Safe and Effective Cynomolgus Monkey GLP—Tox Study with Repetitive Intrathecal Application of a TGFBR2 Targeting LNA-Gapmer Antisense Oligonucleotide as Treatment Candidate for Neurodegenerative Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antisense Oligonucleotide (NVP-13) Characteristics
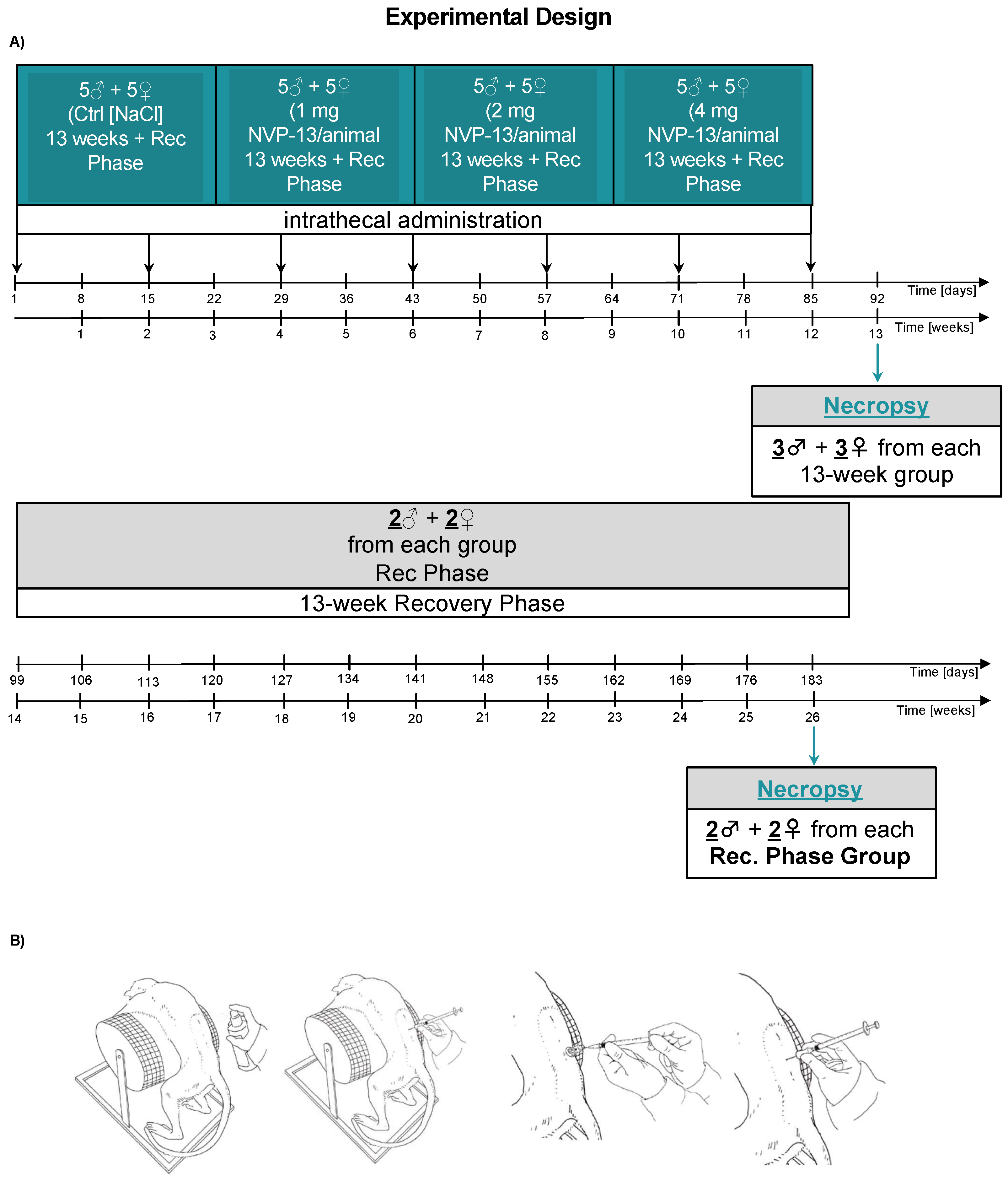
2.2. In Vivo Experimental Design
2.3. Pre-Study Procedures
- European Directive 2001/83/EC and all subsequent amendments
- German Drug Law
- International Conference on Harmonization (ICH) Guideline: Guidance on Non clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals, M3(R2), issued in EMA as CPMP/ICH/286/95.
- ICH-S3A, Toxicokinetics: A Guidance for Assessing Systemic Exposure in Toxicology Studies, issued in EMA as CPMP/ICH/384/95
- ICH-S6, Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals, issued in EMA as
- CPMP/ICH/302/95, and first revision, issued in EMA as CHMP/ICH/731268/1998
- Guideline on repeated dose toxicity, issued by EMA as CPMP/SWP/1042/99 Rev 1
2.4. Dosing Procedure
2.5. Plasma Collection
2.6. Cerebrospinal Fluid Collection
2.7. Tissue Collection
2.7.1. Brain
2.7.2. Spinal Cord
2.7.3. Liver and Kidney
2.8. Clinical Observations
2.9. In Life Parameters
2.10. Clinical Pathology
2.11. End of in Life Phase
2.12. Necropsy, Organ Weights, and Macroscopic Observations
2.13. Histology
2.14. Microscopic Observations
2.15. NVP-13 Concentration in Plasma
2.16. Statistics
3. Results
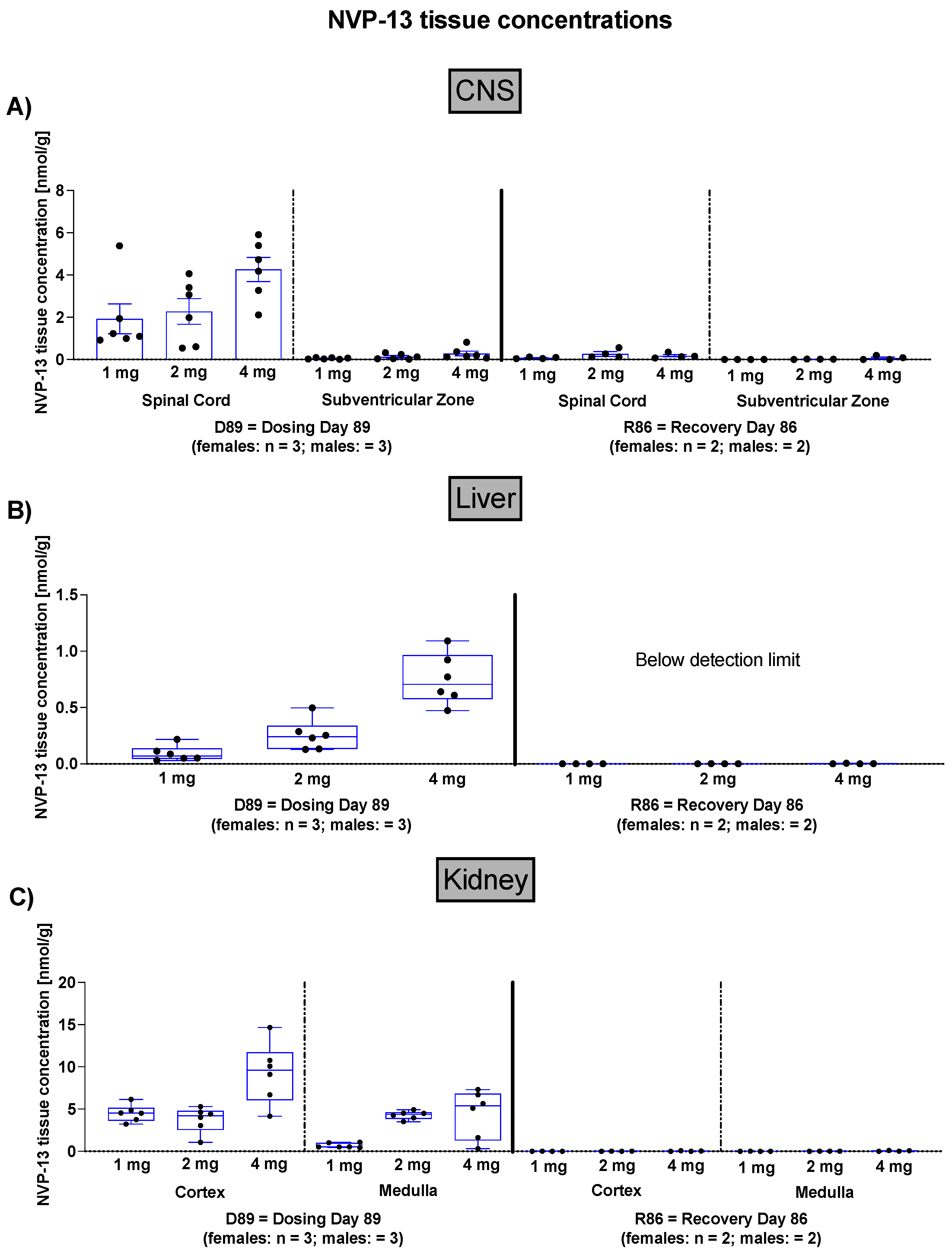
3.1. NVP-13 CNS, Liver, and Kidney Concentrations
3.2. Effects of NVP-13 on Physiological Parameters
3.3. Effects of NVP-13 on Neurobehavioral Parameters
3.4. Effects of NVP-13 on Physical Parameters
3.5. Effects of NVP-13 on Neurological Findings—Spinal Reflexes
3.6. Effects of NVP-13 on Ophthalmic Parameters
3.7. Effects of NVP-13 on Cardiovascular Parameters
- -
- Electrocardiography: An eight-lead ECG measurement (Leads I, II, III, aVR, aVL, V1, and V2) was performed, and the following parameters were measured: heart rate (beats/min), RR, PR, QRS, QT, corrected QT.
- -
- Blood Pressure: Systolic, diastolic, and mean arterial pressures (mmHg) were recorded in all animals by high definition oscillometry (HDO) method.
- -
- Respiratory Rate: Investigations were performed on non-anesthetized, temporarily restrained animals once during the predose phase, in weeks 4 and 13 of the dosing phase, and during the last week of recovery phase by counting respiratory phases for 15 s for calculation of respiratory frequency (respirations/minute). For none of the measured parameters were any adverse or test item-related changes noted (Figure 3).
3.8. Effects of NVP-13 on Hematological Parameters
3.9. Effects of NVP-13 on Coagulation
3.10. Effects of NVP-13 on Clinical Chemistry
3.11. Effects of NVP-13 on Cerebrospinal Fluid Clinical Chemistry and Cell Count
3.12. Effects of NVP-13 on Urine Physiology
3.13. Effects of NVP-13 on Macroscopic Observations and Organ Weights
3.14. Effects of NVP-13 on Microscopic Observations
3.14.1. Central Nervous System
3.14.2. Iliac Lymph Node Changes
3.14.3. Necropsy of Recovery Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, S.; Zitzelsperger, E.; Kuespert, S.; Iberl, S.; Heydn, R.; Johannesen, S.; Petri, S.; Aigner, L.; Thal, D.R.; Hermann, A.; et al. The TGF-β System as a Potential Pathogenic Player in Disease Modulation of Amyotrophic Lateral Sclerosis. Front. Neurol. 2017, 8, 669. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, M.; Rosskopf, M.; Wagner, K.; Klein, B.; Couillard-Despres, S.; Reitsamer, H.A.; Stephan, M.; Nguyen, H.P.; Riess, O.; Bogdahn, U.; et al. Reduction in Subventricular Zone-Derived Olfactory Bulb Neurogenesis in a Rat Model of Huntington’s Disease Is Accompanied by Striatal Invasion of Neuroblasts. PLoS ONE 2015, 10, e0116069. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Galán, L.; Gómez-Pinedo, U.; Guerrero, A.; García-Verdugo, J.M.; Matías-Guiu, J. Amyotrophic lateral sclerosis modifies progenitor neural proliferation in adult classic neurogenic brain niches. BMC Neurol. 2017, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Pinedo, U.; Galán, L.; Matías-Guiu, J.A.; Pytel, V.; Moreno, T.; Guerrero-Sola, A.; Matías-Guiu, J. Notch Signalling in the Hippocampus of Patients with Motor Neuron Disease. Front. Neurosci. 2019, 13, 302. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, M.; Reilmann, R.; Winkler, J.; Bogdahn, U.; Aigner, L. Transforming Growth Factor-Beta Signaling in the Neural Stem Cell Niche: A Therapeutic Target for Huntington’s Disease. Neurol. Res. Int. 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2017. [Google Scholar] [CrossRef] [Green Version]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071–17119. [Google Scholar] [CrossRef]
- Traxinger, K.; Kelly, C.; Johnson, B.A.; Lyles, R.H.; Glass, J.D. Prognosis and epidemiology of amyotrophic lateral sclerosis: Analysis of a clinic population, 1997–2011. Neurol. Clin. Pract. 2013, 3, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Hardiman, O.; Kiernan, M.C.; Chiò, A.; Rix-Brooks, B.; van den Berg, L.H.A. Amyotrophic lateral sclerosis: Moving towards a new classification system. Lancet Neurol. 2016, 15, 1182–1194. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Publ. Group 2014, 10, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, T.K.; Smith, D.H.; Voddi, M.; Perri, B.R.; Stutzmann, J.M. Riluzole, a novel neuroprotective agent, attenuates both neurologic motor and cognitive dysfunction following experimental brain injury in the rat. J. Neurotrauma 1996, 13, 767–780. [Google Scholar] [CrossRef]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2009, 4, 191–206. [Google Scholar]
- Lacomblez, L.; Bensimon, G.; Leigh, P.N.; Guillet, P.; Powe, L.; Durrleman, S.; Delumeau, J.C.; Meininger, V. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology 1996, 47, S242–S250. [Google Scholar] [CrossRef]
- Doble, A. The pharmacology and mechanism of action of riluzole. Neurology 1996, 47, S233–S241. [Google Scholar] [CrossRef]
- Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, M.L.; Zamecnik, P.C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 285–288. [Google Scholar] [CrossRef] [Green Version]
- DeVos, S.L.; Miller, T.M. Antisense oligonucleotides: Treating neurodegeneration at the level of RNA. Neurotherapeutics 2013, 10, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, C.F.; Kordasiewicz, H.B.; Cleveland, D.W. Antisense Drugs Make Sense for Neurological Diseases. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 831–852. [Google Scholar] [CrossRef]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.-H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L. Phase 1–2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Leavitt, B.R.; Landwehrmeyer, G.B.; Wild, E.J.; Saft, C.; Barker, R.A.; Blair, N.F.; Craufurd, D.; Priller, J.; Rickards, H. Targeting Huntingtin Expression in Patients with Huntington’s Disease. N. Engl. J. Med. 2019, 380, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Neil, E.E.; Bisaccia, E.K. Nusinersen: A Novel Antisense Oligonucleotide for the Treatment of Spinal Muscular Atrophy. J. Pediatric Pharmacol. Ther. 2019, 24, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wurster, C.D.; Ludolph, A.C. Antisense oligonucleotides in neurological disorders. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418776932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurster, C.D. Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. J. Neurol. 2019, 266, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Haché, M.; Swoboda, K.J.; Sethna, N.; Farrow-Gillespie, A.; Khandji, A.; Xia, S.; Bishop, K.M. Intrathecal Injections in Children with Spinal Muscular Atrophy. J. Child Neurol. 2016, 31, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Chiriboga, C.A.; Swoboda, K.J.; Darras, B.T.; Iannaccone, S.T.; Montes, J.; Darryl, C.; Norris, D.A.; Bennett, C.F.; Bishop, K.M. Results from a phase 1 study of nusinersen (ISIS-SMN Rx) in children with spinal muscular atrophy. Neurology 2016, 86, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Ikushima, H.; Miyazono, K. TGF-β signal transduction spreading to a wider field: A broad variety of mechanisms for context-dependent effects of TGF-β. Cell Tissue Res. 2011, 347, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Strasen, J.; Sarma, U.; Jentsch, M.; Bohn, S.; Sheng, C.; Horbelt, D.; Knaus, P.; Legewie, S.; Loewer, A. Cell-specific responses to the cytokine TGFβ are determined by variability in protein levels. Mol. Syst. Biol. 2018, 14, e7733. [Google Scholar] [CrossRef] [PubMed]
- Zi, Z.; Chapnick, D.A.; Liu, X. Dynamics of TGF-β/Smad signaling. FEBS Lett. 2012, 586, 1921–1928. [Google Scholar] [CrossRef] [Green Version]
- Aigner, L.; Bogdahn, U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell Tissue Res. 2008, 331, 225–241. [Google Scholar] [CrossRef]
- Blank, U.; Karlsson, S. TGF-β signaling in the control of hematopoietic stem cells. Blood 2015, 125, 3542–3550. [Google Scholar] [CrossRef]
- Cua, D.J.; Kastelein, R.A. TGF-[beta], a ‘double agent’ in the immune pathology war. Nat. Immunol. 2006, 7, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Moustakes, A.; Miyazawa, K. TGF-β in Human Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Galbiati, M.; Crippa, V.; Rusmini, P.; Cristofani, R.; Messi, E.; Piccolella, M.; Tedesco, B.; Ferrari, V.; Casarotto, E.; Chierichetti, M.; et al. Multiple Roles of Transforming Growth Factor Beta in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 13, 4291. [Google Scholar] [CrossRef]
- Kuespert, S.; Heydn, R.; Peters, S.; Wirkert, E.; Meyer, A.L.; Siebörger, M.; Johannesen, S.; Aigner, L.; Bogdahn, U.; Bruun, T.H. Antisense Oligonucleotide in LNA-Gapmer Design Targeting TGFBR2—A Key Single Gene Target for Safe and Effective Inhibition of TGFβ Signaling. Int. J. Mol. Sci. 2020, 21, 1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.; Kuespert, S.; Wirkert, E.; Heydn, R.; Jurek, B.; Johannesen, S.; Hsam, O.; Korte, S.; Ludwig, F.T.; Mecklenburg, L.; et al. Reconditioning the Neurogenic Niche of Adult Non-human Primates by Antisense Oligonucleotide-Mediated Attenuation of TGFβ Signaling. Neurotherapeutics 2021, 1–17. [Google Scholar] [CrossRef]
- Passini, M.A.; Bu, J.; Richards, A.M.; Kinnecom, C.; Sardi, S.P.; Stanek, L.M.; Hua, Y.; Rigo, F.; Matson, J.; Hung, G.; et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011, 3, 72ra18. [Google Scholar] [CrossRef] [Green Version]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef]
- Engelhardt, J.A.; Fant, P.; Guionaud, S.; Henry, S.P.; Leach, M.W.; Louden, C.; Scicchitano, M.S.; Weaver, J.L.; Zabka, T.S.; Frazier, K.S. Scientific and Regulatory Policy Committee Points-to-consider Paper*. Toxicol. Pathol. 2015, 43, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.; Runge, F.; Wozniak, M.M.; Ludwig, F.T.; Smieja, D.; Korytko, P.; Mecklenburg, L. Range of Neurological Signs in Cynomolgus Monkeys After Intrathecal Bolus Administration of Antisense Oligonucleotides. Int. J. Toxicol. 2020, 39, 505–509. [Google Scholar] [CrossRef]
- Korte, S.; Luft, J.; von Keutz, A.; Runge, F.; Mecklenburg, L.; Wozniak, M.M.; Zander, S.; Ludwig, F.T.; Pajaziti, B.; Romeike, A.; et al. Save Your Maximum Tolerated Dose: How to Diagnose Procedure-Related Spinal Cord Lesions After Lumbar Intrathecal Bolus Administration of Oligonucleotides in Cynomolgus Monkeys. Int. J. Toxicol. 2020, 39, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Lenz, B.; Braendli-Baiocco, A.; Engelhardt, J.; Fant, P.; Fischer, H.; Francke, S.; Fukuda, R.; Gröters, S.; Harada, T.; Harleman, H.; et al. Characterizing adversity of lysosomal accumulation in nonclinical toxicity studies: Results from the 5th ESTP international expert workshop. Toxicol. Pathol. 2018, 46, 224–246. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Engelhardt, J.A.; Hung, G.; Yee, J.; Kikkawa, R.; Matson, J.; Tayefeh, B.; Machemer, T.; Giclas, P.C.; Henry, S.P. Effects of Repeated Complement Activation Associated with Chronic Treatment of Cynomolgus Monkeys with 2′-O-Methoxyethyl Modified Antisense Oligonucleotide. Nucleic. Acid Ther. 2016, 26, 236–249. [Google Scholar] [CrossRef]





| Sex | NVP-13 | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| Dose Level (mg/Animal) | 0 | 1 | 2 | 4 | 0 | 1 | 2 | 4 |
| Brain | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| Minimal | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Ganglion, Trigeminal | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Intrathecal, Injection site | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Fibrosis | ||||||||
| Not Present | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 |
| Minimal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Slight | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Intrathecal, Injection site | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Gliosis, NOS | ||||||||
| Not Present | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Minimal | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Intrathecal, Injection site | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Minimal | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Spinal cord, Cervical | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 |
| Minimal | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Spinal cord, Lumbar | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Fibrosis, focal | ||||||||
| Not Present | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 0 |
| Minimal | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Slight | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Spinal cord, Lumbar | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Gliosis, NOS | ||||||||
| Not Present | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 1 |
| Minimal | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 |
| Spinal cord, Lumbar | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 1 |
| Minimal | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 0 |
| Slight | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Spinal cord, Thoracic | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 |
| Minimal | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Spinal nerve roots | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Infiltrate, mononuclear cells | ||||||||
| Not Present | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 2 |
| Minimal | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slight | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Spinal nerve roots | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Neuronolysis, dorsal root ganglia | ||||||||
| Not Present | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 |
| Minimal | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Lymph node, Mesenteric | ||||||||
| Number Examined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Granular macrophages | ||||||||
| Not Present | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 |
| Slight | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, S.; Wirkert, E.; Kuespert, S.; Heydn, R.; Johannesen, S.; Friedrich, A.; Mailänder, S.; Korte, S.; Mecklenburg, L.; Aigner, L.; et al. Safe and Effective Cynomolgus Monkey GLP—Tox Study with Repetitive Intrathecal Application of a TGFBR2 Targeting LNA-Gapmer Antisense Oligonucleotide as Treatment Candidate for Neurodegenerative Disorders. Pharmaceutics 2022, 14, 200. https://doi.org/10.3390/pharmaceutics14010200
Peters S, Wirkert E, Kuespert S, Heydn R, Johannesen S, Friedrich A, Mailänder S, Korte S, Mecklenburg L, Aigner L, et al. Safe and Effective Cynomolgus Monkey GLP—Tox Study with Repetitive Intrathecal Application of a TGFBR2 Targeting LNA-Gapmer Antisense Oligonucleotide as Treatment Candidate for Neurodegenerative Disorders. Pharmaceutics. 2022; 14(1):200. https://doi.org/10.3390/pharmaceutics14010200
Chicago/Turabian StylePeters, Sebastian, Eva Wirkert, Sabrina Kuespert, Rosmarie Heydn, Siw Johannesen, Anita Friedrich, Susanne Mailänder, Sven Korte, Lars Mecklenburg, Ludwig Aigner, and et al. 2022. "Safe and Effective Cynomolgus Monkey GLP—Tox Study with Repetitive Intrathecal Application of a TGFBR2 Targeting LNA-Gapmer Antisense Oligonucleotide as Treatment Candidate for Neurodegenerative Disorders" Pharmaceutics 14, no. 1: 200. https://doi.org/10.3390/pharmaceutics14010200
APA StylePeters, S., Wirkert, E., Kuespert, S., Heydn, R., Johannesen, S., Friedrich, A., Mailänder, S., Korte, S., Mecklenburg, L., Aigner, L., Bruun, T.-H., & Bogdahn, U. (2022). Safe and Effective Cynomolgus Monkey GLP—Tox Study with Repetitive Intrathecal Application of a TGFBR2 Targeting LNA-Gapmer Antisense Oligonucleotide as Treatment Candidate for Neurodegenerative Disorders. Pharmaceutics, 14(1), 200. https://doi.org/10.3390/pharmaceutics14010200






