Internal Mammary Arteries as a Model to Demonstrate Restoration of the Impaired Vasodilation in Hypertension, Using Liposomal Delivery of the CYP1B1 Inhibitor, 2,3′,4,5′-Tetramethoxystilbene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TMS-Loaded Liposomes
2.2. HPLC Analysis of TMS/TMS-Loaded Liposomes Stability
2.3. Physiological Function Studies
2.3.1. Human Internal Mammary Artery (IMA) Functional Studies
2.3.2. Rat Coronary Artery Functional Studies
2.4. Detection of Reactive Oxygen Species in Human Internal Mammary Arteries (IMAs)
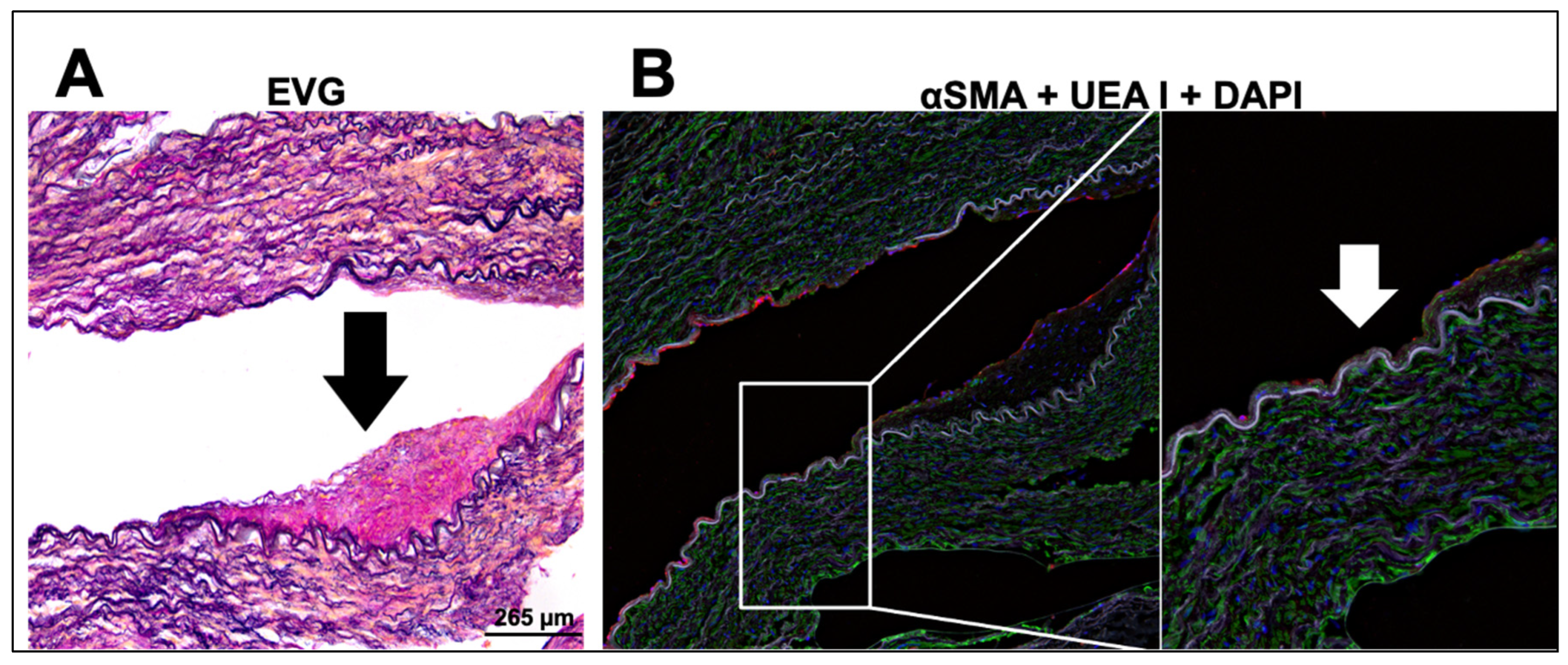
2.5. Immunohistological Analysis of Diseased Human Internal Mammary Arteries (IMAs)
2.6. Statistical Analysis
3. Results
3.1. Synthesis and Characterisation of TMS-Loaded Liposomes
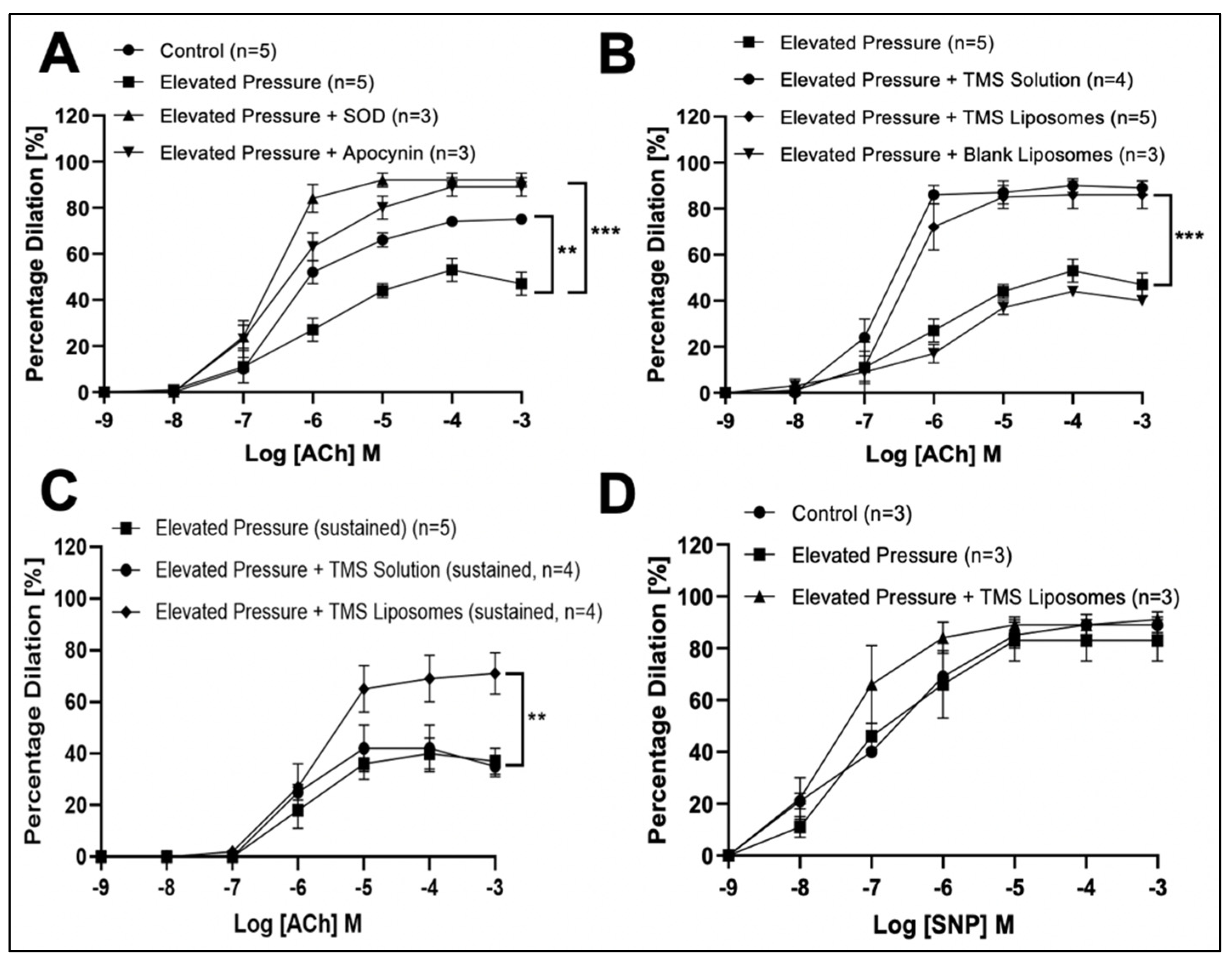
3.2. TMS-Loaded Liposomes Potentiate Endothelial-Dependent Dilator Capacity of Isolated IMAs from CABG Patients Ex Vivo
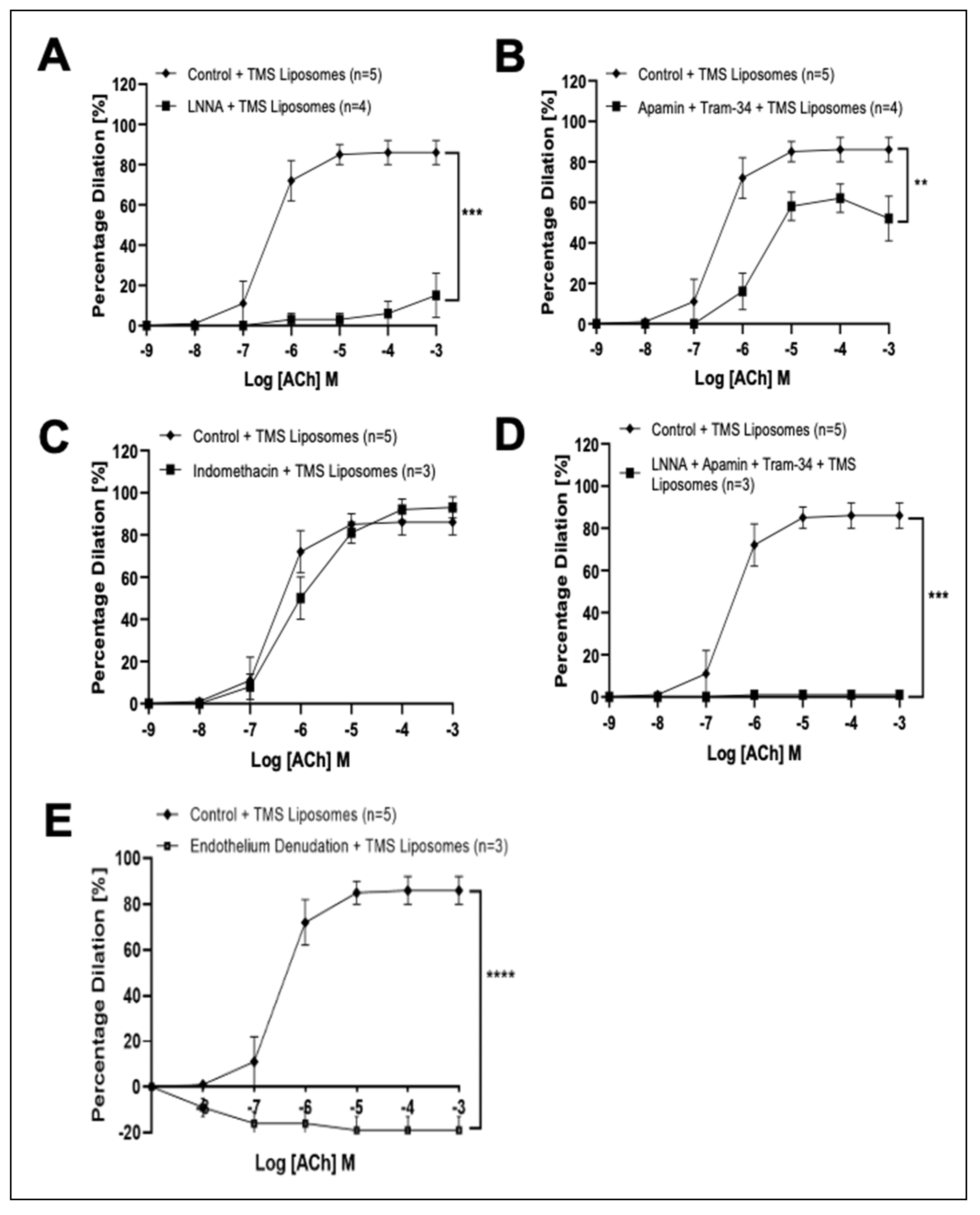
3.3. TMS-Loaded Liposomes Potentiate Endothelial-Dependent Dilation via Activation of NO and Endothelial-Dependent Hyperpolarization (EDH) Pathways, via AMPK in Isolated Rat Coronary Arteries Ex Vivo
3.4. Treatment with TMS-Loaded Liposomes Reduces ROS Moieties, Restoring NO Bioavailability in Isolated Human Internal Mammary Arteries (IMAs) Ex Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease. StatPearls 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554410/ (accessed on 1 July 2022).
- Zang, J.; Liang, J.; Zhuang, X.; Zhang, S.; Liao, X.; Wu, G. Intensive blood pressure treatment in coronary artery disease: Implications from the Systolic Blood Pressure Intervention Trial (SPRINT). J. Hum. Hypertens. 2021, 36, 86–94. [Google Scholar] [CrossRef]
- Poredos, P.; Poredos, A.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef]
- Smith, J.; Lemmey, H.; Borysova, L.; Hiley, C.; Dora, K.; Garland, C. Endothelial Nitric Oxide Suppresses Action-Potential-Like Transient Spikes and Vasospasm in Small Resistance Arteries. Hypertension 2020, 76, 785–794. [Google Scholar] [CrossRef]
- Jennings, B.; Montanez, D.; May, M.; Estes, A.; Fang, X.; Yaghini, F.; Kanu, A.; Malik, K. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc. Drugs Ther. 2014, 28, 145–161. [Google Scholar] [CrossRef]
- Sahan-Firat, S.; Jennings, B.; Yaghini, F.; Song, C.; Estes, A.; Fang, X.; Farjana, N.; Khan, A.; Malik, K. 2,3′,4,5′-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: Contribution of cytochrome P-450 1B1. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H1891–H1901. [Google Scholar] [CrossRef]
- Thirunavukkarasu, S.; Khan, N.; Song, C.; Ghafoor, H.; Brand, D.; Gonzalez, F.; Malik, K. Cytochrome P450 1B1 Contributes to the Development of Angiotensin II–Induced Aortic Aneurysm in Male Apoe−/− Mice. Am. J. Pathol. 2016, 186, 2204–2219. [Google Scholar] [CrossRef]
- Malik, K.; Jennings, B.; Yaghini, F.; Sahan-Firat, S.; Song, C.; Estes, A.; Fang, X. Contribution of cytochrome P450 1B1 to hypertension and associated pathophysiology: A novel target for antihypertensive agents. Prostaglandins Other Lipid Mediat. 2012, 98, 69–74. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Z.; He, G. Difference in Endothelium-Derived Hyperpolarizing Factor–Mediated Hyperpolarization and Nitric Oxide Release between Human Internal Mammary Artery and Saphenous Vein. Circulation 2000, 102, Iii-296–Iii-301. [Google Scholar]
- Pompilio, G.; Rossoni, G.; Alamanni, F.; Tartara, P.; Barajon, I.; Rumio, C.; Manfredi, B.; Biglioli, P. Comparison of endothelium-dependent vasoactivity of internal mammary arteries from hypertensive, hypercholesterolemic, and diabetic patients. Ann. Thorac. Surg. 2001, 72, 1290–1297. [Google Scholar] [CrossRef]
- Archer, S.; Gragasin, F.; Wu, X.; Wang, S.; McMurtry, S.; Kim, D.; Platonov, M.; Koshal, A.; Hashimoto, K.; Campbell, W.; et al. Endothelium-Derived Hyperpolarizing Factor in Human Internal Mammary Artery Is 11,12-Epoxyeicosatrienoic Acid and Causes Relaxation by Activating Smooth Muscle BKCa Channels. Circulation 2003, 107, 769–776. [Google Scholar] [CrossRef]
- Fonseca, D.; Antunes, P.; Cotrim, M. Endothelium-dependent vasoactivity of the human internal mammary artery. Coron. Artery Dis. 2014, 25, 266–274. [Google Scholar] [CrossRef]
- Ruel, M.; Kulik, A. Suboptimal Medical Therapy After Coronary Revascularization. J. Am. Coll. Cardiol. 2018, 71, 603–605. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Feng, Y.; Clayton, J.; Yake, W.; Li, J.; Wang, W.; Winne, L. Resveratrol Derivative, Trans-3,5,4′-Trimethoxystilbene Sensitizes Osteosarcoma Cells to Apoptosis via ROS-Induced Caspases Activation. Oxidative Med. Cell. Longev. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Nguyen, C.; Kotturi, H.; Waris, G.; Mohammed, A.; Chandrakesan, P.; May, R. (Z)-3,5,4′-Trimethoxystilbene Limits Hepatitis C and Cancer Pathophysiology by Blocking Microtubule Dynamics and Cell-Cycle Progression. Cancer Res. 2016, 76, 4887–4896. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.; Rogers, K.; Delehanty, J. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Zaabalawi, A.; Astley, C.; Renshall, L.; Beards, F.; Lightfoot, A.; Degens, H.; Whitehead, D.; Alexander, Y.; Harris, L.; Azzawi, M. Tetramethoxystilbene-Loaded Liposomes Restore Reactive-Oxygen-Species-Mediated Attenuation of Dilator Responses in Rat Aortic Vessels Ex vivo. Molecules 2019, 24, 4360. [Google Scholar] [CrossRef]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.; Agemy, L.; Teesalu, T.; Glazier, J.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef]
- Muir, A.; McKeown, P.; Bayraktutan, U. Role of Gender, Smoking Profile, Hypertension, and Diabetes on Saphenous Vein and Internal Mammary Artery Endothelial Relaxation in Patients with Coronary Artery Bypass Grafting. Oxidative Med. Cell. Longev. 2010, 3, 199–205. [Google Scholar] [CrossRef]
- Astley, C.; Houacine, C.; Zaabalawi, A.; Wilkinson, F.; Lightfoot, A.; Alexander, Y.; Whitehead, D.; Singh, K.; Azzawi, M. Nanostructured Lipid Carriers Deliver Resveratrol, Restoring Attenuated Dilation in Small Coronary Arteries, via the AMPK Pathway. Biomedicines 2021, 9, 1852. [Google Scholar] [CrossRef]
- Touyz, R.; Anagnostopoulou, A.; Camargo, L.; Rios, F.; Montezano, A. Vascular Biology of Superoxide-Generating NADPH Oxidase 5—Implications in Hypertension and Cardiovascular Disease. Antioxid. Redox Signal. 2019, 30, 1027–1040. [Google Scholar] [CrossRef] [Green Version]
- Foudi, N.; Louedec, L.; Cachina, T.; Brink, C.; Norel, X. Selective cyclooxygenase-2 inhibition directly increases human vascular reactivity to norepinephrine during acute inflammation. Cardiovasc. Res. 2008, 81, 269–277. [Google Scholar] [CrossRef]
- Bishop-Bailey, D.; Pepper, J.; Haddad, E.; Newton, R.; Larkin, S.; Mitchell, J. Induction of Cyclooxygenase-2 in Human Saphenous Vein and Internal Mammary Artery. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1644–1648. [Google Scholar] [CrossRef]
- Xu, Y.; Henning, R.; Lipsic, E.; van Buiten, A.; van Gilst, W.; Buikema, H. Acetylcholine stimulated dilatation and stretch induced myogenic constriction in mesenteric artery of rats with chronic heart failure. Eur. J. Heart Fail. 2007, 9, 144–151. [Google Scholar] [CrossRef]
- Hoopes, S.; Garcia, V.; Edin, M.; Schwartzman, M.; Zeldin, D. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015, 120, 9–16. [Google Scholar] [CrossRef]
- Savoui, G.; Craciun, C.; Cristescu, C.; Serban, C.; Ionescu, D.; Fira-Mladinescu, O.; Noveanu, L.; Muntean, D.; Mihalas, G. In Vitro Effects of Nitric Oxide Synthase Inhibition by L-NAME on Human Internal Mammary Arteries Rings. Studia Univ. 2009, 19, 103–107. [Google Scholar]
- Edwards, J.; McCarthy, C.; Wenceslau, C. The Obligatory Role of the Acetylcholine-Induced Endothelium-Dependent Contraction in Hypertension: Can Arachidonic Acid Resolve this Inflammation. Curr. Pharm. Des. 2020, 26, 3723–3732. [Google Scholar] [CrossRef]
- Mikstacka, R.; Wierzchowski, M.; Dutkiewicz, Z.; Gielara-Korzańska, A.; Korzański, A.; Teubert, A. 3,4,2′-Trimethoxy-trans-stilbene—A potent CYP1B1 inhibitor. MedChemComm 2014, 5, 496–501. [Google Scholar] [CrossRef]
- Fan, C.; Joshi, J.; Li, F.; Xu, B.; Khan, M.; Yang, J.; Zhu, W. Nanoparticle-Mediated Drug Delivery for Treatment of Ischemic Heart Disease. Front. Bioeng. Biotechnol. 2020, 8, 687. [Google Scholar] [CrossRef]
- Glukhova, O. Liposome Drug Delivery System across Endothelial Plasma Membrane: Role of Distance between Endothelial Cells and Blood Flow Rate. Molecules 2020, 25, 1875. [Google Scholar] [CrossRef]
- Koning, G.; Schiffelers, R.; Wauben, M.; Kok, R.; Mastrobattista, E.; Molema, G. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate–containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006, 54, 1198–1208. [Google Scholar] [CrossRef]
- Shimokawa, H.; Godo, S. Nitric oxide and endothelium-dependent hyperpolarization mediated by hydrogen peroxide in health and disease. Basic Clin. Pharmacol. Toxicol. 2020, 127, 92–101. [Google Scholar] [CrossRef]
- Nishijima, Y.; Zheng, X.; Lund, H.; Suzuki, M.; Mattson, D.; Zhang, D. Characterization of blood pressure and endothelial function in TRPV4-deficient mice with l-NAME- and angiotensin II-induced hypertension. Physiol. Rep. 2014, 2, e00199. [Google Scholar] [CrossRef]
- Ndiaye, M.; Chataigneau, T.; Chataigneau, M.; Schini-Kerth, V. Red wine polyphenols induce EDHF-mediated relaxations in porcine coronary arteries through the redox-sensitive activation of the PI3-kinase/Akt pathway. Br. J. Pharmacol. 2004, 142, 1131–1136. [Google Scholar] [CrossRef]
- Rodríguez, C.; Muñoz, M.; Contreras, C.; Prieto, D. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, H.; Wang, P.; Zhao, Y.; Li, Q.; Wei, X.; Cui, Y.; Sun, J.; Shang, Q.; Liu, D.; et al. Dietary Curcumin Ameliorates Aging-Related Cerebrovascular Dysfunction through the AMPK/Uncoupling Protein 2 Pathway. Cell. Physiol. Biochem. 2013, 32, 1167–1177. [Google Scholar] [CrossRef]
- Chen, C.; Kassan, A.; Castañeda, D.; Gabani, M.; Choi, S.; Kassan, M. Metformin prevents vascular damage in hypertension through the AMPK/ER stress pathway. Hypertens. Res. 2019, 42, 960–969. [Google Scholar] [CrossRef]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef]
- Price, N.; Gomes, A.; Ling, A.; Duarte, F.; Martin-Montalvo, A.; North, B.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Enkhjargal, B.; Godo, S.; Sawada, A.; Suvd, N.; Saito, H.; Noda, K.; Satoh, K.; Shimokawa, H. Endothelial AMP-Activated Protein Kinase Regulates Blood Pressure and Coronary Flow Responses Through Hyperpolarization Mechanism in Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1505–1513. [Google Scholar] [CrossRef]
- Kong, B.; Man, R.; Gao, Y.; Vanhoutte, P.; Leung, S. Reduced activity of SKCa and Na-K ATPase underlies the accelerated impairment of EDH -type relaxations in mesenteric arteries of aging spontaneously hypertensive rats. Pharmacol. Res. Perspect. 2015, 3, e00150. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Schubert, K.; Blodow, S.; Kreutz, C.; Erdogmus, S.; Wiedenmann, M.; Qiu, J.; Fey, T.; Ruth, P.; Lubomirov, L.; et al. AMPK Dilates Resistance Arteries via Activation of SERCA and BKCa Channels in Smooth Muscle. Hypertension 2015, 66, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, N.; Chen, K.; Li, C.; Croft, K.; Keaney, J. Chronic activation of AMP-activated protein kinase prevents 20-hydroxyeicosatetraenoic acid-induced endothelial dysfunction. Clin. Exp. Pharmacol. Physiol. 2011, 38, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, J.; Srinivasan, S.; Bruno, N.; Parent, A.; Hughes, T.; Pollock, J.; Gjyshi, O.; Cavett, V.; Nowak, J.; Garcia-Ordonez, R.; et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. eLife 2014, 3, e02057. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.; Bilia, A.; Bergonzi, M. Stealth and Cationic Nanoliposomes as Drug Delivery Systems to Increase Andrographolide BBB Permeability. Pharmaceutics 2018, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A. Insight into Cellular Uptake, and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Yang, J.; Bahreman, A.; Daudey, G.; Bussmann, J.; Olsthoorn, R.; Kros, A. Drug Delivery via Cell Membrane Fusion Using Lipopeptide Modified Liposomes. ACS Cent. Sci. 2016, 2, 621–630. [Google Scholar] [CrossRef]
- Farid, M.; Faber, T.; Dietrich, D.; Lamprecht, A. Cell membrane fusing liposomes for cytoplasmic delivery in brain endothelial cells. Colloids Surf. B Biointerfaces 2020, 194, 111193. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaabalawi, A.; Renshall, L.; Beards, F.; Lightfoot, A.P.; Degens, H.; Alexander, Y.; Hasan, R.; Bilal, H.; Graf, B.A.; Harris, L.K.; et al. Internal Mammary Arteries as a Model to Demonstrate Restoration of the Impaired Vasodilation in Hypertension, Using Liposomal Delivery of the CYP1B1 Inhibitor, 2,3′,4,5′-Tetramethoxystilbene. Pharmaceutics 2022, 14, 2046. https://doi.org/10.3390/pharmaceutics14102046
Zaabalawi A, Renshall L, Beards F, Lightfoot AP, Degens H, Alexander Y, Hasan R, Bilal H, Graf BA, Harris LK, et al. Internal Mammary Arteries as a Model to Demonstrate Restoration of the Impaired Vasodilation in Hypertension, Using Liposomal Delivery of the CYP1B1 Inhibitor, 2,3′,4,5′-Tetramethoxystilbene. Pharmaceutics. 2022; 14(10):2046. https://doi.org/10.3390/pharmaceutics14102046
Chicago/Turabian StyleZaabalawi, Azziza, Lewis Renshall, Frances Beards, Adam P. Lightfoot, Hans Degens, Yvonne Alexander, Ragheb Hasan, Haris Bilal, Brigitte A. Graf, Lynda K. Harris, and et al. 2022. "Internal Mammary Arteries as a Model to Demonstrate Restoration of the Impaired Vasodilation in Hypertension, Using Liposomal Delivery of the CYP1B1 Inhibitor, 2,3′,4,5′-Tetramethoxystilbene" Pharmaceutics 14, no. 10: 2046. https://doi.org/10.3390/pharmaceutics14102046
APA StyleZaabalawi, A., Renshall, L., Beards, F., Lightfoot, A. P., Degens, H., Alexander, Y., Hasan, R., Bilal, H., Graf, B. A., Harris, L. K., & Azzawi, M. (2022). Internal Mammary Arteries as a Model to Demonstrate Restoration of the Impaired Vasodilation in Hypertension, Using Liposomal Delivery of the CYP1B1 Inhibitor, 2,3′,4,5′-Tetramethoxystilbene. Pharmaceutics, 14(10), 2046. https://doi.org/10.3390/pharmaceutics14102046






