Efficacy and Safety of Novel Aspirin Formulations: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aspirin Formulations
2.3. Study Design
2.4. Endoscopy and Collection of Gastric Mucosal Samples for Histopathology
2.5. Statistical Analysis
3. Results
3.1. Dissolution Studies
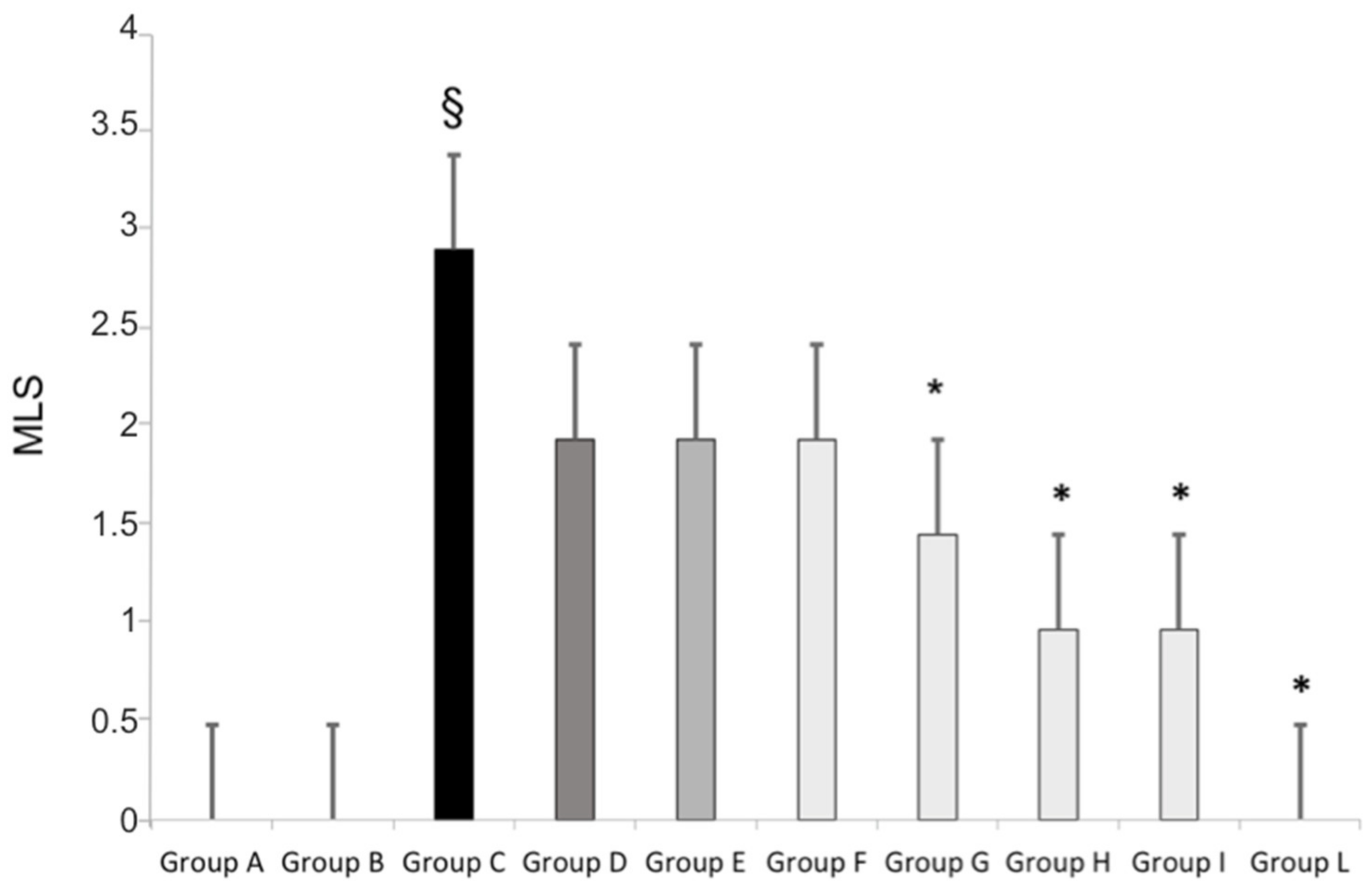
3.2. Gastric Mucosal Injury Induced by Standard Aspirin or Aspirin Micronized and Cogrinded with Collagen
3.3. Gastric Micro-Vessel Vasodilatation Induced by Standard as Well as Micronized and Collagen-Cogrinded Aspirin
3.4. TXB2 and Urinary 11-Dehydro-TX B2 Determinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awtry, E.H.; Loscalzo, J. Aspirin. Circulation 2000, 101, 1206–1218. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, C.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed] [Green Version]
- Schrör, K. Aspirin and platelets: The antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin. Thromb. Hemost. 1997, 23, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Angerson, W.J.; Knill-Jones, R.P.; Blatchford, O. Upper gastrointestinal haemorrhage associated with low-dose aspirin and anti-thrombotic drugs—A 6-year analysis and comparison with non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 2005, 22, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.; Lanas, A.; Arroyo, M.T.; Ferreira, I.J. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment. Pharmacol. Ther. 2002, 16, 1945–1953. [Google Scholar] [CrossRef]
- Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy–I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994, 308, 81–106. [Google Scholar] [CrossRef]
- Warner, T.D.; Nylander, S.; Whatling, C. Anti-platelet therapy: Cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br. J. Clin. Pharmacol. 2011, 72, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Schoen, R.T.; Vender, R.J. Mechanisms of nonsteroidal anti- inflammatory drug-induced gastric damage. Am. J. Med. 1989, 86, 449–458. [Google Scholar] [CrossRef]
- Halter, F. Mechanism of gastrointestinal toxicity of NSAIDs. Scand. J. Rheumatol. Suppl. 1988, 73, 16–21. [Google Scholar] [CrossRef]
- Yeomans, N.D.; Lanas, A.I.; Talley, N.J.; Thomson, A.B.R.; Daneshjoo, R.; Eriksson, B.; Appelman-Eszczuk, S.; Långström, G.; Naesdal, J.; Serrano, P.; et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment. Pharmacol. Ther. 2005, 22, 795–801. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Stevens, W.; Haynes, R.; Aung, T.; Chen, F.; Buck, G.; Collins, R.; Armitage, J.; The ASCEND Study Collaborative Group. ASCEND: A Study of Cardiovascular Events iN Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am. Heart J. 2018, 198, 135–144. [Google Scholar] [CrossRef]
- Derry, S.; Loke, Y.K. Risk of gastrointestinal haemorrhage with long term use of aspirin: Meta-analysis. BMJ 2000, 321, 1183–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilotto, A.; Franceschi, M.; Leandro, G.; Paris, F.; Cascavilla, L.; Longo, M.G.; Niro, V.; Andriulli, A.; Scarcelli, C.; Di Mario, F. Proton-pump inhibitors reduce the risk of uncomplicated peptic ulcer in elderly either acute or chronic users of aspirin/non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 2004, 20, 1091–1097. [Google Scholar] [CrossRef]
- Huang, M.; Han, M.; Han, W.; Kuang, L. Proton pump inhibitors versus histamine-2 receptor blockers for stress ulcer prophylaxis in patients with sepsis: A retrospective cohort study. J. Int. Med. Res. 2021, 49, 03000605211025130. [Google Scholar] [CrossRef] [PubMed]
- Dotevall, G.; Ekenved, G. The absorption of acetylsalicylic acid from the stomach in relation to intragastric pH. Scand. J. Gastroenterol. 1976, 11, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.R.; Hunt, J.N. Relationship between pH and absorption of acetylsalicylic acid from the stomach. Gut 1969, 10, 77–78. [Google Scholar]
- Mitra, A.; Kesisoglou, F. Impaired drug absorption due to high stomach pH: A review of strategies for mitigation of such effect to enable pharmaceutical product development. Mol. Pharm. 2013, 10, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Rosano, G.; Malara, N.; Di Fabrizio, E.; Vitale, C.; Coluccio, M.; Maiuolo, J.; Wasti, A.A.; Muscoli, C.; Gliozzi, M.; et al. Aspirin wears smart. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Lavie, C.J.; Howden, C.W.; Scheiman, J.; Tursi, J. Upper Gastrointestinal Toxicity Associated With Long-Term Aspirin Therapy: Consequences and Prevention. Curr. Probl. Cardiol. 2017, 42, 146–164. [Google Scholar] [CrossRef]
- Sostres, C.; Lanas, A. Gastrointestinal effects of aspirin. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 385–394. [Google Scholar] [CrossRef]
- García Rodríguez, L.A.; Vora, P.; Brobert, G.; Soriano-Gabarrò, M.; Cea Soriano, L. Bleeding associated with low-dose aspirin: Comparison of data from the COMPASS randomized controlled trial and routine clinical practice. Int. J. Cardiol. 2020, 318, 21–24. [Google Scholar] [CrossRef]
- Cea Soriano, L.; Lanas, A.; Soriano-Gabarró, M.; García Rodríguez, L.A. Incidence of Upper and Lower Gastrointestinal Bleeding in New Users of Low-Dose Aspirin. Clin. Gastroenterol. Hepatol. 2019, 17, 887–895.e6. [Google Scholar] [CrossRef] [Green Version]
- Cea Soriano, L.; Gaist, D.; Soriano-Gabarró, M.; García Rodríguez, L.A. Incidence of intracranial bleeds in new users of low-dose aspirin: A cohort study using The Health Improvement Network. J. Thromb. Haemost. 2017, 15, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: A population-based cohort study. Lancet 2017, 390, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Scheiman, J.M. Strategies to reduce the GI risks of antiplatelet therapy. Rev. Cardiovasc. Med. 2005, 6 (Suppl. 4), S23–S31. [Google Scholar] [PubMed]
- Zhao, C.; Wang, J.; Xiao, Q. Efficacy of Teprenone for Prevention of NSAID-Induced Gastrointestinal Injury: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 647494. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Sugimoto, M.; Kodaira, C.; Yamade, M.; Shirai, N.; Ikuma, M.; Tanaka, T.; Sugimura, H.; Hishida, A.; Furuta, T. Relationship between low-dose aspirin-induced gastric mucosal injury and intragastric pH in healthy volunteers. Dig. Dis. Sci. 2009, 55, 1627–1636. [Google Scholar] [CrossRef]
- Kitay, A.M.; Ferstl, F.S.; Geibel, J.P. Induction of Secretagogue Independent Gastric Acid Secretion via a Novel Aspirin-Activated Pathway. Front. Physiol. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Lanas, A.; Wu, P.; Medin, J.; Mills, E. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. J. Clin. Gastroenterol. Hepatol. 2011, 9, 762–768.e6. [Google Scholar] [CrossRef]
- Lanza, F.L.; Royer, G.L., Jr.; Nelson, R.S.; Chen, T.T.; Seckman, C.E.; Rack, M.F. A comparative endoscopic evaluation of the damaging effects of nonsteroidal anti-inflammatory agents on the gastric and duodenal mucosa. Am. J. Gastroenterol. 1981, 75, 17–21. [Google Scholar]

| Group | N. | Age (Years) | Gender (Male/Female) | Body Weight (Kg) | Body Mass Index (Kg/m2) | Smoking | Concomitant Treatment |
|---|---|---|---|---|---|---|---|
| A—placebo oral | 20 | 32 ± 6 | 10 M and 10 F | 68 ± 8 | 23 ± 2 | 0 | 0 |
| B—placebo sublingual | 20 | 35 ± 4 | 10 M and 10 F | 66 ± 7 | 25 ± 4 | 0 | 0 |
| C—oral standard aspirin 100 mg | 20 | 34 ± 4 | 9 M and 11 F | 66 ± 6 | 24 ± 4 | 0 | 0 |
| D—oral standard aspirin 50 mg | 20 | 34 ± 5 | 11 M and 9 F | 68 ± 7 | 23 ± 5 | 0 | 0 |
| E—sublingual standard aspirin 100 mg | 20 | 34 ± 5 | 11 M and 9 F | 65 ± 8 | 23 ± 3 | 0 | 0 |
| F—sublingual standard aspirin 50 mg | 20 | 35 ± 5 | 9 M and 11 F | 68 ± 8 | 24 ± 3 | 0 | 0 |
| G—oral micronized collagen-cogrinded aspirin 100 mg | 20 | 33 ± 4 | 10 M and 10 F | 67 ± 6 | 24 ± 4 | 0 | 0 |
| H—oral micronized collagen-cogrinded aspirin 50 mg | 20 | 34 ± 5 | 11 M and 9 F | 66 ± 6 | 25 ± 3 | 0 | 0 |
| I—sublingual micronized collagen-cogrinded aspirin 100 mg | 20 | 35 ± 4 | 10 M and 10 F | 66 ± 8 | 25 ± 4 | 0 | 0 |
| L—sublingual micronized collagen-cogrinded aspirin 50 mg | 20 | 34 ± 4 | 9 M and 11 F | 68 ± 8 | 23 ± 5 | 0 | 0 |
| Group | Serum TXB2 Time 0 | Serum TXB2 7 Days | Urinary 11-dehydro-TXB2 Time 0 | Urinary 11-dehydro-TXB2 7 Days |
|---|---|---|---|---|
| A—placebo oral | 302 ± 44 | 278 ± 48 | 485 ± 54 | 490 ± 58 |
| B—placebo sublingual | 298 ± 38 | 281 ± 45 | 498 ± 50 | 486 ± 46 |
| C—oral standard aspirin 100 mg | 286 ± 40 | 38 ± 12 * | 485 ± 52 | 86 ± 26 * |
| D—oral standard aspirin 50 mg | 295 ± 38 | 71 ± 18 * | 505 ± 48 | 108 ± 27 * |
| E—sublingual standard aspirin 100 mg | 304 ± 42 | 48 ± 14 * | 495 ± 50 | 95 ± 18 * |
| F—sublingual standard aspirin 50 mg | 302 ± 35 | 70 ± 15 * | 502 ± 46 | 118 ± 22 * |
| G—oral micronized collagen-cogrinded aspirin 100 mg | 300 ± 44 | 36 ± 12 * | 494 ± 54 | 77 ± 16 * |
| H—oral micronized collagen-cogrinded aspirin 50 mg | 286 ± 40 | 64 ± 15 * | 506 ± 54 | 106 ± 20 * |
| I—sublingual micronized collagen-cogrinded aspirin 100 mg | 290 ± 44 | 30 ± 14 * | 496 ± 48 | 66 ± 18 * |
| L—sublingual micronized collagen-cogrinded aspirin 50 mg | 302 ± 38 | 46 ± 20 * | 502 ± 48 | 88 ± 18 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollace, R.; Gliozzi, M.; Macrì, R.; Tavernese, A.; Musolino, V.; Carresi, C.; Maiuolo, J.; Muscoli, C.; Tomino, C.; Rosano, G.M.; et al. Efficacy and Safety of Novel Aspirin Formulations: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceutics 2022, 14, 187. https://doi.org/10.3390/pharmaceutics14010187
Mollace R, Gliozzi M, Macrì R, Tavernese A, Musolino V, Carresi C, Maiuolo J, Muscoli C, Tomino C, Rosano GM, et al. Efficacy and Safety of Novel Aspirin Formulations: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceutics. 2022; 14(1):187. https://doi.org/10.3390/pharmaceutics14010187
Chicago/Turabian StyleMollace, Rocco, Micaela Gliozzi, Roberta Macrì, Annamaria Tavernese, Vincenzo Musolino, Cristina Carresi, Jessica Maiuolo, Carolina Muscoli, Carlo Tomino, Giuseppe Maria Rosano, and et al. 2022. "Efficacy and Safety of Novel Aspirin Formulations: A Randomized, Double-Blind, Placebo-Controlled Study" Pharmaceutics 14, no. 1: 187. https://doi.org/10.3390/pharmaceutics14010187
APA StyleMollace, R., Gliozzi, M., Macrì, R., Tavernese, A., Musolino, V., Carresi, C., Maiuolo, J., Muscoli, C., Tomino, C., Rosano, G. M., Fini, M., Volterrani, M., Silvestrini, B., & Mollace, V. (2022). Efficacy and Safety of Novel Aspirin Formulations: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceutics, 14(1), 187. https://doi.org/10.3390/pharmaceutics14010187








