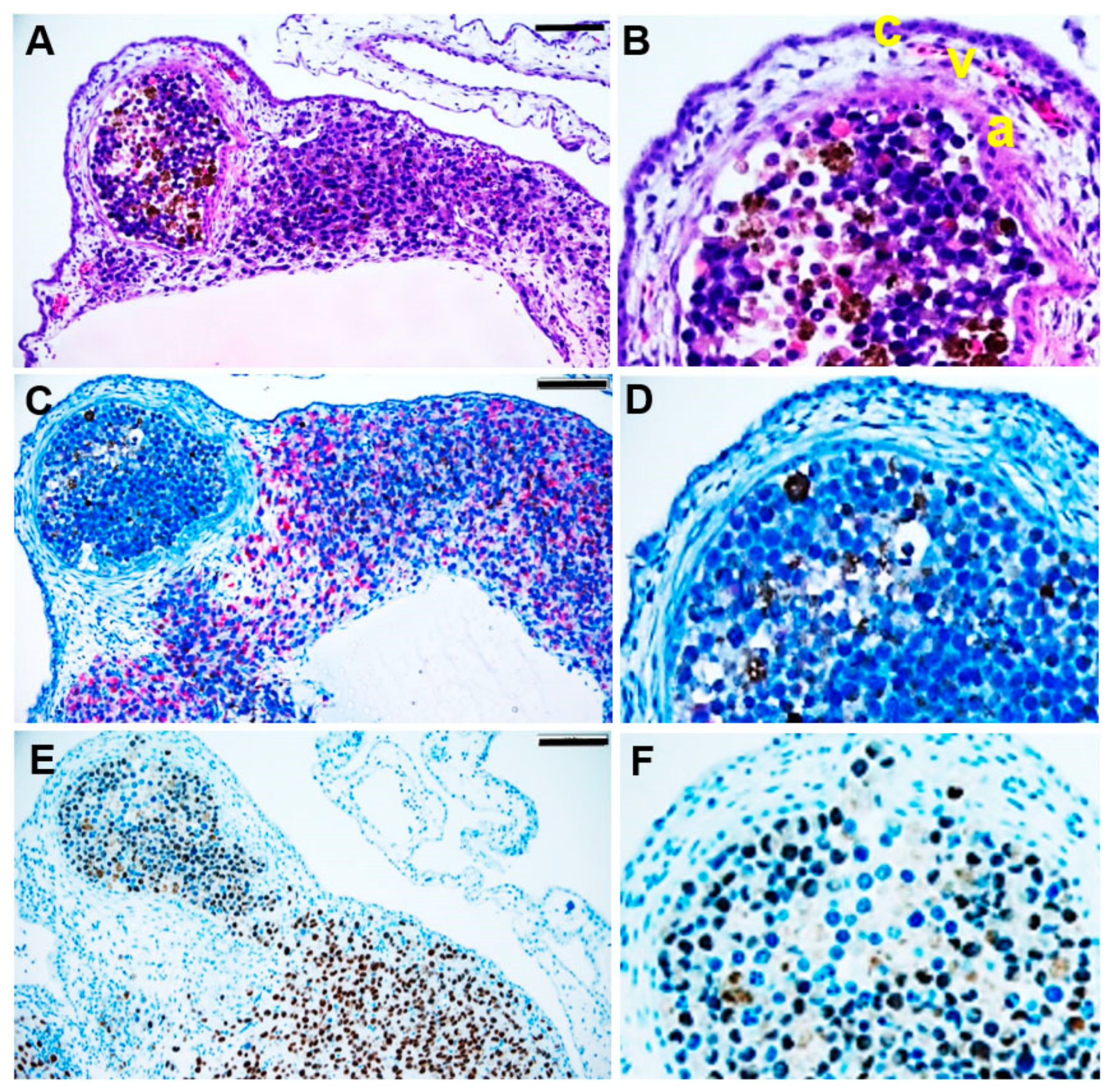
2.4. Immunohistochemistry
2.5. Fluorescence Microscopy
2.6. Image Analysis
2.7. Data Statistics
3. Results
3.1. Chick Embryo Viability Pretreatments of the CAM
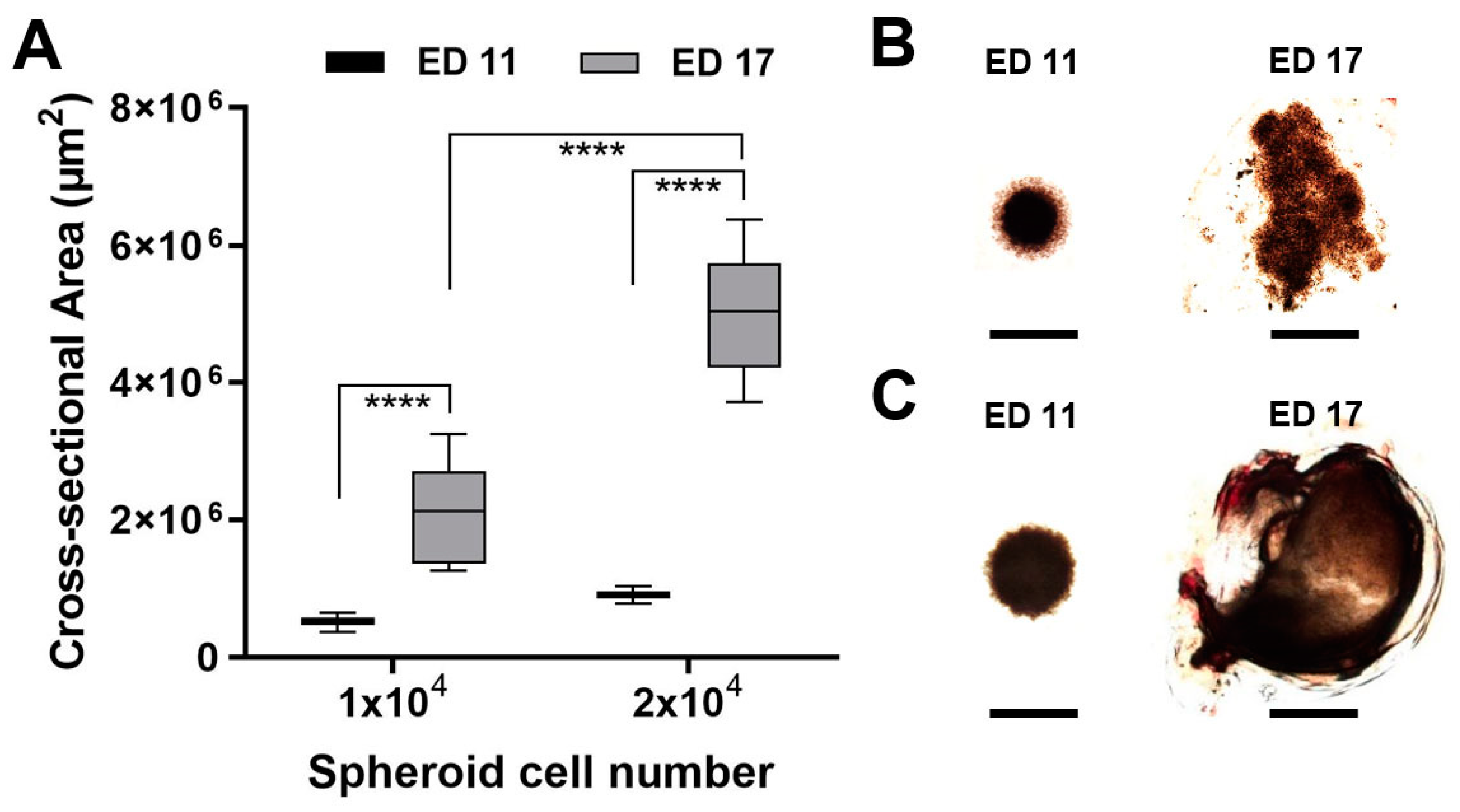
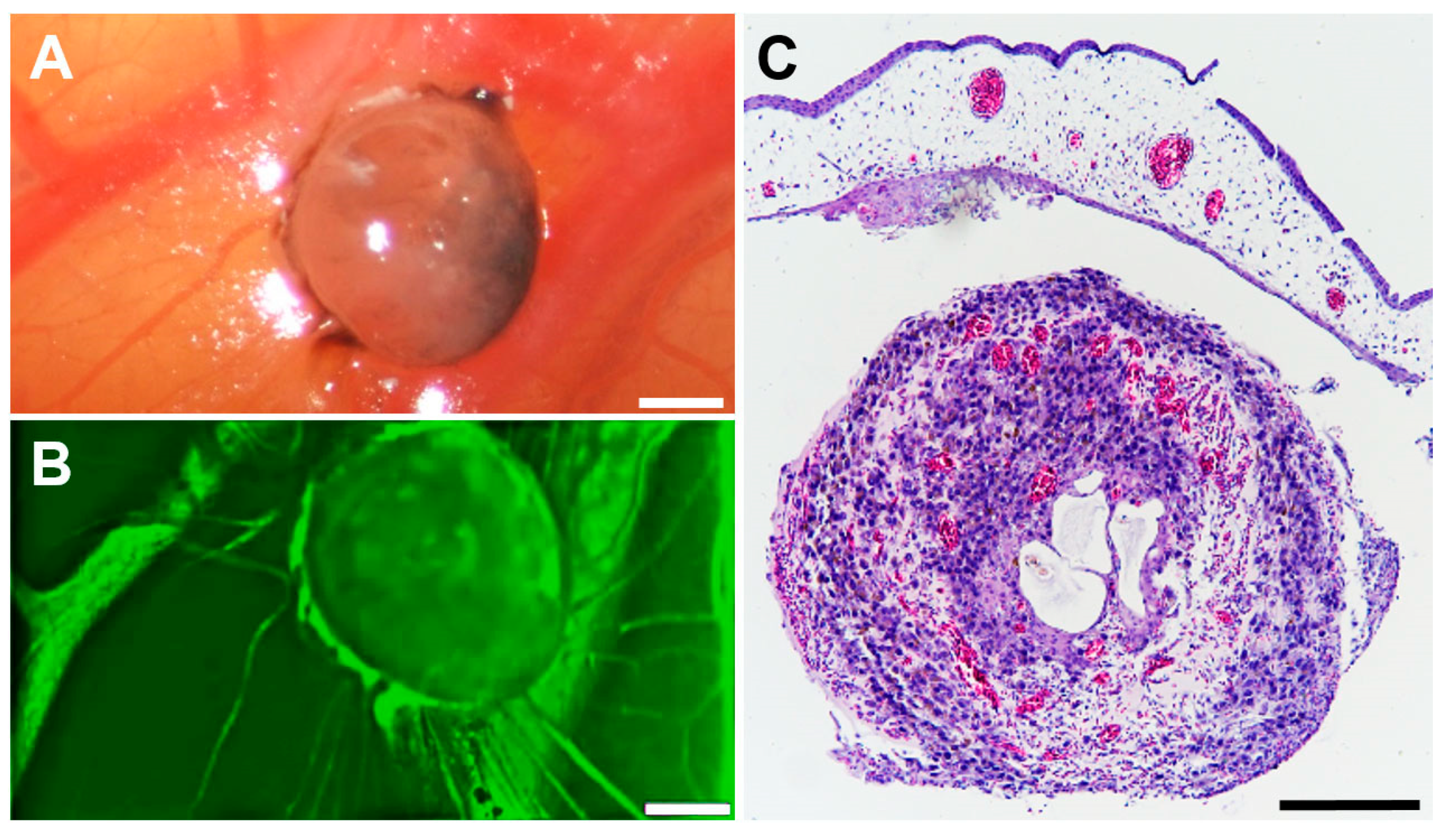
3.2. Tumour Spheroid Attachment Rate
3.3. Tumour Spheroid Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schüler, A.O.; Bornfeld, N. Current therapy aspects of intraocular tumours. Ophthalmologe 2000, 97, 207–222. [Google Scholar] [PubMed]
- Virgili, G.; Parravano, M.; Gatta, G. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef]
- Grabowska, A.; Abelarias, J.; Peralta, J.; Asencio, M.; Garcia-Cabezas, M.A.; Escabias-Del Pozo, M.; Nevado, J.; Vallespin, E.; Solera, J.; Martinez Pilar, P.; et al. Uveal melanoma in a 19-month-old child. J. AAPOS 2011, 15, 606–608. [Google Scholar] [CrossRef]
- Al-Jamal, R.T.; Cassoux, N.; Kivelä, T.T. Survival of young patients with posterior uveal melanoma. JAMA Ophthalmol. 2019, 137, 1091. [Google Scholar] [CrossRef]
- Font, R.L.; Croxatto, J.O.; Rao, N.A. AFIP Atlas of Tumour Pathology, 4th ed.; American Registry of Pathology: Washington, DC, USA, 2006; p. 52. [Google Scholar]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; Fouchardiere, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Smith, J.H.; Padnick-Silver, L.; Newlin, A.; Rhodes, K.; Rubinstein, W.S. Genetik study of familial uveal melanoma: Association of uveal and cutaneous melanoma across GenoMEL. Cancer Res. 2006, 66, 9818–9828. [Google Scholar]
- Singh, A.D.; Damato, B.; Howard PHarbour, J.W. Uveal melanoma: Genetic aspects. Ophthalmol. Clin. N. Am. 2005, 18, 85–97. [Google Scholar] [CrossRef]
- Williams, E.A.; Montesion, M.; Shah, N.; Sharaf, R.; Pavlick, D.C.; Sokol, E.S.; Alexander, B.; Venstrom, J.; Elvin, J.A.; Ross, J.S.; et al. Melanoma with in-frame deletion of MAP2K1: A distinct molecular subtype of cutaneous melanoma mutually exclusive from BRAF, NRAS, and NF1 mutations. Mod. Pathol. 2020, 33, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 3, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Coupland, S.E.; Lake, S.L.; Zeschnigk, M.; Damato, B.E. Molecular Pathology of Uveal Melanoma. Eye 2013, 27, 230–242. [Google Scholar] [CrossRef]
- Shain, A.H.; Bagger, M.M.; Yu, R.; Chang, D.; Liu, S.; Vemula, S.; Weier, J.F.; Wadt, K.; Heegaard, S.; Bastian, B.C.; et al. The Genetic Evolution of Metastatic Uveal Melanoma. Nat. Genet. 2019, 51, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2018, 33, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Jankovic, B.D.; Isakovic, K.; Lukic, M.L.; Vujanovic, N.L.; Petrovic, S.; Markovic, B.M. Immunological capacity of the chicken embryo. Relationship between the maturation of lymphoid tissues and the occurrence of cell-mediated immunity in the developing chicken embryo. Immunology 1975, 29, 497–508. [Google Scholar] [PubMed]
- Koop, S.; Khokha, R.; Schmidt, E.E.; MacDonald, I.C.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumour growth. Cancer Res 1994, 54, 4791–4797. [Google Scholar]
- Koop, S.; Mac Donald, I.C.; Luzzi, K.; Schmidt, E.E.; Morris, V.L.; Grattan, M.; Khokha, R.; Chambers, A.F.; Groom, A.C. Fate of melanoma cells entering the microcirculation: Over 80% survive and extravasate. Cancer Res 1995, 55, 2520–2523. [Google Scholar]
- Kalirai, H.; Shahidipour, H.; Coupland, S.E.; Luyten, G. Use of the Chick Embryo Model in Uveal Melanoma. Ocul. Oncol. Pathol. 2015, 1, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyten, G.P.; Mooy, C.M.; De Jong, P.T.; Hoogeveen, A.T.; Luider, T.M. A Chicken Embryo Model to Study the Growth of Human Uveal Melanoma. Biochem. Biophys. Res. Commun. 1993, 192, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Fiorentzis, M.; Viestenz, A.; Siebolts, U.; Seitz, B.; Coupland, S.E.; Heinzelmann, J. The Potential Use of Electrochemotherapy in the Treatment of Uveal Melanoma: In Vitro Results in 3D Tumour Cultures and In Vivo Results in a Chick Embryo Model. Cancers 2019, 11, 1344. [Google Scholar] [CrossRef] [Green Version]
- Fiorentzis, M.; Sokolenko, E.A.; Bechrakis, N.E.; Ting, S.; Schmid, K.W.; Sak, A.; Stuschke, M.; Seitz, B.; Berchner-Pfannschmidt, U. Electrochemotherapy with Bleomycin Enhances the Radiosensitivity of Uveal Melanomas: First In Vitro Results in 3D Cultures of Primary Uveal Melanoma Cell Lines. Cancers 2021, 12, 3086. [Google Scholar] [CrossRef]
- Kunz, P.; Schenker, A.; Sähr, H.; Lehner, B.; Fellenberg, J. Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma. PLoS ONE 2019, 14, e0215312. [Google Scholar] [CrossRef] [PubMed]
- Hessemer, V.; Dick, B. Viscoelastic substances in cataract surgery. Principles and current overview. Klin. Monbl. Augenheilkd. 1996, 209, 55–61. [Google Scholar] [CrossRef]
- Mads Borries, M.; Barooji, Y.F.; Yennek, S.; Grapin-Botton, A.; Berg-Sørensen, K.; Oddershede, L.B. Quantification of Visco-Elastic Properties of a Matrigel for Organoid Development as a Function of Polymer Concentration. Front. Phys. 2020, 8, 579168. [Google Scholar] [CrossRef]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef] [PubMed]
- Sys, G.M.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The in ovo CAM-assay as a xenograft model for sarcoma. J. Vis. Exp. 2013, 77, 50522. [Google Scholar] [CrossRef]
- De Waard-Siebinga, I.; Blom, D.J.; Griffioen, M.; Schrier, P.I.; Hoogendoorn, E.; Beverstock, G.; Danen, E.H.; Jager, M.J. Establishment and characterization of an uveal-melanoma cell line. Int. J. Cancer 1995, 62, 155–161. [Google Scholar] [CrossRef]
- Jager, M.J.; Magner, J.A.; Ksander, B.R.; Dubovy, S.R. Uveal Melanoma Cell Lines: Where do they come from? (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2016, 114, T5. [Google Scholar]
- Griewank, K.G.; Yu, X.; Khalili, J.; Sozen, M.M.; Stempke-Hale, K.; Bernatchez, C.; Wardell, S.; Bastian, B.C.; Woodman, S.E. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell Melanoma Res. 2012, 25, 182–187. [Google Scholar] [CrossRef] [Green Version]


| Parameters Analysed | Conditions Tested |
|---|---|
| Number of cells for the spheroids (92.1, Mel270, UPMM3, UPMD2 cell lines) | 10 × 103 cells |
| 20 × 103 cells | |
| Day of spheroid transplantation | D5 |
| D7 | |
| D8 | |
| D11 | |
| Cleansing of the eggs | distilled water |
| ethanol 70% | |
| Fixation of spheroid | plastic ring, 2 mm |
| plastic ring made from lab plastic pipets | |
| plastic ring and matrix | |
| without fixation | |
| Laceration of the CAM for the implantation of the spheroids | 30-gauge cannula |
| Nr. 11 scalpel | |
| 25-gauge vitrectomy membrane scraper | |
| Matrix | Matrigel |
| Hyalon | |
| Hyalon GV | |
| Provisc | |
| Viscoat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolenko, E.A.; Berchner-Pfannschmidt, U.; Ting, S.C.; Schmid, K.W.; Bechrakis, N.E.; Seitz, B.; Tsimpaki, T.; Kraemer, M.M.; Fiorentzis, M. Optimisation of the Chicken Chorioallantoic Membrane Assay in Uveal Melanoma Research. Pharmaceutics 2022, 14, 13. https://doi.org/10.3390/pharmaceutics14010013
Sokolenko EA, Berchner-Pfannschmidt U, Ting SC, Schmid KW, Bechrakis NE, Seitz B, Tsimpaki T, Kraemer MM, Fiorentzis M. Optimisation of the Chicken Chorioallantoic Membrane Assay in Uveal Melanoma Research. Pharmaceutics. 2022; 14(1):13. https://doi.org/10.3390/pharmaceutics14010013
Chicago/Turabian StyleSokolenko, Ekaterina A., Utta Berchner-Pfannschmidt, Saskia C. Ting, Kurt W. Schmid, Nikolaos E. Bechrakis, Berthold Seitz, Theodora Tsimpaki, Miriam Monika Kraemer, and Miltiadis Fiorentzis. 2022. "Optimisation of the Chicken Chorioallantoic Membrane Assay in Uveal Melanoma Research" Pharmaceutics 14, no. 1: 13. https://doi.org/10.3390/pharmaceutics14010013






