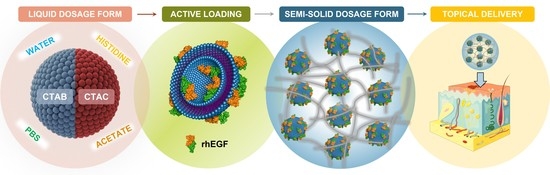
DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use
Abstract
:1. Introduction
2. Materials and Methods
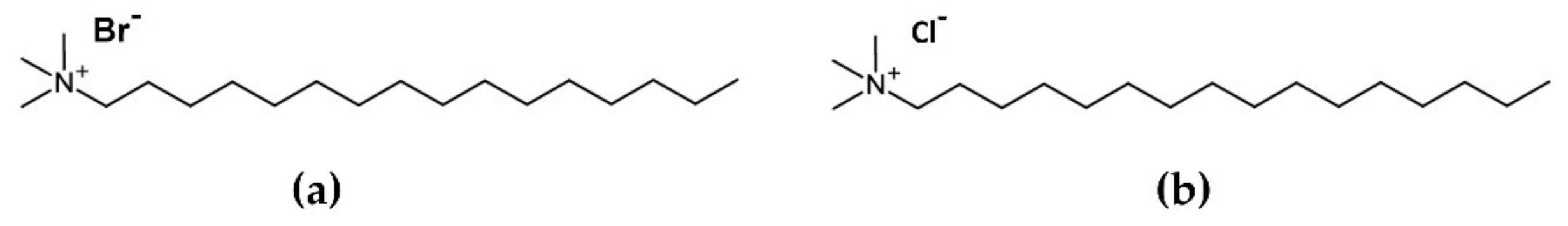
2.1. Materials
2.2. Preparation of DELOS Nanovesicles
2.3. Preparation of Hydrogels Enriched with DELOS Nanovesicles
2.4. DELOS Nanovesicles and Hydrogels Characterization
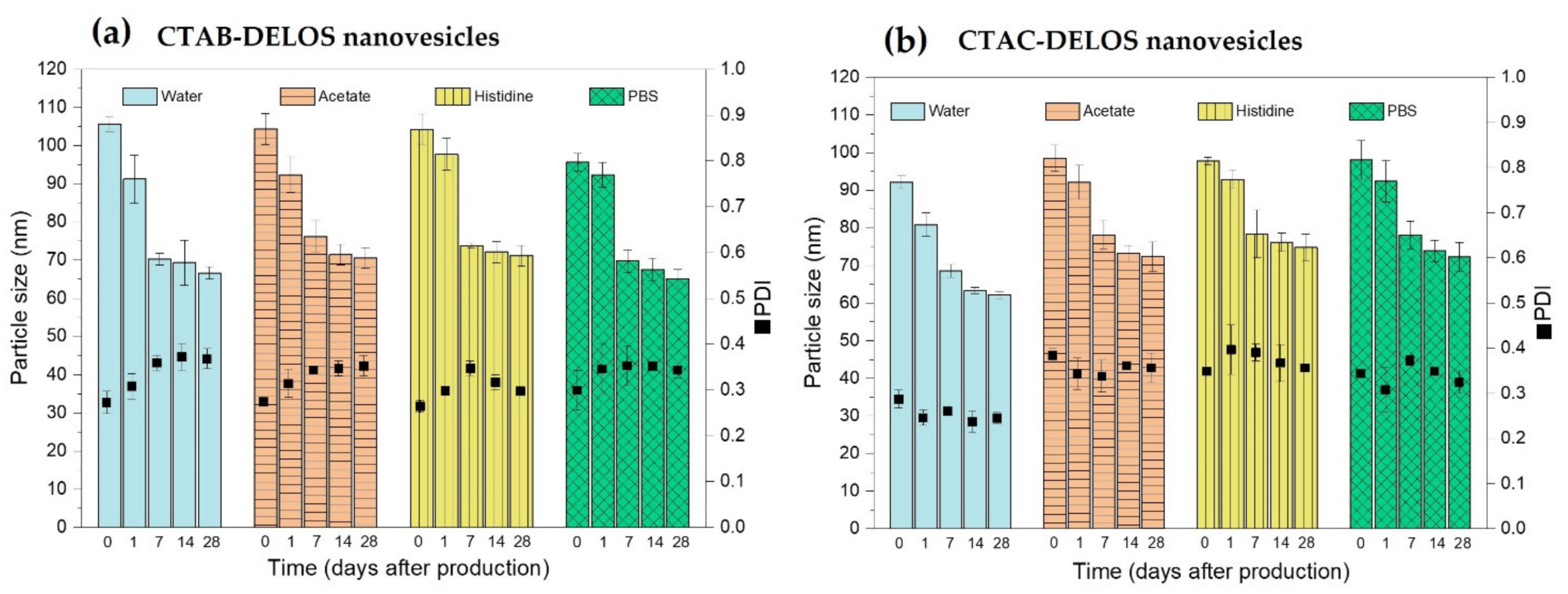
2.4.1. Particle Size, Polydispersity Index and Zeta Potential Assessment
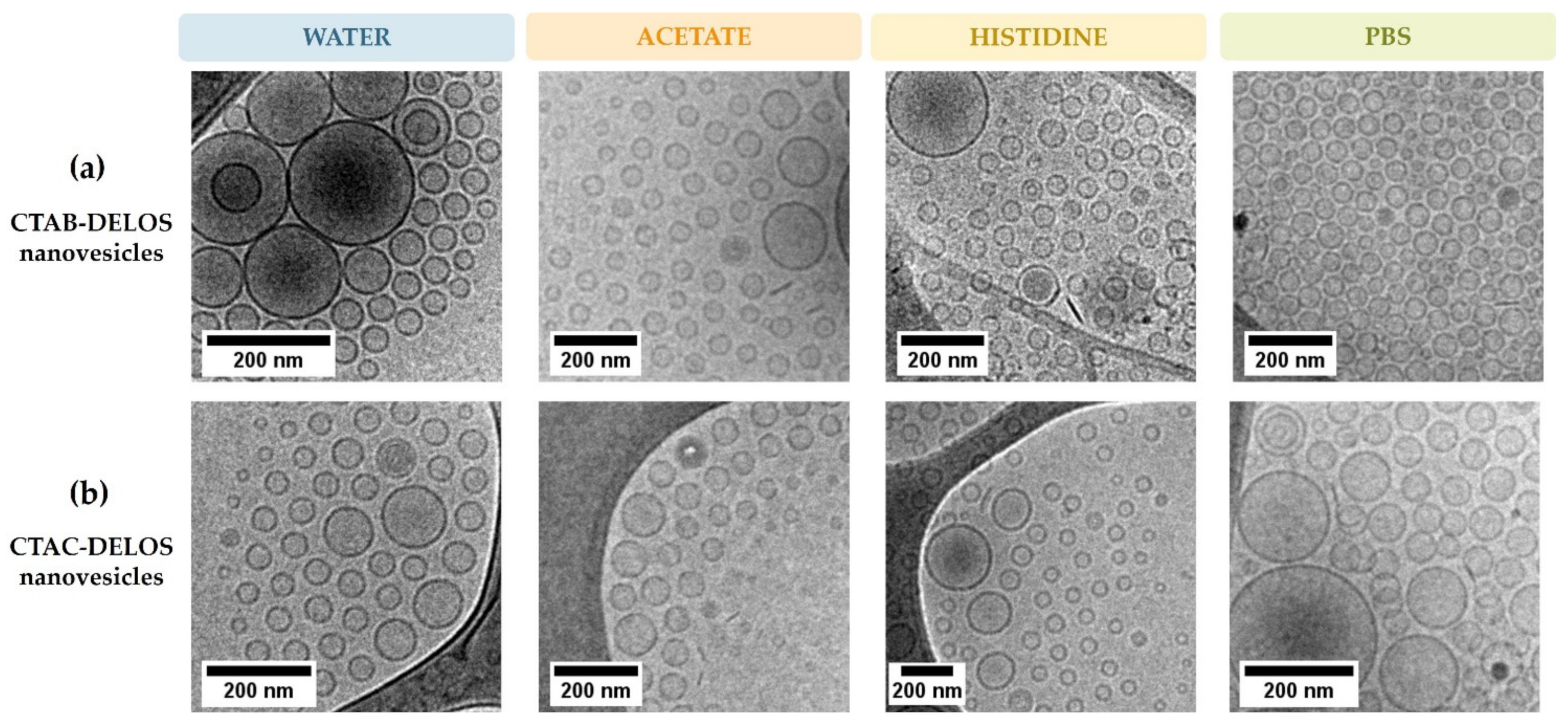
2.4.2. Morphological Characterization by Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.4.3. Hydrogel Microscopical Appearance
2.4.4. Hydrogel Rheological Properties
2.4.5. Absorption and Fluorescence Spectroscopy Measurements of Dye-Labelled DELOS Nanovesicles
2.4.6. Confocal Microscopic Characterization of Dye-Labelled DELOS Nanovesicles-Based Hydrogels
2.4.7. In Vitro rhEGF Biological Activity by Cell Proliferation Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Development of DELOS Nanovesicle Suspensions with Different Surfactant Counterions and Dispersant Media—Preparation and Physicochemical Characterization
3.2. Semi-Solid Dosage Form: Hydrogels Enriched with DELOS Nanovesicles
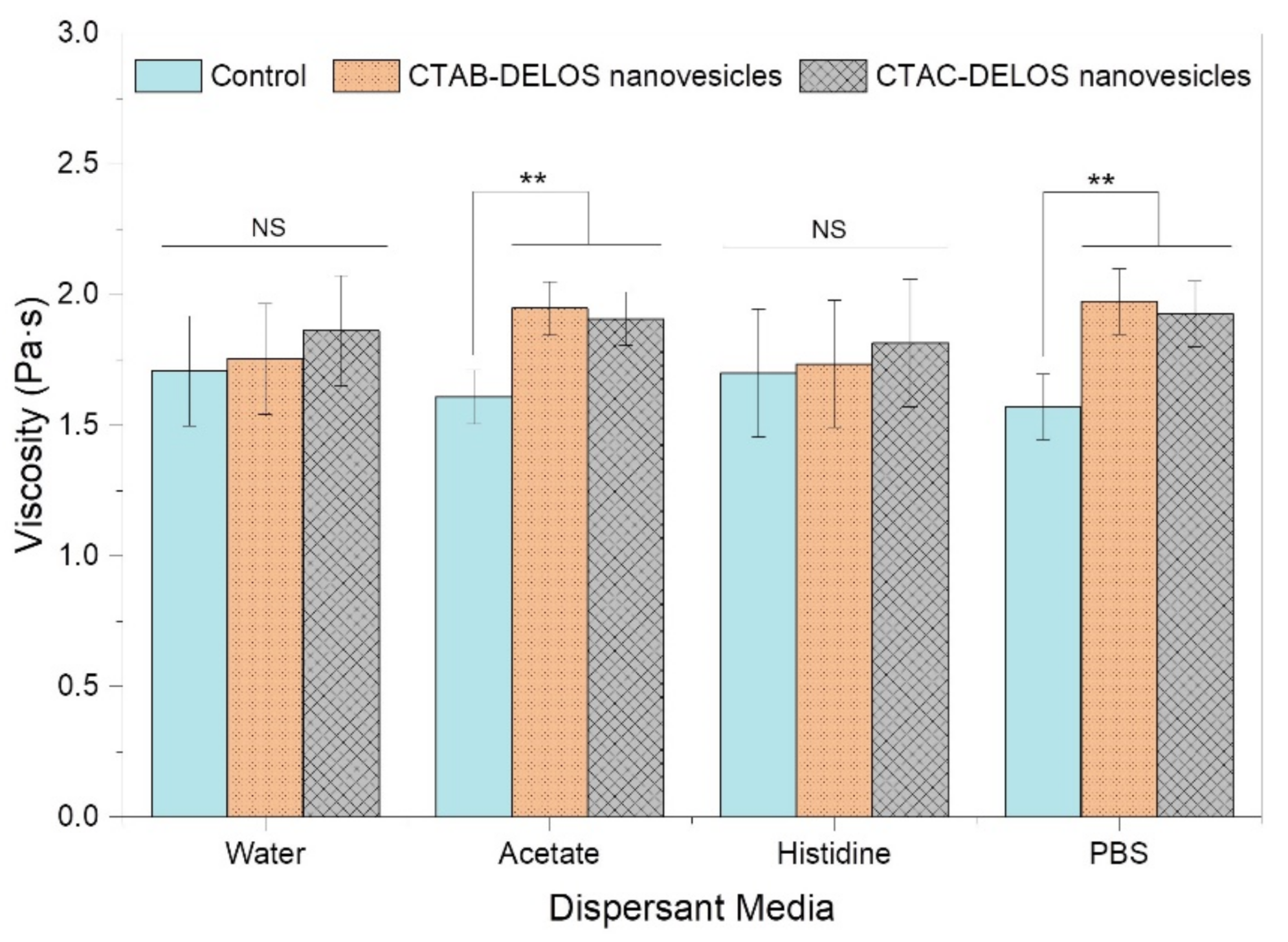
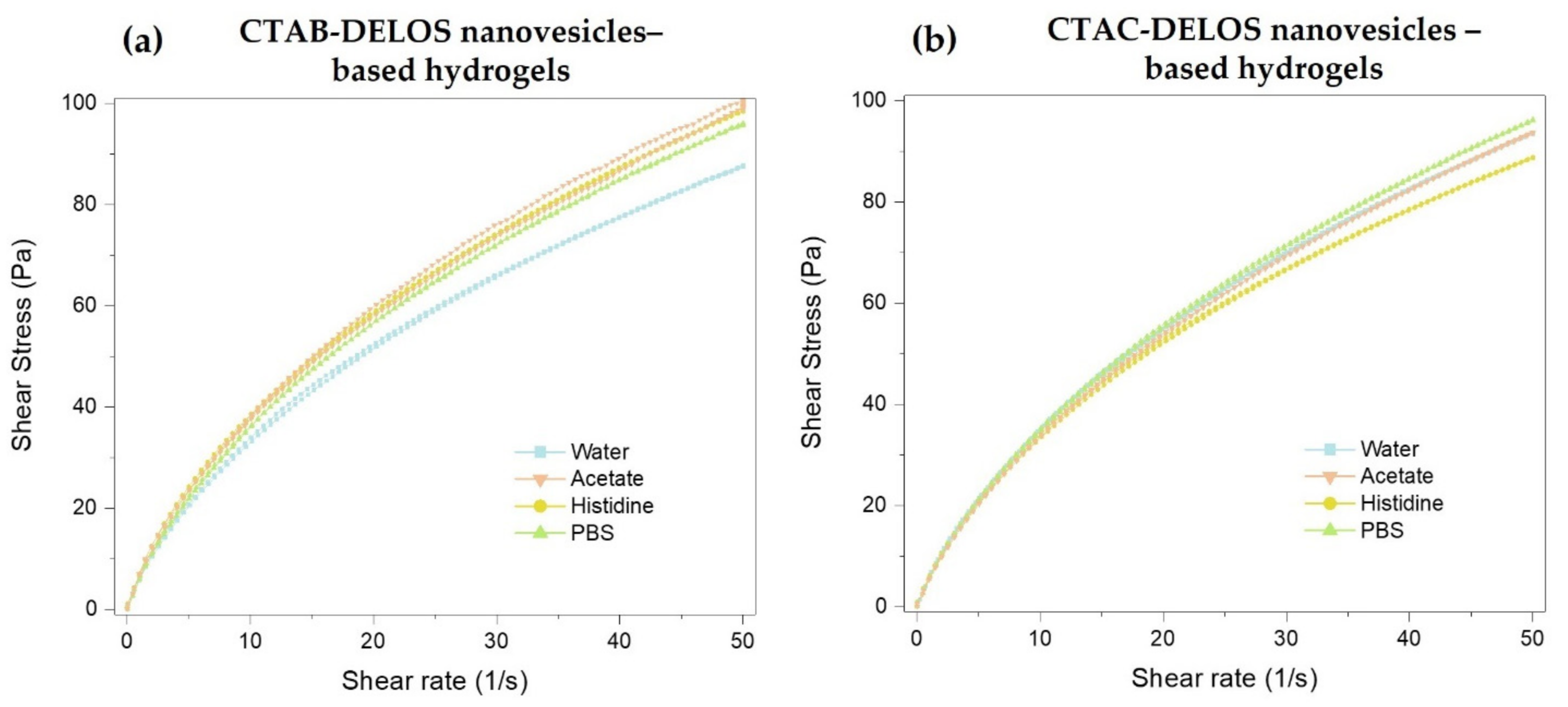
3.2.1. Preparation and Physicochemical Characterization of DELOS Nanovesicles-Based Hydrogels
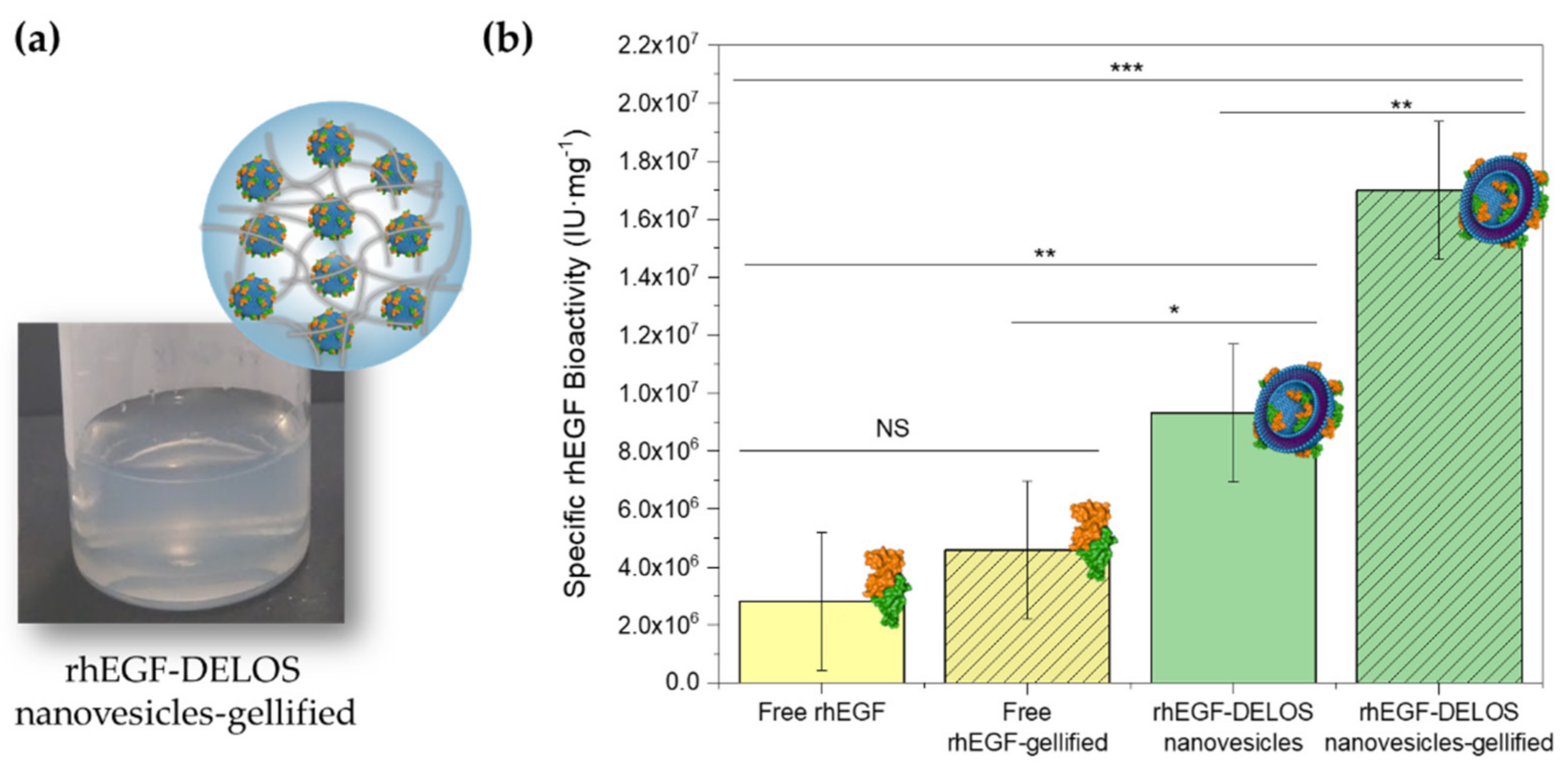
3.2.2. Integrity of DELOS Nanovesicles When Gellified
3.2.3. In Vitro Protein-Specific Bioactivity of Gellified rhEGF-DELOS Nanovesicles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, A.K.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Tadwee, I.K.; Gore, S.; Giradkar, P. Advances in Topical Drug Delivery System: A Review. Int. J. Pharm. Res. Allied Sci. 2011, 1, 14–23. [Google Scholar]
- Bhowmik, D.; Gopinath, H.; Pragati Kumar, B.; Duraivel, S.; Sampath Kumar, K.P. Recent advantaces in novel topical drug delivery system. Pharma Innov. 2012, 1, 12–31. [Google Scholar]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, I.; Elizondo, E.; Esteban, O.; Corchero, J.L.; Melgarejo, M.; Pulido, D.; Córdoba, A.; Moreno, E.; Unzueta, U.; Vazquez, E.; et al. Multifunctional Nanovesicle-Bioactive Conjugates Prepared by a One-Step Scalable Method Using CO2-Expanded Solvents. Nano Lett. 2013, 13, 3766–3774. [Google Scholar] [CrossRef]
- Merlo-Mas, J.; Tomsen-Melero, J.; Corchero, J.L.; González-Mira, E.; Font, A.; Pedersen, J.N.; García-Aranda, N.; Cristóbal-Lecina, E.; Alcaina-Hernando, M.; Mendoza, R.; et al. Application of Quality by Design to the robust preparation of a liposomal GLA formulation by DELOS-susp method. J. Supercrit. Fluids 2021, 173, 105204. [Google Scholar] [CrossRef]
- Ferrer-Tasies, L.; Santana, H.; Cabrera-Puig, I.; González-Mira, E.; Ballell-Hosa, L.; Castellar-Álvarez, C.; Córdoba, A.; Merlo-Mas, J.; Gerónimo, H.; Chinea, G.; et al. Recombinant Human Epidermal Growth Factor/Quatsome Nanoconjugates: A Robust Topical Delivery System for Complex Wound Healing. Adv. Ther. 2021, 4, 2000260. [Google Scholar] [CrossRef]
- Ferrer-Tasies, L.; Moreno-Calvo, E.; Cano-Sarabia, M.; Aguilella-Arzo, M.; Angelova, A.; Lesieur, S.; Ricart, S.; Faraudo, J.; Ventosa, N.; Veciana, J. Quatsomes: Vesicles Formed by Self-Assembly of Sterols and Quaternary Ammonium Surfactants. Langmuir 2013, 29, 6519–6528. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Cui, F.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Yu, Z.-G.; Zhu, M.-Y.; Shen, L.-Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients, 6th ed.; Libros Digitales-Pharmaceutical Press: London, UK; Washington, DC, USA, 2009. [Google Scholar]
- Bioworld. CTAB MSDS|bioWORLD. 2017. Available online: https://www.bio-world.com/msds/40330044/CTAB.html (accessed on 1 July 2018).
- Levin, J.; Maibach, H.I. 13 Human Skin Buffering Capacity. In Handbook of Cosmetic Science and Technology; CRC Press: Oxford, NY, USA, 2014. [Google Scholar]
- Levin, J.; Maibach, H. Human skin buffering capacity: An overview. Skin Res. Technol. 2008, 14, 121–126. [Google Scholar] [CrossRef]
- Tomsen-Melero, J.; Passemard, S.; García-Aranda, N.; Díaz-Riascos, Z.V.; González-Rioja, R.; Pedersen, J.N.; Lyngsø, J.; Merlo-Mas, J.; Cristóbal-Lecina, E.; Corchero, J.L.; et al. Impact of Chemical Composition on the Nanostructure and Biological Activity of α-Galactosidase-Loaded Nanovesicles for Fabry Disease Treatment. ACS Appl. Mater. Interfaces 2021, 13, 7825–7838. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Finney, D.J. Statistical Method in Biological Assay; Mathematics in Medicine Series; Arnold Hodder: London, UK, 1978. [Google Scholar]
- Nakata, K.; Tsuchido, T.; Matsumura, Y. Antimicrobial cationic surfactant, cetyltrimethylammonium bromide, induces superoxide stress in Escherichia coli cells. J. Appl. Microbiol. 2011, 110, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lim, S.H.; Choi, J.S.; Park, Y. Antibacterial properties of cetyltrimethylammonium bromide-stabilized green silver nanoparticles against methicillin-resistant Staphylococcus aureus. Arch. Pharmacal Res. 2015, 38, 1906–1912. [Google Scholar] [CrossRef]
- Pang, S.; Willis, L.; Andersen, F.A. Final report on the safety assessment of Cetrimonium Chloride, Cetrimonium Bromide and Steartrimonium Chloride. Int. J. Toxicol. 1997, 16, 195–220. [Google Scholar] [CrossRef]
- Ash, M. Handbook of Preservatives; Synapse Info Resources: Endicott, NY, USA, 2004. [Google Scholar]
- Findlay, B.; Szelemej, P.; Zhanel, G.G.; Schweizer, F. Guanidylation and Tail Effects in Cationic Antimicrobial Lipopeptoids. PLoS ONE 2012, 7, e41141. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Zakharova, L.Y. Self-assembling and biological properties of single-chain dicationic pyridinium-based surfactants. Colloids Surf. B Biointerfaces 2019, 175, 351–357. [Google Scholar] [CrossRef] [PubMed]
- FDA. Cetrimonium Chloride UNII UC9PE95IBP. 2021. Available online: https://fda.report/UNII/UC9PE95IBP (accessed on 11 January 2022).
- Sorensen, H.H.; Skriver, L.; Hoelgaard, A.R. A Stabilized Pharmaceutical Formulation Comprising Growth Hormone and Histidine. DK Patent EP1197222A2, 17 April 2002. [Google Scholar]
- Sek, D. Breaking old habits: Moving away from commonly used buffers in pharmaceuticals. Eur. Pharm. Rev. 2012, 7, 37–41. [Google Scholar]
- Strickley, R.G.; Lambert, W.J. A review of Formulations of Commercially Available Antibodies. J. Pharm. Sci. 2021, 110, 2590–2608.e56. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, E.; Moreno, E.; Cabrera, I.; Córdoba, A.; Sala, S.; Veciana, J.; Ventosa, N. Liposomes and Other Vesicular Systems: Structural Characteristics, Methods of Preparation, and Use in Nanomedicine. Prog. Mol. Biol. Transl. Sci. 2011, 104, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.P.; Brown, S.B.; Griffiths, C.E.; Weller, R.B.; Gibbs, N.K. Feeding filaggrin: Effects of l-histidine supplementation in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, E.; Kim, Y. l-histidine and l-carnosine accelerate wound healing via regulation of corticosterone and PI3K/Akt phosphorylation in d-galactose-induced aging models in vitro and in vivo. J. Funct. Foods 2019, 58, 227–237. [Google Scholar] [CrossRef]
- Santana, H.; González, Y.; Campana, P.T.; Noda, J.; Amarantes, O.; Itri, R.; Beldarraín, A.; Páez, R. Screening for stability and compatibility conditions of recombinant human epidermal growth factor for parenteral formulation: Effect of pH, buffers, and excipients. Int. J. Pharm. 2013, 452, 52–62. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H.C. The pH of the Skin Surface and Its Impact on the Barrier Function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Bigliardi, P.L. Role of Skin pH in Psoriasis. Curr. Probl. Dermatol. 2018, 54, 108–114. [Google Scholar] [CrossRef]
- Proksch, E. Buffering Capacity. Curr. Probl. Dermatol. 2018, 54, 11–18. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; James, G.; Marks, J.; Shank, R.C.; et al. Safety Assessment of Citric Acid, Inorganic Citrate Salts, and Alkyl Citrate Esters as Used in Cosmetics. Int. J. Toxicol. 2014, 33, 16S–46S. [Google Scholar] [CrossRef]
- Proksch, E.; Soeberdt, M.; Neumann, C.; Kilic, A.; Reich, H.; Abels, C. Influence of Buffers of Different pH and Composition on the Murine Skin Barrier, Epidermal Proliferation, Differentiation, and Inflammation. Skin Pharmacol. Physiol. 2019, 32, 328–336. [Google Scholar] [CrossRef]
- Bernstein, E. Use of Citric Acid and Low Concentrations of Alpha-Hydroxy Acids for Superficial Skin Treatment. U.S. Patent Application No. 10/689,002, 20 October 2003. [Google Scholar]
- Weil, I. Skin Treatment Composition Containing Monoester of Citric Acid. U.S. Patent No. 5,047,166, 10 September 1991. [Google Scholar]
- Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Methyl Acetate. Int. J. Toxicol. 2012, 31, 112S–136S. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.C.; Pirie, A.A.; Ford, L.V.; Callaghan, C.L.; McTurk, K.; Lucy, D.; Scrimger, D.G. The use of phosphate buffered saline for the recovery of cells and spermatozoa from swabs. Sci. Justice 2006, 46, 179–184. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Yuet, P.K.; Blankschtein, D. Molecular-Thermodynamic Modeling of Mixed Cationic/Anionic Vesicles. Langmuir 1996, 12, 3802–3818. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128140321. [Google Scholar]
- Rossetti, M.; Stella, L.; Morlà-Folch, J.; Bobone, S.; Boloix, A.; Baranda, L.; Moscone, D.; Roldán, M.; Veciana, J.; Segura, M.F.; et al. Engineering DNA-Grafted Quatsomes as Stable Nucleic Acid-Responsive Fluorescent Nanovesicles. Adv. Funct. Mater. 2021, 31, 2103511. [Google Scholar] [CrossRef]
- Vargas-Nadal, G.; Muñoz-Ubeda, M.; Alamo, P.; Arnal, M.M.; Céspedes, V.; Köber, M.; Gonzalez, E.; Ferrer-Tasies, L.; Vinardell, M.P.; Mangues, R.; et al. MKC-Quatsomes: A stable nanovesicle platform for bio-imaging and drug-delivery applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102136. [Google Scholar] [CrossRef]
- Boloix, A.; Feiner-Gracia, N.; Köber, M.; Repetto, J.; Pascarella, R.; Soriano, A.; Masanas, M.; Segovia, N.; Vargas-Nadal, G.; Merlo-Mas, J.; et al. Engineering pH-Sensitive Stable Nanovesicles for Delivery of MicroRNA Therapeutics. Small 2021, 2101959. [Google Scholar] [CrossRef]
- Pramanick, S.; Singodia, D.; Chandel, V. Excipient Selection in Parenteral Formulation Development. Pharma Times 2013, 45, 65–77. [Google Scholar]
- Patel, N.A.; Patel, N.J.; Patel, R.P. Design and evaluation of transdermal drug delivery system for curcumin as an anti-inflammatory drug. Drug Dev. Ind. Pharm. 2009, 35, 234–242. [Google Scholar] [CrossRef]
- Laffleur, F.; Strasdat, B. Gelatin-based formulations for dermal application. Eur. Polym. J. 2019, 118, 542–550. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Nikolić, S.; Calpena, A.C.; Egea, M.A.; Souto, E.B.; García, M.L. Improved and Safe Transcorneal Delivery of Flurbiprofen by NLC and NLC-Based Hydrogels. J. Pharm. Sci. 2012, 101, 707–725. [Google Scholar] [CrossRef]
- Eskens, O.; Villani, G.; Amin, S. Rheological investigation of thermoresponsive alginate-methylcellulose gels for epidermal growth factor formulation. Cosmetics 2021, 8, 3. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. 2015, 22, 969–987. [Google Scholar] [CrossRef] [Green Version]
- Calixto, G.; Yoshii, A.C.; Silva, H.R.E.; Cury, B.S.F.; Chorilli, M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef]
- Ardizzone, A.; Kurhuzenkau, S.; Illa-Tuset, S.; Faraudo, J.; Bondar, M.; Hagan, D.; Van Stryland, E.W.; Painelli, A.; Sissa, C.; Feiner, N.; et al. Nanostructuring Lipophilic Dyes in Water Using Stable Vesicles, Quatsomes, as Scaffolds and Their Use as Probes for Bioimaging. Small 2018, 14, 1703851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morla-Folch, J.; Vargas-Nadal, G.; Zhao, T.; Sissa, C.; Ardizzone, A.; Kurhuzenkau, S.; Köber, M.; Uddin, M.; Painelli, A.; Veciana, J.; et al. Dye-Loaded Quatsomes Exhibiting FRET as Nanoprobes for Bioimaging. ACS Appl. Mater. Interfaces 2020, 12, 20253–20262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, R.; Dai, Z. Multicolor nanobubbles for FRET/ultrasound dual-modal contrast imaging. Nanoscale 2018, 10, 20347–20353. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Moon, Y.; Jang, Y.; Lee, K.T.; Lee, C.; Jun, Y.; Lee, S. Molecular mechanisms of atlastin-mediated ER membrane fusion revealed by a FRET-based single-vesicle fusion assay. Sci. Rep. 2017, 7, 8700. [Google Scholar] [CrossRef] [Green Version]
- Gravier, J.; Sancey, L.; Rustique, E.; Passirani, C.; Benoît, J.-P.; Coll, J.-L.; Texier, I. FRET imaging approaches for in vitro and in vivo characterization of synthetic lipid nanoparticles. Mol. Pharm. 2014, 11, 3133–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The blood clearance kinetics and pathway of polymeric micelles in cancer drug delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef] [PubMed]
- Illa-Tuset, S. Molecular Modelling of Quatsome Nanovesicles. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2019. [Google Scholar]
- Delledonne, A.; Morla-Folch, J.; Anzola, M.; Bertocchi, F.; Vargas-Nadal, G.; Köber, M.; Sissa, C.; Ventosa, N.; Painelli, A. Increasing resonance energy transfer upon dilution: A counterintuitive observation in CTAB micelles. J. Mater. Chem. C 2021, 9, 10952–10964. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Academic/Plenum, Kluwer: New York, NY, USA, 1999. [Google Scholar]


| Vesicle Membrane Components | Dispersant Media 1 |
|---|---|
| Cholesterol:CTAB (CTAB-DELOS nanovesicles) | Water pH ca. 7.0 Sodium citrate buffer, pH = 5.0 (5 mM) Sodium acetate buffer, pH = 5.0 (5 mM) Histidine buffer, pH = 7.0 (5 mM) PBS buffer, pH = 7.4 (5 mM) |
| Cholesterol:CTAC (CTAC-DELOS nanovesicles) | Water pH ca. 7.0 Sodium citrate buffer, pH = 5.0 (5 mM) Sodium acetate buffer, pH = 5.0 (5 mM) Histidine buffer, pH = 7.0 (5 mM) PBS buffer, pH = 7.4 (5 mM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballell-Hosa, L.; González-Mira, E.; Santana, H.; Morla-Folch, J.; Moreno-Masip, M.; Martínez-Prieto, Y.; Revuelta, A.; Di Mauro, P.P.; Veciana, J.; Sala, S.; et al. DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics 2022, 14, 199. https://doi.org/10.3390/pharmaceutics14010199
Ballell-Hosa L, González-Mira E, Santana H, Morla-Folch J, Moreno-Masip M, Martínez-Prieto Y, Revuelta A, Di Mauro PP, Veciana J, Sala S, et al. DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics. 2022; 14(1):199. https://doi.org/10.3390/pharmaceutics14010199
Chicago/Turabian StyleBallell-Hosa, Lídia, Elisabet González-Mira, Hector Santana, Judit Morla-Folch, Marc Moreno-Masip, Yaima Martínez-Prieto, Albert Revuelta, Primiano Pio Di Mauro, Jaume Veciana, Santi Sala, and et al. 2022. "DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use" Pharmaceutics 14, no. 1: 199. https://doi.org/10.3390/pharmaceutics14010199
APA StyleBallell-Hosa, L., González-Mira, E., Santana, H., Morla-Folch, J., Moreno-Masip, M., Martínez-Prieto, Y., Revuelta, A., Di Mauro, P. P., Veciana, J., Sala, S., Ferrer-Tasies, L., & Ventosa, N. (2022). DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics, 14(1), 199. https://doi.org/10.3390/pharmaceutics14010199