Liposomal Encapsulation Increases the Efficacy of Azithromycin against Chlamydia trachomatis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. C. trachomatis Strains
2.3. Preparation of AZT-Liposomes
2.4. Size Measurements of AZT-Liposomes
2.5. Zeta Potential Measurements of AZT-Liposomes
2.6. Liposomal Bilayer Elasticity Determination
2.7. AZT Encapsulation Efficiency (EE) Determination
2.8. Cytotoxicity Measurement by 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT) Assay
2.9. Culture of HeLa 229 Cells and Direct qPCR Measurement of the Impact of AZT-Liposomes on C. trachomatis Growth
2.10. Statistical Analysis
3. Results
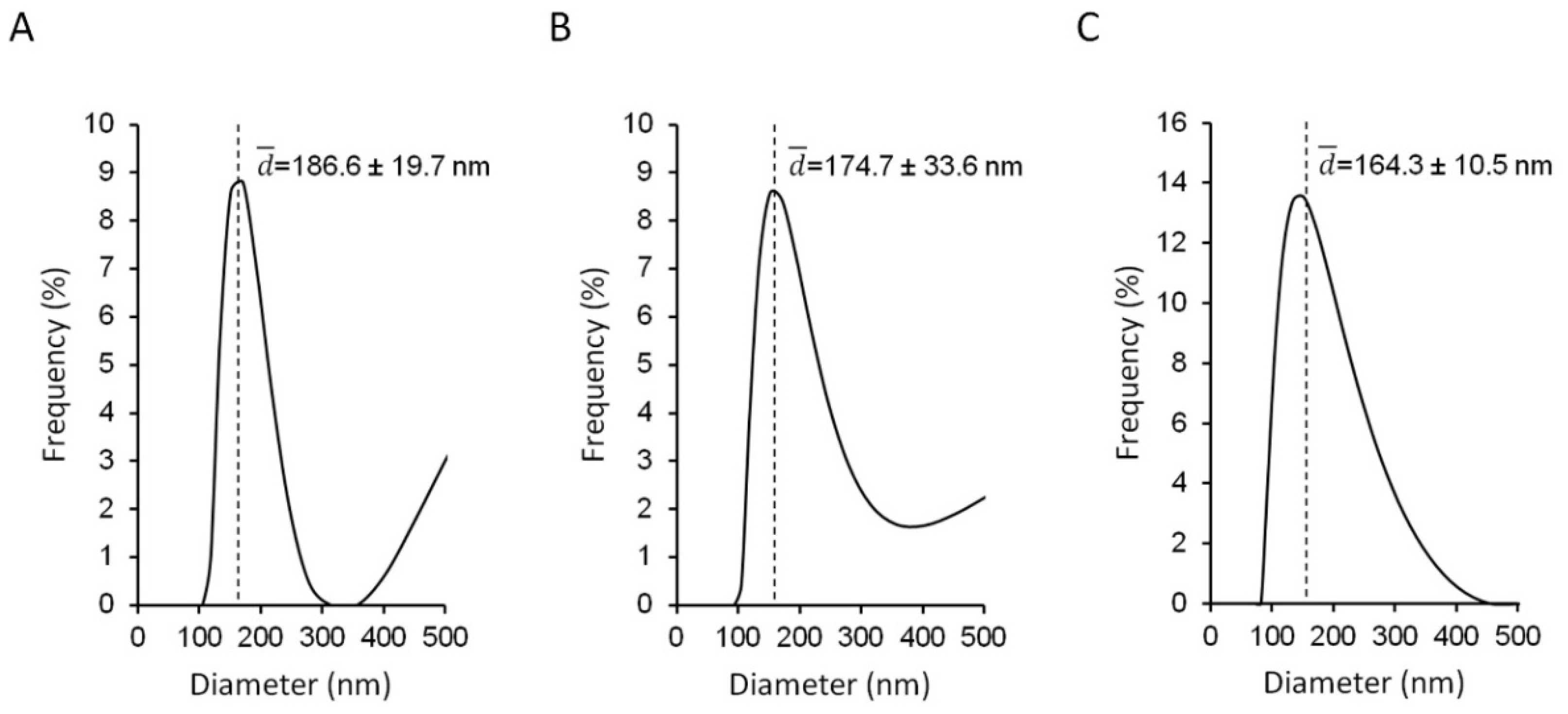
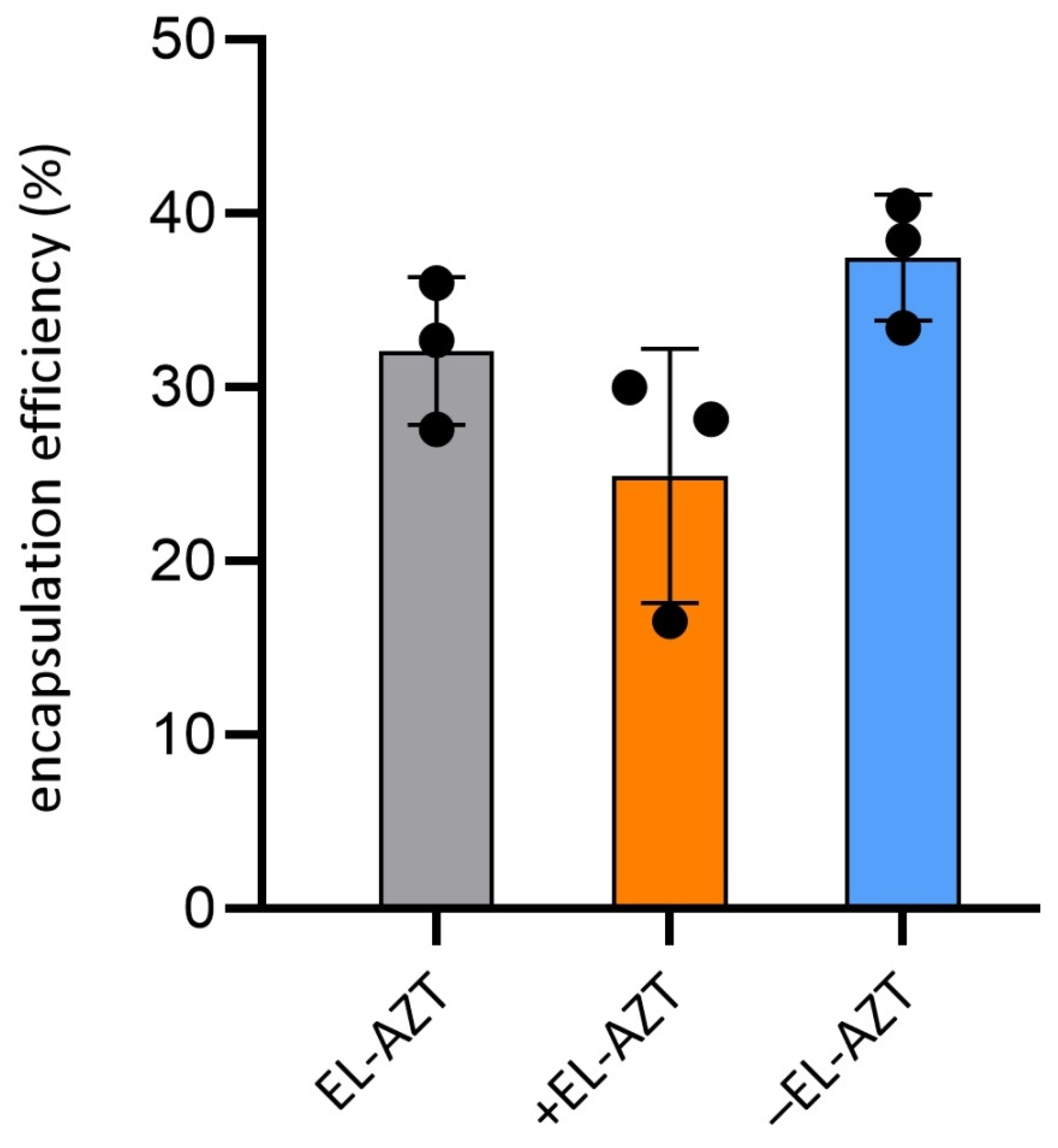
3.1. Physico-Chemical Properties of the AZT-Liposomes
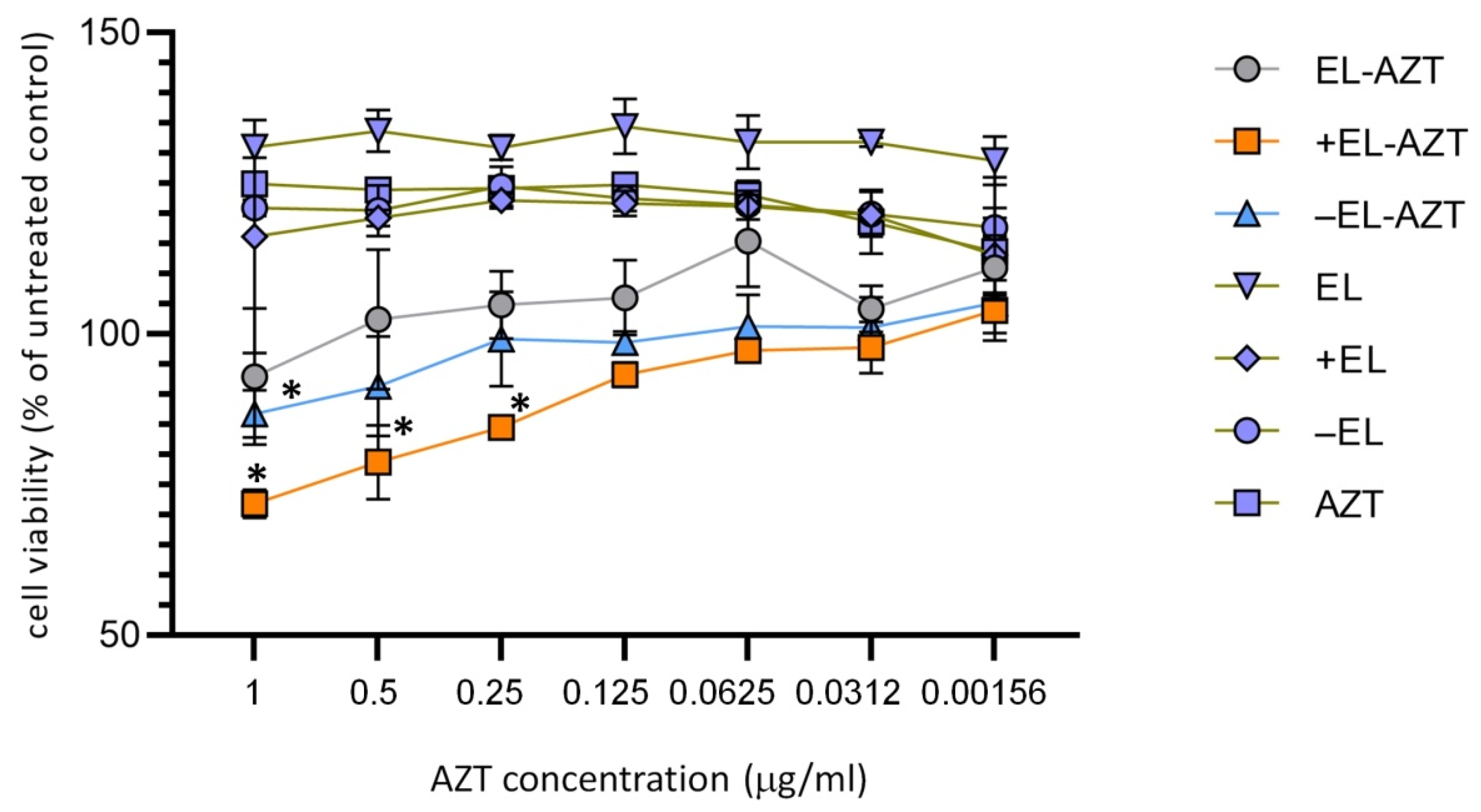
3.2. Cytotoxicity Measurement of the of AZT-Liposomes on HeLa 229 Cells
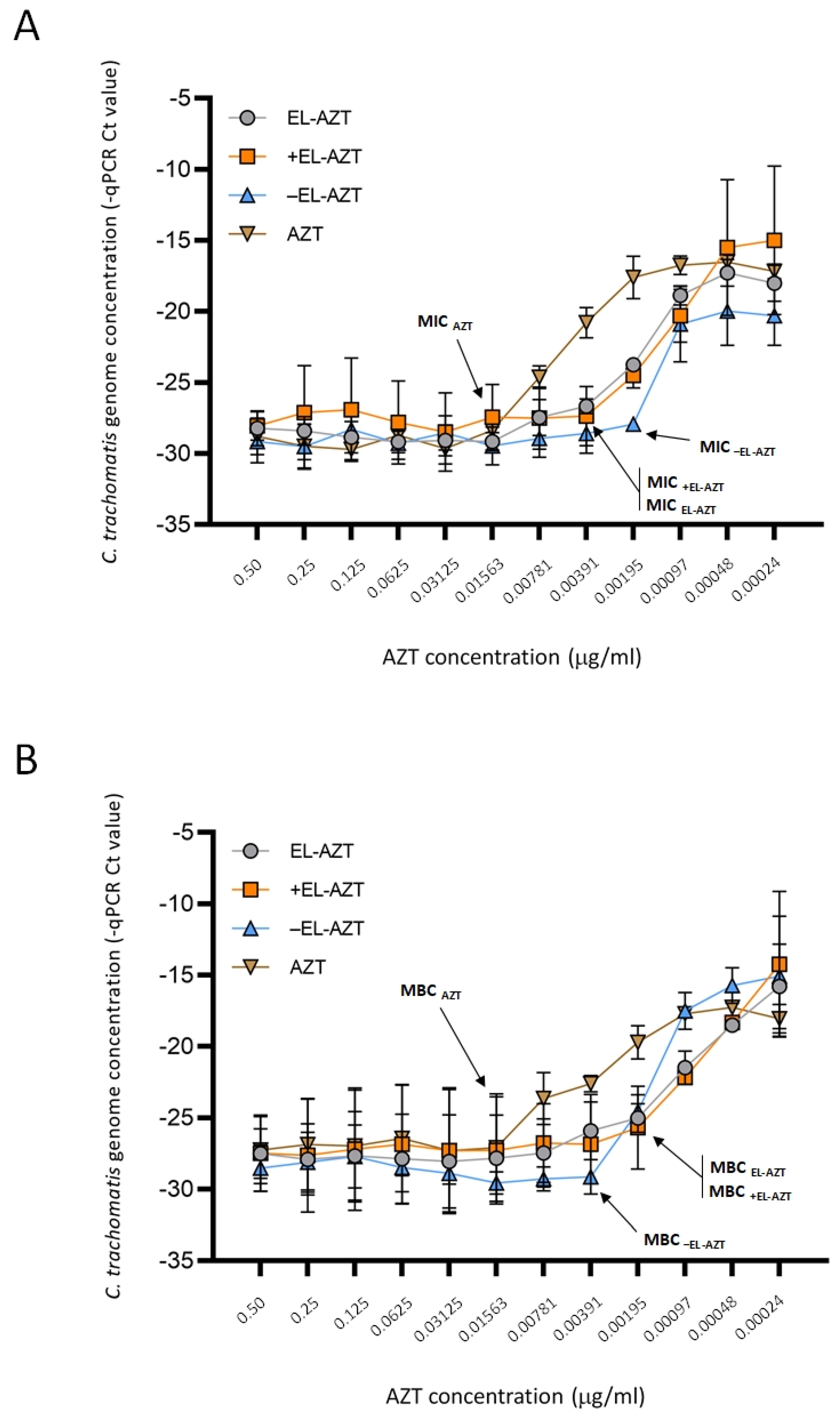
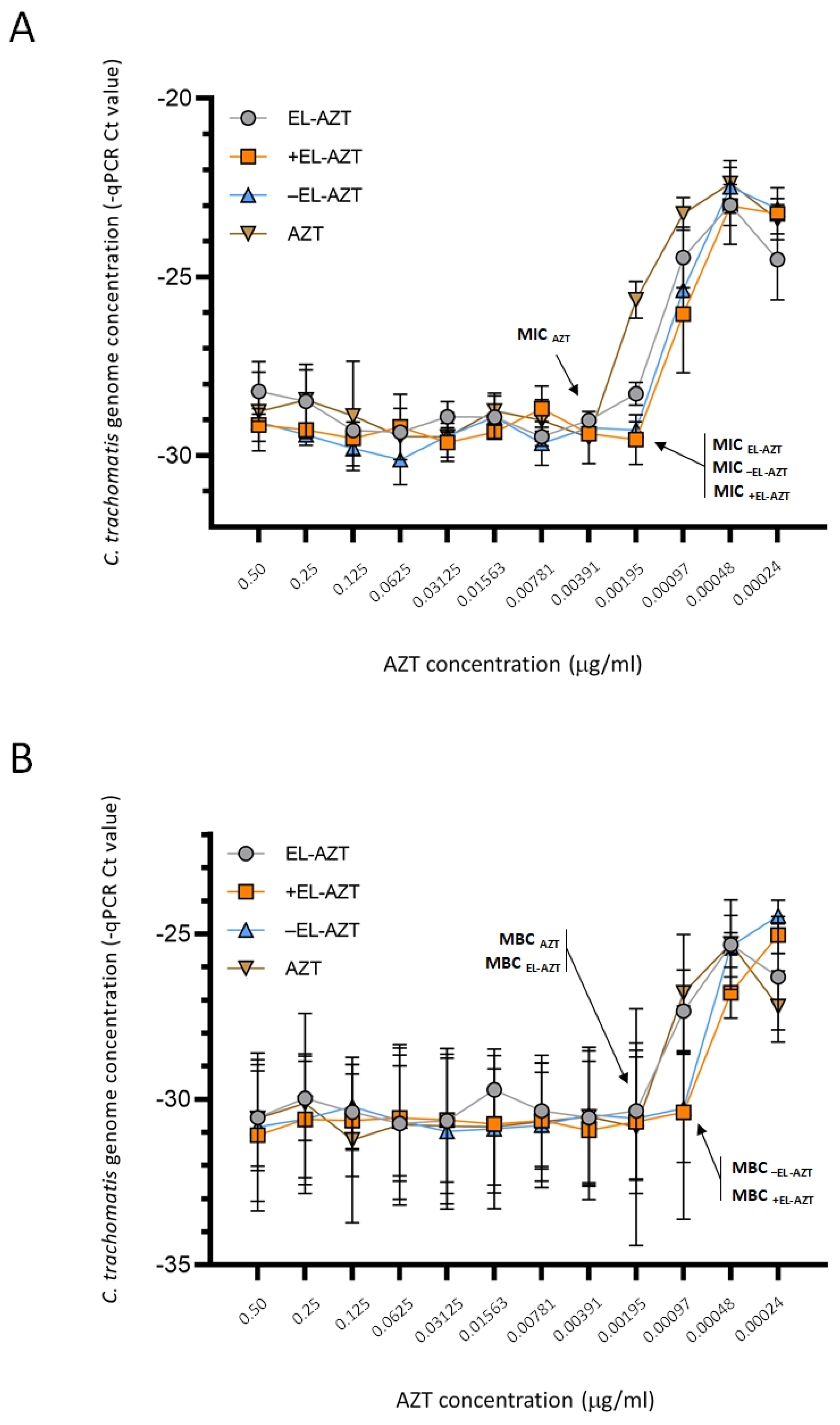
3.3. Impact of AZT-Liposomes on C. trachomatis Growth
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trachoma Key Facts (World Health Organization). Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Trachoma (accessed on 20 December 2021).
- Smolarczyk, K.; Mlynarczyk-Bonikowska, B.; Rudnicka, E.; Szukiewicz, D.; Meczekalski, B.; Smolarczyk, R.; Pieta, W. The Impact of Selected Bacterial Sexually Transmitted Diseases on Pregnancy and Female Fertility. Int. J. Mol. Sci. 2021, 22, 2170. [Google Scholar] [CrossRef]
- Gladue, R.P.; Snider, M.E. Intracellular Accumulation of Azithromycin by Cultured Human Fibroblasts. Antimicrob. Agents Chemother. 1990, 34, 1056–1060. [Google Scholar] [CrossRef] [Green Version]
- Challenges in Treating Chlamydia Trachomatis, Including Rectal Infections: Is It Time to Go Back to Doxycycline?—S. Lena Kang-Birken. 2021. Available online: https://journals.sagepub.com/doi/10.1177/10600280211029945 (accessed on 5 July 2021).
- Kong, F.Y.S.; Tabrizi, S.N.; Fairley, C.K.; Phillips, S.; Fehler, G.; Law, M.; Vodstrcil, L.A.; Chen, M.; Bradshaw, C.S.; Hocking, J.S. Higher Organism Load Associated with Failure of Azithromycin to Treat Rectal Chlamydia. Epidemiol. Infect. 2016, 144, 2587–2596. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; You, C.; Cao, J.; Jiang, Y.; Liu, Y.; Liu, Q. High Treatment Failure Rate Is Better Explained by Resistance Gene Detection than by Minimum Inhibitory Concentration in Patients with Urogenital Chlamydia Trachomatis Infection. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 96, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoz, K.M.; Rockey, D.D. Antibiotic Resistance in Chlamydiae. Future Microbiol. 2010, 5, 1427–1442. [Google Scholar] [CrossRef] [Green Version]
- Vanić, Ž.; Jøraholmen, M.W.; Škalko-Basnet, N. Nanomedicines for the Topical Treatment of Vulvovaginal Infections: Addressing the Challenges of Antimicrobial Resistance. Adv. Drug Deliv. Rev. 2021, 178, 113855. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as Delivery Systems for Antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef]
- Rukavina, Z.; Vanić, Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, E18. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Sachetelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Sénéchal, S.; Lagacé, J. Demonstration of a Fusion Mechanism between a Fluid Bactericidal Liposomal Formulation and Bacterial Cells. Biochim. Biophys. Acta 2000, 1463, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Mol. Basel Switz. 2021, 26, 2047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, Y.; Khalil, H.; Wang, R.; Lu, T.; Zhao, W.; Zhang, Y.; Chen, J.; Chen, T. Fusion between Fluid Liposomes and Intact Bacteria: Study of Driving Parameters and in Vitro Bactericidal Efficacy. Int. J. Nanomed. 2016, 11, 4025–4036. [Google Scholar] [CrossRef] [Green Version]
- Vanić, Z.; Holæter, A.-M.; Skalko-Basnet, N. (Phospho)Lipid-Based Nanosystems for Skin Administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic Liposomes as Novel Carriers: Recent Advances in Drug Delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-Z.; Hao, X.-L.; Zhao, N.; Han, W.-X.; Zhai, X.-F.; Zhao, Q.; Wang, Y.-E.; Zhou, Y.-Q.; Cheng, Y.-C.; Yue, Y.-H.; et al. Propylene Glycol-Embodying Deformable Liposomes as a Novel Drug Delivery Carrier for Vaginal Fibrauretine Delivery Applications. J. Control. Release Off. J. Control. Release Soc. 2016, 226, 107–114. [Google Scholar] [CrossRef]
- Rukavina, Z.; Šegvić Klarić, M.; Filipović-Grčić, J.; Lovrić, J.; Vanić, Ž. Azithromycin-Loaded Liposomes for Enhanced Topical Treatment of Methicillin-Resistant Staphyloccocus Aureus (MRSA) Infections. Int. J. Pharm. 2018, 553, 109–119. [Google Scholar] [CrossRef]
- Vanić, Ž.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Amidžić Klarić, D.; Bogdanov, A.; Raffai, T.; Virok, D.P.; et al. Azithromycin-Liposomes as a Novel Approach for Localized Therapy of Cervicovaginal Bacterial Infections. Int. J. Nanomed. 2019, 14, 5957–5976. [Google Scholar] [CrossRef] [Green Version]
- Bonicelli, M.G.; Giansanti, L.; Ierino, M.; Mancini, G. Interaction of Cationic Liposomes with Cell Membrane Models. J. Colloid Interface Sci. 2011, 355, 1–8. [Google Scholar] [CrossRef]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanić, Ž.; Škalko-Basnet, N. Liposomes Augment Biological Benefits of Curcumin for Multitargeted Skin Therapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Jurstrand, M.; Falk, L.; Fredlund, H.; Lindberg, M.; Olcén, P.; Andersson, S.; Persson, K.; Albert, J.; Bäckman, A. Characterization of Chlamydia Trachomatis Omp1 Genotypes among Sexually Transmitted Disease Patients in Sweden. J. Clin. Microbiol. 2001, 39, 3915–3919. [Google Scholar] [CrossRef] [Green Version]
- Sabet, S.F.; Simmons, J.; Caldwell, H.D. Enhancement of Chlamydia Trachomatis Infectious Progeny by Cultivation of HeLa 229 Cells Treated with DEAE-Dextran and Cycloheximide. J. Clin. Microbiol. 1984, 20, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavelić, Z.; Skalko-Basnet, N.; Jalsenjak, I. Characterisation and in Vitro Evaluation of Bioadhesive Liposome Gels for Local Therapy of Vaginitis. Int. J. Pharm. 2005, 301, 140–148. [Google Scholar] [CrossRef]
- Vanić, Ž.; Hurler, J.; Ferderber, K.; Golja Gašparović, P.; Škalko-Basnet, N.; Filipović-Grčić, J. Novel Vaginal Drug Delivery System: Deformable Propylene Glycol Liposomes-in-Hydrogel. J. Liposome Res. 2014, 24, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Eszik, I.; Lantos, I.; Önder, K.; Somogyvári, F.; Burián, K.; Endrész, V.; Virok, D.P. High Dynamic Range Detection of Chlamydia Trachomatis Growth by Direct Quantitative PCR of the Infected Cells. J. Microbiol. Methods 2016, 120, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Palac, Z.; Engesland, A.; Flaten, G.E.; Škalko-Basnet, N.; Filipović-Grčić, J.; Vanić, Ž. Liposomes for (Trans)Dermal Drug Delivery: The Skin-PVPA as a Novel in Vitro Stratum Corneum Model in Formulation Development. J. Liposome Res. 2014, 24, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrett, S.; Golding, M.; Williams, W.P. A Simple Method for the Preparation of Liposomes for Pharmaceutical Applications: Characterization of the Liposomes. J. Pharm. Pharmacol. 1991, 43, 154–161. [Google Scholar] [CrossRef]
- Pavelić, Z.; Skalko-Basnet, N.; Schubert, R.; Jalsenjak, I. Liposomal Gels for Vaginal Drug Delivery. Methods Enzymol. 2004, 387, 287–299. [Google Scholar] [CrossRef]
- Pavelić, Z.; Skalko-Basnet, N.; Filipović-Grcić, J.; Martinac, A.; Jalsenjak, I. Development and in Vitro Evaluation of a Liposomal Vaginal Delivery System for Acyclovir. J. Control. Release Off. J. Control. Release Soc. 2005, 106, 34–43. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, A.; Oh, Y.-K.; Kim, C.-K. Effect of Edge Activators on the Formation and Transfection Efficiency of Ultradeformable Liposomes. Biomaterials 2005, 26, 205–210. [Google Scholar] [CrossRef]
- Vanić, Ž.; Hafner, A.; Bego, M.; Škalko-Basnet, N. Characterization of Various Deformable Liposomes with Metronidazole. Drug Dev. Ind. Pharm. 2013, 39, 481–488. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Montenez, J.P.; Piret, J.; Tulkens, P.M.; Courtoy, P.J.; Mingeot-Leclercq, M.P. Interaction of the Macrolide Azithromycin with Phospholipids. I. Inhibition of Lysosomal Phospholipase A1 Activity. Eur. J. Pharmacol. 1996, 314, 203–214. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yeh, M.-K. Clinical Development of Liposome-Based Drugs: Formulation, Characterization, and Therapeutic Efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Agacfidan, A.; Moncada, J.; Schachter, J. In Vitro Activity of Azithromycin (CP-62,993) against Chlamydia Trachomatis and Chlamydia Pneumoniae. Antimicrob. Agents Chemother. 1993, 37, 1746–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati, M.; Rodrìguez Fermepin, M.; Olmo, A.; D’Apote, L.; Cevenini, R. Comparative In-Vitro Activity of Moxifloxacin, Minocycline and Azithromycin against Chlamydia spp. J. Antimicrob. Chemother. 1999, 43, 825–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, L.E.; Gaydos, C.A.; Quinn, T.C. In Vitro Evaluation of Activities of Azithromycin, Erythromycin, and Tetracycline against Chlamydia Trachomatis and Chlamydia Pneumoniae. Antimicrob. Agents Chemother. 1992, 36, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Belland, R.J.; Nelson, D.E.; Virok, D.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Beatty, W.L.; Caldwell, H.D. Transcriptome Analysis of Chlamydial Growth during IFN-Gamma-Mediated Persistence and Reactivation. Proc. Natl. Acad. Sci. USA 2003, 100, 15971–15976. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Lawrence, A.; Kroon, S.; Vodstrcil, L.A.; Phillips, S.; Hocking, J.S.; Timms, P.; Huston, W.M. Chlamydial Clinical Isolates Show Subtle Differences in Persistence Phenotypes and Growth in Vitro. Access Microbiol. 2021, 3, 000204. [Google Scholar] [CrossRef]
- Aboud, H.M.; Ali, A.A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of Carvedilol for Intranasal Delivery: Formulation, Characterization and in Vivo Evaluation. Drug Deliv. 2016, 23, 2471–2481. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid Vesicles for Skin Delivery of Drugs: Reviewing Three Decades of Research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Cserháti, T. Alkyl Ethoxylated and Alkylphenol Ethoxylated Nonionic Surfactants: Interaction with Bioactive Compounds and Biological Effects. Environ. Health Perspect. 1995, 103, 358–364. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Shokri, J.; Dashbolaghi, A.; Hassan-Zadeh, D.; Ghafourian, T.; Barzegar-Jalali, M. The Enhancement Effect of Surfactants on the Penetration of Lorazepam through Rat Skin. Int. J. Pharm. 2003, 250, 359–369. [Google Scholar] [CrossRef]


| Liposomes (Code) | EPC (mg) | EPG (mg) | DODAB (mg) | AZT (mg) | Tween 80 (mg) | PB, pH 7.5 (mL) |
|---|---|---|---|---|---|---|
| +EL-AZT | 190 | - | 10 | 10 | 50 | 10 |
| −EL-AZT | 190 | 10 | - | 10 | 50 | 10 |
| EL-AZT | 200 | - | - | 10 | 50 | 10 |
| +EL | 190 | - | 10 | - | 50 | 10 |
| −EL | 190 | 10 | - | - | 50 | 10 |
| EL | 200 | - | - | - | 50 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, A.; Janovák, L.; Vraneš, J.; Meštrović, T.; Ljubin-Sternak, S.; Cseh, Z.; Endrész, V.; Burián, K.; Vanić, Ž.; Virok, D.P. Liposomal Encapsulation Increases the Efficacy of Azithromycin against Chlamydia trachomatis. Pharmaceutics 2022, 14, 36. https://doi.org/10.3390/pharmaceutics14010036
Bogdanov A, Janovák L, Vraneš J, Meštrović T, Ljubin-Sternak S, Cseh Z, Endrész V, Burián K, Vanić Ž, Virok DP. Liposomal Encapsulation Increases the Efficacy of Azithromycin against Chlamydia trachomatis. Pharmaceutics. 2022; 14(1):36. https://doi.org/10.3390/pharmaceutics14010036
Chicago/Turabian StyleBogdanov, Anita, László Janovák, Jasmina Vraneš, Tomislav Meštrović, Sunčanica Ljubin-Sternak, Zsuzsanna Cseh, Valéria Endrész, Katalin Burián, Željka Vanić, and Dezső P. Virok. 2022. "Liposomal Encapsulation Increases the Efficacy of Azithromycin against Chlamydia trachomatis" Pharmaceutics 14, no. 1: 36. https://doi.org/10.3390/pharmaceutics14010036
APA StyleBogdanov, A., Janovák, L., Vraneš, J., Meštrović, T., Ljubin-Sternak, S., Cseh, Z., Endrész, V., Burián, K., Vanić, Ž., & Virok, D. P. (2022). Liposomal Encapsulation Increases the Efficacy of Azithromycin against Chlamydia trachomatis. Pharmaceutics, 14(1), 36. https://doi.org/10.3390/pharmaceutics14010036











