Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of Myrosinase-Core Streptavidin (MYR-coreSA) Encoding Plasmid
2.3. Expression and Purification of MYR-coreSA Recombinant Protein
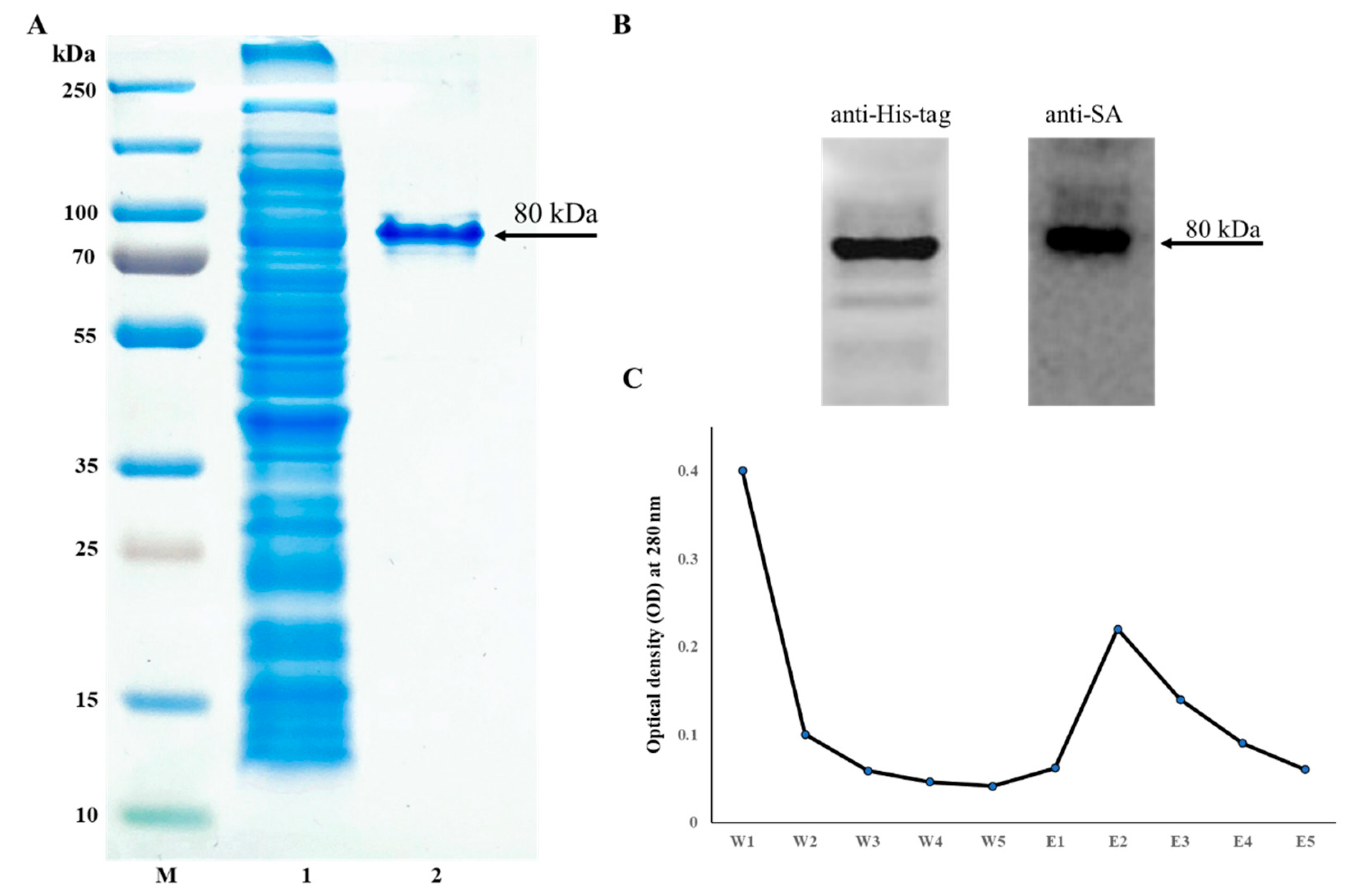
2.4. SDS-PAGE, Western Blot, and BCA Assay
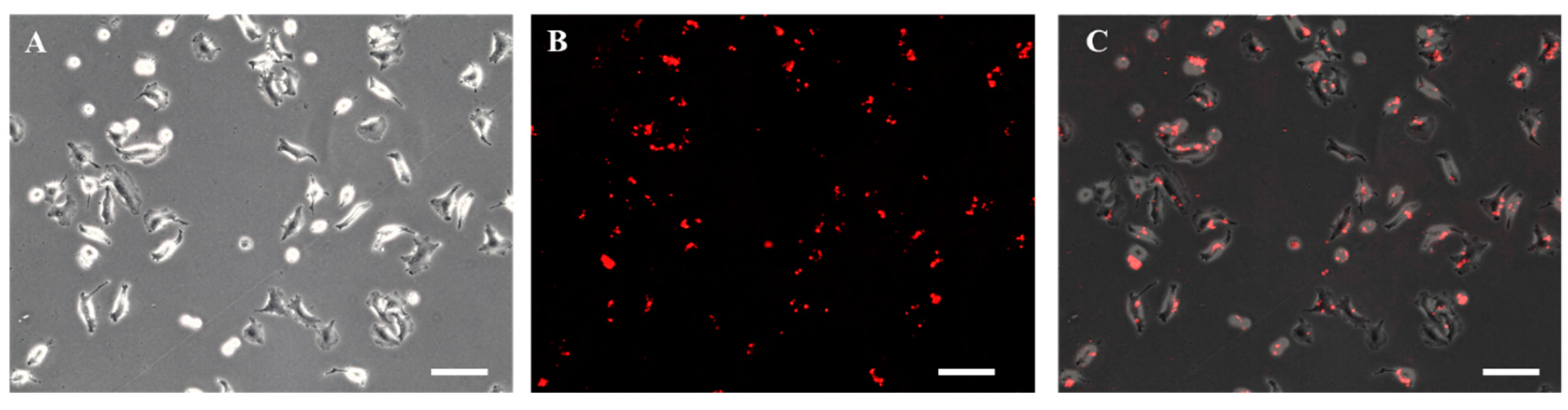
2.5. Surface Biotinylation and MYR-coreSA Tethering on A549 Cancer Cells
2.6. Enzyme Activity Assay
2.7. Cytotoxicity Assay
2.8. Apoptosis Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Expression, Purification, and Characterization of MYR-coreSA Fusion Protein
3.2. Biotinylation of Cell Membrane Surface
3.3. Anticancer Activity of Sinigrin on Myrosinase-Tethered A549 Cells
3.4. AITC-Induced Cell Apoptosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, R.; Iwasaki, M.; Hara, A.; Inoue, M.; Sasazuki, S.; Sawada, N.; Yamaji, T.; Shimazu, T.; Tsugane, S. Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: The Japan Public Health Center-based Prospective Study. Cancer Causes Control 2013, 24, 2117–2128. [Google Scholar] [CrossRef]
- Han, B.; Li, X.; Yu, T. Cruciferous vegetables consumption and the risk of ovarian cancer: A meta-analysis of observational studies. Diagn. Pathol. 2014, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.Y.; Yang, Y.L.; Yan, H.; Huang, Q.; Liu, K.J.; Zhang, S. Phenethyl isothiocyanate suppresses the metastasis of ovarian cancer associated with the inhibition of CRM1-mediated nuclear export and mTOR-STAT3 pathway. Cancer Biol. Ther. 2017, 18, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dey, M. Dietary Phenethyl Isothiocyanate Protects Mice from Colitis Associated Colon Cancer. Int. J. Mol. Sci. 2017, 18, 1908. [Google Scholar] [CrossRef] [Green Version]
- Izzotti, A.; Larghero, P.; Cartiglia, C.; Longobardi, M.; Pfeffer, U.; Steele, V.E.; De Flora, S. Modulation of microRNA expression by budesonide, phenethyl isothiocyanate and cigarette smoke in mouse liver and lung. Carcinogenesis 2010, 31, 894–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khor, T.O.; Keum, Y.S.; Lin, W.; Kim, J.H.; Hu, R.; Shen, G.; Xu, C.; Gopalakrishnan, A.; Reddy, B.; Zheng, X.; et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006, 66, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Powolny, A.A.; Bommareddy, A.; Hahm, E.-R.; Normolle, D.P.; Beumer, J.H.; Nelson, J.B.; Singh, S.V. Chemopreventative Potential of the Cruciferous Vegetable Constituent Phenethyl Isothiocyanate in a Mouse Model of Prostate Cancer. JNCI J. Natl. Cancer Inst. 2011, 103, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, K.C.; Lu, C.C.; Tang, Y.J.; Chiang, J.H.; Kuo, D.H.; Chen, F.A.; Chen, I.L.; Yang, J.S. Allyl isothiocyanate inhibits cell metastasis through suppression of the MAPK pathways in epidermal growth factor-stimulated HT29 human colorectal adenocarcinoma cells. Oncol. Rep. 2014, 31, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Li, P.; Xue, Z. Effect of allyl isothiocyanate on the viability and apoptosis of the human cervical cancer heLa cell line in vitro. Oncol. Lett. 2018, 15, 8756–8760. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K. Allyl isothiocyanate, a’ constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis 2003, 24, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Yin, M.C.; Chiu, H.Y.; Yang, J.S. Sensitivity of allyl isothiocyanate to induce apoptosis via ER stress and the mitochondrial pathway upon ROS production in colorectal adenocarcinoma cells. Oncol. Rep. 2020, 44, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.S.; Chen, T.; Wong, Y.S. Allyl isothiocyanate induces G2/M arrest in human colorectal adenocarcinoma SW620 cells through down-regulation of Cdc25B and Cdc25C. Mol. Med. Rep. 2010, 3, 1023–1030. [Google Scholar] [CrossRef]
- Tsai, S.C.; Huang, W.W.; Huang, W.C.; Lu, C.C.; Chiang, J.H.; Peng, S.F.; Chung, J.G.; Lin, Y.H.; Hsu, Y.M.; Amagaya, S.; et al. ERK-modulated intrinsic signaling and G2/M phase arrest contribute to the induction of apoptotic death by allyl isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int. J. Oncol. 2012, 41, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Kim, G.H. Allyl Isothiocyanate Influences Cell Adhesion, Migration and Metalloproteinase Gene Expression in SK-Hep1 Cells. Exp. Biol. Med. 2009, 234, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Tang, L.; Li, Y.; Geng, F.; Paonessa, J.D.; Chen, S.C.; Wong, M.K.K.; Zhang, Y. Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis 2010, 31, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Tang, L.; Li, Y.; Yang, L.; Choi, K.S.; Kazim, A.L.; Zhang, Y. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. J. Biol. Chem. 2011, 286, 32259–32267. [Google Scholar] [CrossRef] [Green Version]
- Sávio, A.L.V.; da Silva, G.N.; Salvadori, D.M.F. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil). Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2015, 771, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Chen, K.; Lu, C.; Lan, Y.; Lai, C.; Chung, Y.; Yang, J.L.Y.-C. Allyl isothiocyanate triggers G2/M phase arrest and apoptosis in human brain malignant glioma GBM 8401 cells through a mitochondria-dependent pathway. Oncol. Rep. 2010, 24, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.Y.; Tsai, F.J.; Bau, D.T.; Hsu, Y.M.; Yang, J.S.; Tu, M.G.; Chiang, S. lun Potential effects of allyl isothiocyanate on inhibiting cellular proliferation and inducing apoptotic pathway in human cisplatin-resistant oral cancer cells. J. Formos. Med. Assoc. 2020, 120, 515–523. [Google Scholar] [CrossRef]
- Zhang, Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2004, 555, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.E.; Morra, M.J. Simultaneous Quantification of Sinigrin, Sinalbin, and Anionic Glucosinolate Hydrolysis Products in Brassica juncea and Sinapis alba Seed Extracts Using Ion Chromatography. J. Agric. Food Chem. 2014, 62, 10687–10693. [Google Scholar] [CrossRef] [PubMed]
- Dübel, S.; Breitling, F.; Kontermann, R.; Schmidt, T.; Skerra, A.; Little, M. Bifunctional and multimeric complexes of streptavidin fused to single chain antibodies (scFv). J. Immunol. Methods 1995, 178, 201–209. [Google Scholar] [CrossRef]
- Andersson, D.; Chakrabarty, R.; Bejai, S.; Zhang, J.; Rask, L.; Meijer, J. Myrosinases from root and leaves of Arabidopsis thaliana have different catalytic properties. Phytochemistry 2009, 70, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-H.; Lin, Y.; Zhang, Z.-L.; Zhuan, F.; Liu, A.-A.; Xie, M.; Tian, Z.-Q.; Zhang, Z.; Wang, H.; Pang, D.-W. Surface Labeling of Enveloped Viruses Assisted by Host Cells. ACS Chem. Biol. 2012, 7, 683–688. [Google Scholar] [CrossRef]
- Tarar, A.; Alyami, E.M.; Peng, C.A. Mesenchymal stem cells anchored with thymidine phosphorylase for doxifluridine-mediated cancer therapy. RSC Adv. 2021, 11, 1394–1403. [Google Scholar] [CrossRef]
- Tripathi, K.; Hussein, U.K.; Anupalli, R.; Barnett, R.; Bachaboina, L.; Scalici, J.; Rocconi, R.P.; Owen, L.B.; Piazza, G.A.; Palle, K. Allyl isothiocyanate induces replication-associated DNA damage response in NSCLC cells and sensitizes to ionizing radiation. Oncotarget 2015, 6, 5237–5252. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, T.H.; Liu, B.L.; Wu, L.C.; Chen, Y.C.; Tzeng, Y.M.; Hsu, S.L. Destruxin B isolated from entomopathogenic fungus Metarhizium anisopliae induces apoptosis via a Bcl-2 family-dependent mitochondrial pathway in human nonsmall cell lung cancer cells. Evid. -Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.D.; Pagidas, K. Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell proliferation and induces apoptosis in human ovarian cancer cells. Anticancer Res. 2010, 30, 2519–2523. [Google Scholar]
- Hacker, G. The morphology of apoptosis. Cell Tissue Res. 2000, 301, 5–17. [Google Scholar] [CrossRef]
- Tarar, A.; Alyami, E.M.; Peng, C.-A. Efficient expression of soluble recombinant protein fused with core-streptavidin in bacterial strain with T7 expression system. Methods Protoc. 2020, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, X.; Chang, K.; Liu, X.; Xiong, J. Allyl isothiocyanate inhibits the proliferation of renal carcinoma cell line GRC-1 by inducing an imbalance between Bcl2 and Bax. Med. Sci. Monit. 2016, 22, 4283–4288. [Google Scholar] [CrossRef] [Green Version]
- Savio, A.L.V.; da Silva, G.N.; de Camargo, E.A.; Salvadori, D.M.F. Cell cycle kinetics, apoptosis rates, DNA damage and TP53 gene expression in bladder cancer cells treated with allyl isothiocyanate (mustard essential oil). Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2014, 762, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Stennicke, H.R.; Salvesen, G.S. Properties of the caspases. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1998, 1387, 17–31. [Google Scholar] [CrossRef]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell. Longev. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Li, Y.; Wade, K.L.; Paonessa, J.D.; Fahey, J.W.; Zhang, Y. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis 2010, 31, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Iglesias, M.J.; Novío, S.; García, C.; Pérez-Muñuzuri, E.; Soengas, P.; Cartea, E.; Velasco, P.; Freire-Garabal, M. Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. Int. J. Mol. Sci. 2019, 20, 4977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef] [Green Version]
- Encinas-Basurto, D.; Juarez, J.; Valdez, M.A.; Burboa, M.G.; Barbosa, S.; Taboada, P. Targeted Drug Delivery Via Human Epidermal Growth Factor Receptor for Sustained Release of Allyl Isothiocyanate. Curr. Top. Med. Chem. 2018, 18, 1252–1260. [Google Scholar] [CrossRef]
- Chang, W.; Chen, B.; Inbaraj, B.S.; Chien, J. Preparation of allyl isothiocyanate nanoparticles, their anti-inflammatory activity towards RAW 264.7 macrophage cells and anti-proliferative effect on HT1376 bladder cancer cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef]






| Myrosinase (MYR) | |
| Forward primer | 5′-GCCATGGATATCATGAAGCTTCTTATGCTCGCCTTTG-3′ |
| Reverse primer | 5′-GAATTCGGATCCTGCATCTGCAAGACTCTTCCGATC-3′ |
| Core Streptavidin (coreSA) | |
| Forward primer | 5′-AGATCCGAATTCGGTGCTGCTGAAGCAGGT-3′ |
| Reverse primer | 5′-ATTATACTCGAGGGAGGCGGCGGACGGCTT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarar, A.; Alyami, E.M.; Peng, C.-A. Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin. Pharmaceutics 2022, 14, 144. https://doi.org/10.3390/pharmaceutics14010144
Tarar A, Alyami EM, Peng C-A. Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin. Pharmaceutics. 2022; 14(1):144. https://doi.org/10.3390/pharmaceutics14010144
Chicago/Turabian StyleTarar, Ammar, Esmael M. Alyami, and Ching-An Peng. 2022. "Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin" Pharmaceutics 14, no. 1: 144. https://doi.org/10.3390/pharmaceutics14010144
APA StyleTarar, A., Alyami, E. M., & Peng, C. -A. (2022). Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin. Pharmaceutics, 14(1), 144. https://doi.org/10.3390/pharmaceutics14010144








