Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Preparation of the Systems
2.2.2. Identification of the systems of RA with Cyclodextrins
X-ray Powder Diffraction
Differential Scanning Calorimetry
Fourier Transform Infrared Spectroscopy
Studies of Interactions of RA and Cyclodextrins
2.2.3. Chromatographic Studies of Changes of RA Concentrations
2.2.4. The Solubility Studies of RA
2.2.5. The Dissolution Studies of RA
2.2.6. Membrane Permeability of RA
2.2.7. Biological Activity of RA
Antioxidant Activity of RA
Inhibition of Enzymes by RA influencing the Development of Neurodegenerative Diseases
2.2.8. Statistical Analysis
3. Results
3.1. The Preparation of the Systems of RA with Cyclodextrins
3.2. X-ray Powder Diffraction
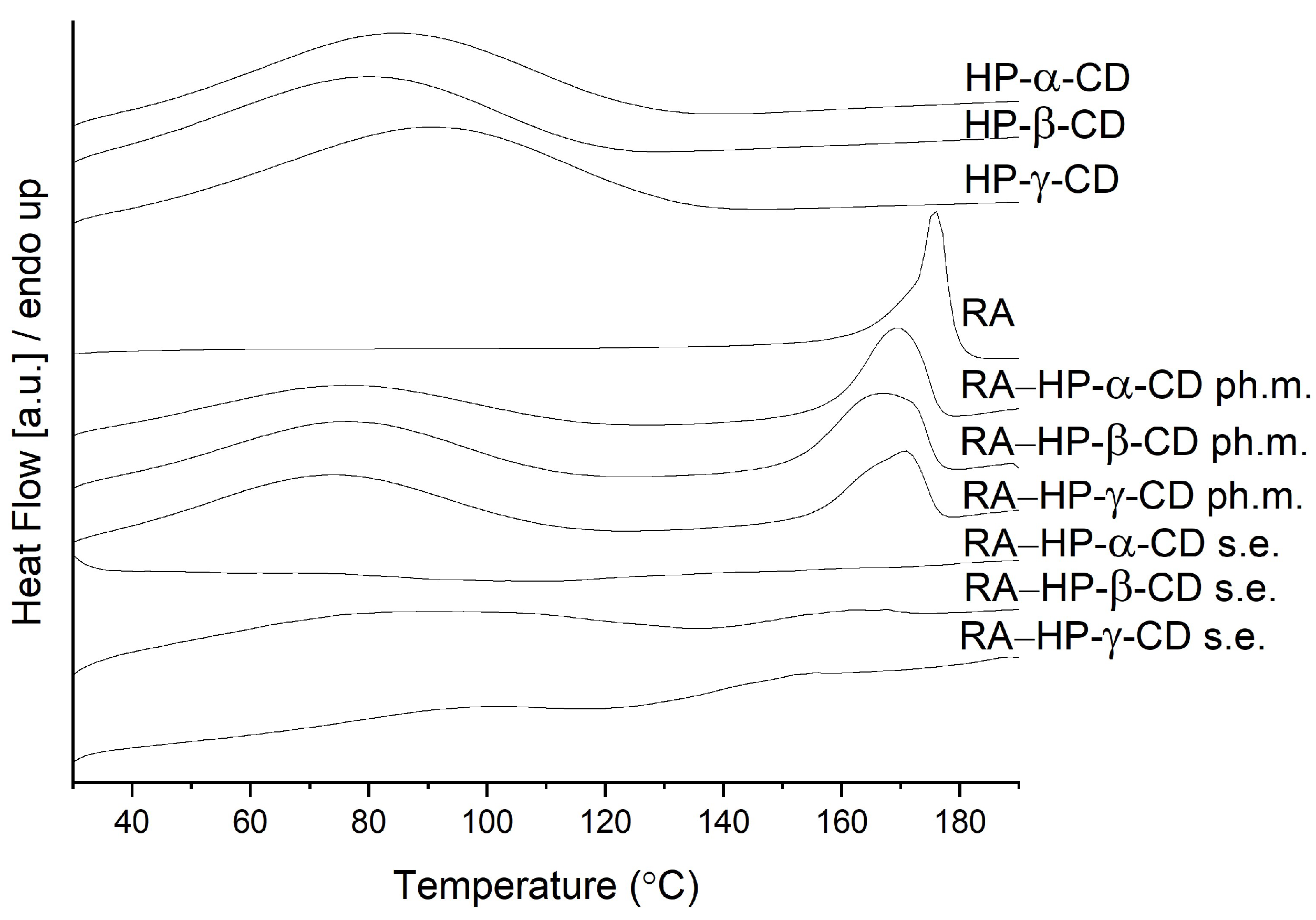
3.3. Differential Scanning Calorimetry
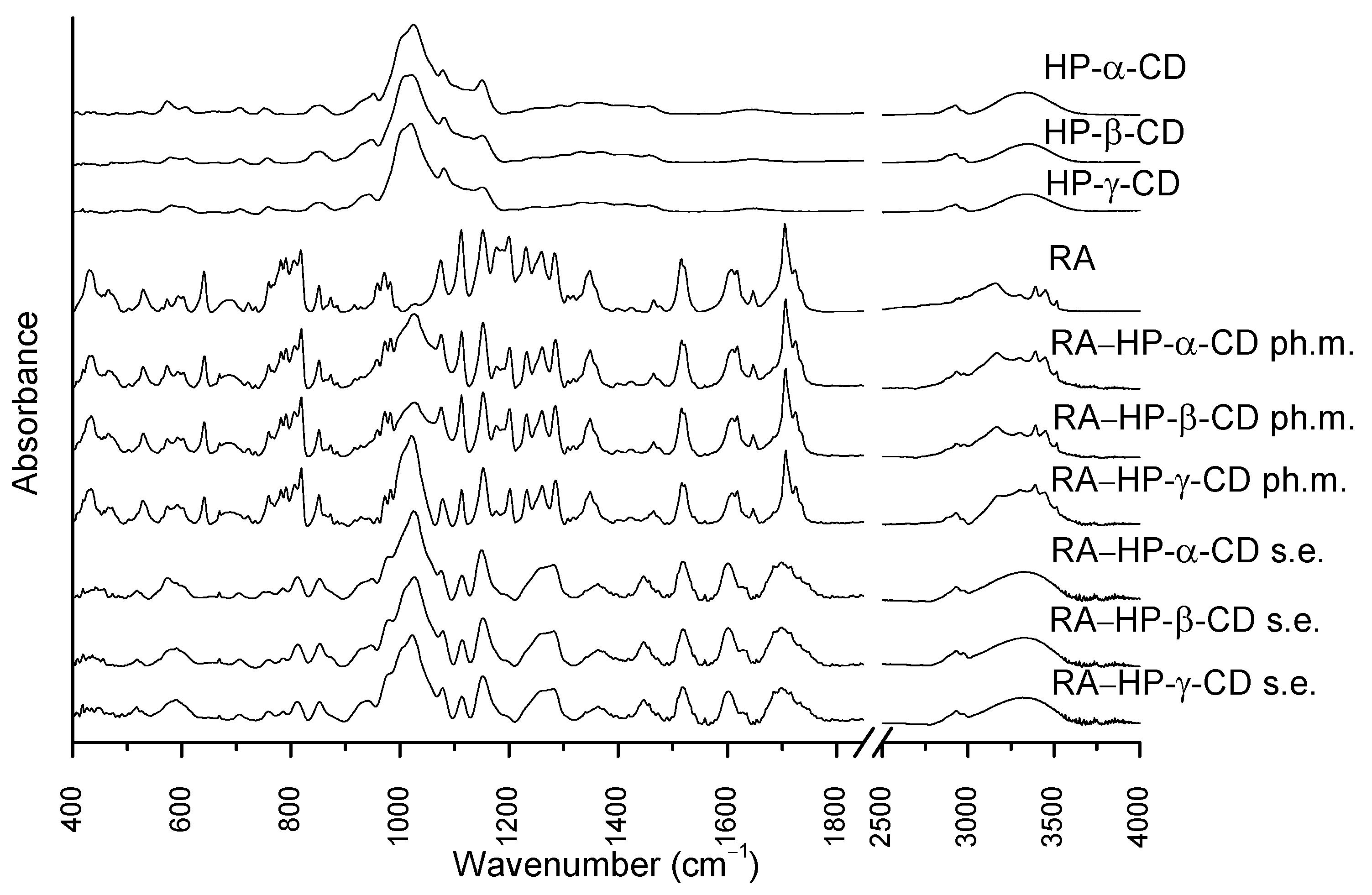
3.4. Fourier Transform Infrared Spectroscopy
3.5. Studies of RA Interactions with Cyclodextrins
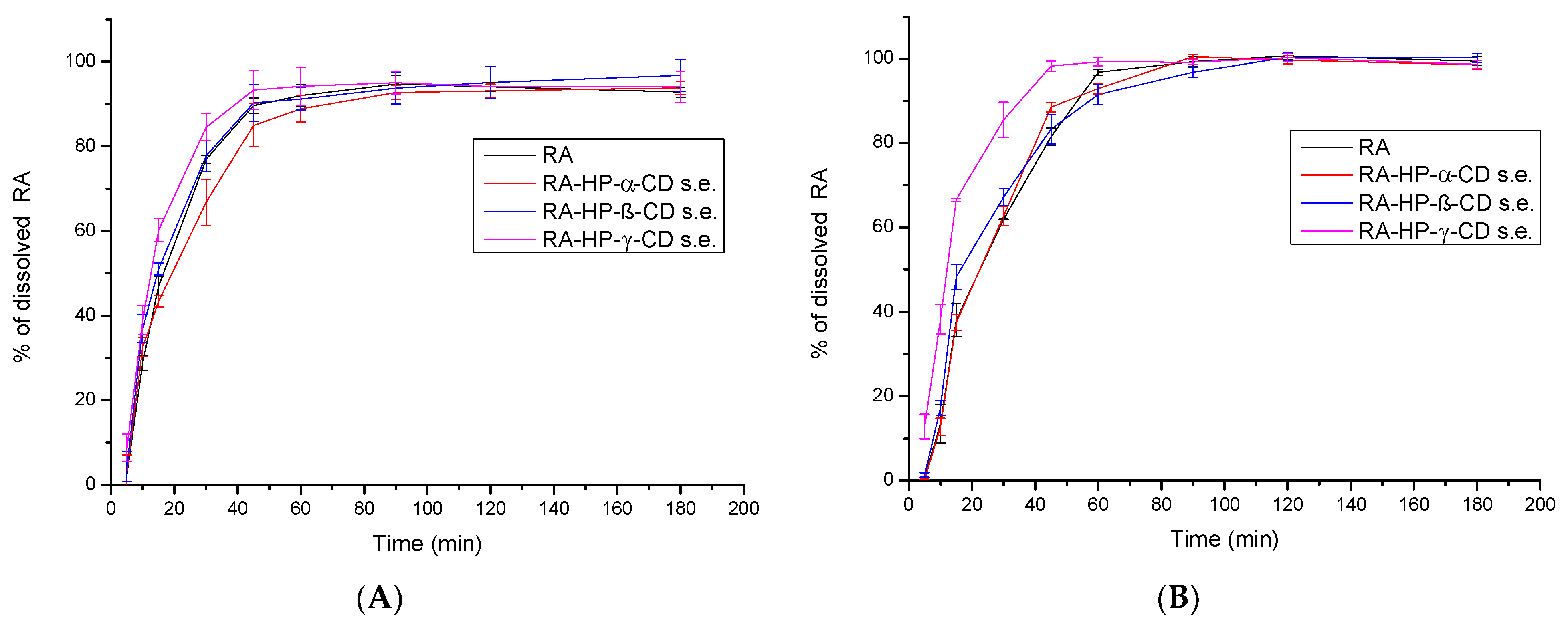
3.6. The Solubility Study of RA
3.7. The Apparent Solubility Study of RA
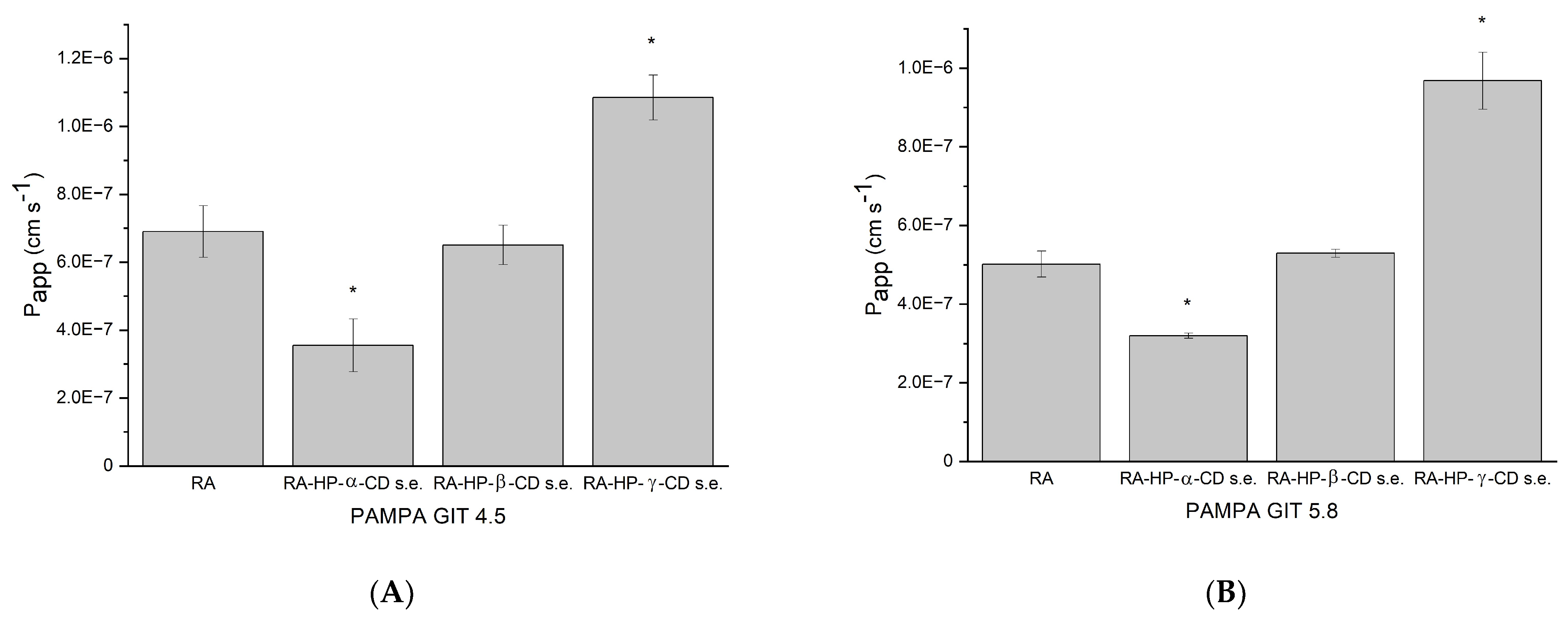
3.8. The Permeability Study of RA
3.9. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | acetylcholinesterase |
| ANOVA | one-way analysis of variance |
| ATCI | acetylthiocholine iodide |
| ATR-FTIR | Attenuated Total Reflectance Fourier Transform Infrared spectroscopy |
| AUC | area under the curve |
| BBB | Blood–brain barrier |
| BChE | butyrylcholinesterase |
| BCS | Biopharmaceutics Classification System |
| BTCI | butyrylthiocholine iodide |
| CD | cyclodextrin |
| CUPRAC | cupric reducing antioxidant capacity |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DSC | differential scanning calorimetry |
| DTNB | 5,5′-dithio-bis-(2-nitrobenzoic) acid |
| FRAP | ferric reducing antioxidant power |
| GIT | gastrointestinal tract |
| HE-β-CD | 2-hydroxyethyl-β-cyclodextrin |
| HPLC | high-performance liquid chromatography |
| HP-β-CD | 2-hydroxypropyl-β-cyclodextrin |
| LLOD | lower limit of detection |
| LLOQ | lower limit of quantification |
| M-β-CD | methyl-β-cyclodextrin |
| PAMPA | parallel artificial membrane permeability assay |
| RA | rosmarinic acid |
| RPM | rotations per minute |
| TNB | 3-carboxy-4-nitrothiolate |
| TPTZ | 2,4,6-tris(2-pyridyl)-1,3,5-triazine |
| XRPD | X-ray powder diffraction |
References
- Runtuwene, J.; Cheng, K.-C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.R.; Inui, A. Rosmarinic Acid Ameliorates Hyperglycemia and Insulin Sensitivity in Diabetic Rats, Potentially by Modulating the Expression of PEPCK and GLUT4. Drug Des. Devel. Ther. 2016, 10, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Hossan, M.S.; Rahman, S.; Bashar, A.B.M.; Jahan, R.; Nahian, A.; Rahmatullah, M. Rosmarinic Acid: A Review of Its Anticancer Action. WORLD J. Pharm. Pharm. Sci. 2014, 3, 57–70. [Google Scholar]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and Anti-Inflammatory Effects of Rosmarinic Acid in an Experimental Murine Model of Japanese Encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3370. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. An Overview of the Neuroprotective Potential of Rosmarinic Acid and Its Association with Nanotechnology-Based Delivery Systems: A Novel Approach to Treating Neurodegenerative Disorders. Neurochem. Int. 2019, 122, 47–58. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. Inhibitory Effects of Rosmarinic Acid Extracts on Porcine Pancreatic Amylase in Vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar] [PubMed]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of Rosmarinic Acid on Atopic Dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic Acid-Induced Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Woottisin, N.; Sukprasert, S.; Kulsirirat, T.; Tharavanij, T.; Sathirakul, K. Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia Laurifolia Leaf Water Extract in a Caco-2 Cell Model. Molecules 2022, 27, 3884. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Rui, T.; Kang, A.; Li, G.; Fu, T.; Li, J.; Di, L.; Cai, B. Pharmacokinetics of Rosmarinic Acid in Rats by LC-MS/MS: Absolute Bioavailability and Dose Proportionality. RSC Adv. 2017, 7, 9057–9063. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Iwasa, K.; Nagai, T.; Kobayashi, S.; Nakamura, H.; Yamada, M. Pharmacokinetics, Safety and Tolerability of Melissa Officinalis Extract Which Contained Rosmarinic Acid in Healthy Individuals: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0126422. [Google Scholar] [CrossRef]
- Dhiman, P.; Bhatia, M. Pharmaceutical Applications of Cyclodextrins and Their Derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 171–186. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.-S.; Lee, S.; Oh, H.B. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Shen, J.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Aliphatic Polyester Nanofibers Functionalized with Cyclodextrins and Cyclodextrin-Guest Inclusion Complexes. Polymers 2018, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Özyürek, M.; Tufan, A.N.; Güçlü, K.; Apak, R. Spectroscopic Study and Antioxidant Properties of the Inclusion Complexes of Rosmarinic Acid with Natural and Derivative Cyclodextrins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Fateminasab, F.; Bordbar, A.K.; Asadi, B.; Shityakov, S.; Zare Karizak, A.; Mohammadpoor-Baltork, I.; Saboury, A.A. Modified β-Cyclodextrins: Rosmarinic Acid Inclusion Complexes as Functional Food Ingredients Show Improved Operations (Solubility, Stability and Antioxidant Activity). Food Hydrocoll. 2022, 131, 107731. [Google Scholar] [CrossRef]
- Veras, K.S.; Silveira Fachel, F.N.; Delagustin, M.G.; Teixeira, H.F.; Barcellos, T.; Henriques, A.T.; Bassani, V.L.; Koester, L.S. Complexation of Rosmarinic Acid with Hydroxypropyl-β-Cyclodextrin and Methyl-β-Cyclodextrin: Formation of 2:1 Complexes with Improved Antioxidant Activity. J. Mol. Struct. 2019, 1195, 582–590. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-Cyclodextrin as an Effective Carrier of Curcumin–Piperine Nutraceutical System with Improved Enzyme Inhibition Properties. J. Enzyme Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wahlgren, M.; Axenstrand, M.; Håkansson, Å.; Marefati, A.; Lomstein Pedersen, B. In Vitro Methods to Study Colon Release: State of the Art and An Outlook on New Strategies for Better In-Vitro Biorelevant Release Media. Pharmaceutics 2019, 11, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hens, B.; Tsume, Y.; Bermejo Sanz, M.; Paixao, P.; Koenigsknecht, M.; Baker, J.; Hasler, W.; Lionberger, R.; Fan, J.; Dickens, J.; et al. Low Buffer Capacity and Alternating Motility Along The Human Gastrointestinal Tract: Implications for In Vivo Dissolution and Absorption of Ionizable Drugs. Mol. Pharm. 2017, 14, 4281–4294. [Google Scholar] [CrossRef] [PubMed]
- Zorić, Z.; Markić, J.; Pedisić, S.; Bučević-Popović, V.; Generalić-Mekinić, I.; Grebenar, K.; Kulišić-Bilušić, T. Stability of Rosmarinic Acid in Aqueous Extracts from Different Lamiaceae Species after in Vitro Digestion with Human Gastrointestinal Enzymes. Food Technol. Biotechnol. 2016, 54, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, F.; Kuiper, J.; Fotaki, N. Fed-State Gastric Media and Drug Analysis Techniques: Current Status and Points to Consider. Eur. J. Pharm. Biopharm. 2016, 107, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Prior, A.; Frutos, P.; Correa, C. Comparison of Dissolution Profiles: Current Guidelines. In Proceedings of the VI Congreso SEFIG, Granada, Spain, 9–11 February 2003. [Google Scholar]
- Fischer, H.; Kansy, M.; Avdeef, A.; Senner, F. Permeation of Permanently Positive Charged Molecules through Artificial Membranes--Influence of Physico-Chemical Properties. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2007, 31, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High Throughput Artificial Membrane Permeability Assay for Blood-Brain Barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total Antioxidant Capacity Assay of Human Serum Using Copper(II)-Neocuproine as Chromogenic Oxidant: The CUPRAC Method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of Pentacyclic Triterpenoids-Rich Callus Extract of Chaenomeles Japonica (Thunb) Lindl. Ex Spach on Viability, Morphology, and Proliferation of Normal Human Skin Fibroblasts. Mol. Basel Switz. 2018, 23, 3009. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lim, T.Y.; Lim, Y.Y.; Yule, C.M. Evaluation of Antioxidant, Antibacterial and Anti-Tyrosinase Activities of Four Macaranga Species. Food Chem. 2009, 114, 594–599. [Google Scholar] [CrossRef]
- Veras, K.S.; Fachel, F.N.S.; Pittol, V.; Garcia, K.R.; Bassani, V.L.; dos Santos, V.; Henriques, A.T.; Teixeira, H.F.; Koester, L.S. Compatibility Study of Rosmarinic Acid with Excipients Used in Pharmaceutical Solid Dosage Forms Using Thermal and Non-Thermal Techniques. Saudi Pharm. J. 2019, 27, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Zhu, X.; Sun, Y.; Su, L.; Yu, H.; Liu, D.; Li, Y.; Du, Y.; Liu, R.; et al. Tanshinone IIA-Loaded Micelles Functionalized with Rosmarinic Acid: A Novel Synergistic Anti-Inflammatory Strategy for Treatment of Atherosclerosis. J. Pharm. Sci. 2022, 11, 2827–2838. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah, E. Rosmarinic Acid-Loaded Electrospun Nanofibers: In Vitro Release Kinetic Study and Bioactivity Assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Madureira, A.; Sarmento, B.; Gomes, A.; Pintado, M.; Fonte, P. Evaluation of the Interactions between Rosmarinic Acid and Bovine Milk Casein. RSC Adv. 2015, 5, 88529–88538. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Lula, I.S.; Sinisterra, R.D.; Bassani, V.L. Quercetin/β-Cyclodextrin Solid Complexes Prepared in Aqueous Solution Followed by Spray-Drying or by Physical Mixture. AAPS PharmSciTech 2009, 10, 235–242. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water Soluble Complexes of Curcumin with Cyclodextrins: Characterization by FT-Raman Spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Yan, H.-H.; Zhang, J.-Q.; Ren, S.-H.; Xie, X.-G.; Huang, R.; Jin, Y.; Lin, J. Experimental and Computational Studies of Naringin/Cyclodextrin Inclusion Complexation. J. Incl. Phenom. Macrocycl. Chem. 2017, 88, 15–26. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Rosmarinic Acid (CAS 20283-92-5). Available online: https://www.caymanchem.com/product/70900 (accessed on 3 August 2022).
- Wüst Zibetti, A.; Aydi, A.; Claumann, C.A.; Eladeb, A.; Adberraba, M. Correlation of Solubility and Prediction of the Mixing Properties of Rosmarinic Acid in Different Pure Solvents and in Binary Solvent Mixtures of Ethanol+water and Methanol+water from (293.2 to 318.2) K. J. Mol. Liq. 2016, 216, 370–376. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-Dissolving Antioxidant Curcumin/Cyclodextrin Inclusion Complex Electrospun Nanofibrous Webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Sá Couto, A.; Ryzhakov, A.; Larsen, K.L.; Loftsson, T. Interaction of Native Cyclodextrins and Their Hydroxypropylated Derivatives with Carbamazepine in Aqueous Solution. Evaluation of Inclusion Complexes and Aggregates Formation. ACS Omega 2019, 4, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, S.; Berdejo, D.; Pagán, E.; García-Gonzalo, D.; Pagán, R. Modified Cyclodextrin Type and Dehydration Methods Exert a Significant Effect on the Antimicrobial Activity of Encapsulated Carvacrol and Thymol. J. Sci. Food Agric. 2021, 101, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, X.; Yang, G.; Feng, W.; Zong, L.; Zhao, L.; Ye, F.; Fu, Y. Antibacterial Perillaldehyde/Hydroxypropyl-γ-Cyclodextrin Inclusion Complex Electrospun Polymer-Free Nanofiber: Improved Water Solubility, Thermostability, and Antioxidant Activity. Ind. Crops Prod. 2022, 176, 114300. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Meenatchi, V.; Lin, L. Encapsulation of Essential Oil Components with Methyl-β-Cyclodextrin Using Ultrasonication: Solubility, Characterization, DPPH and Antibacterial Assay. Ultrason. Sonochem. 2020, 64, 104997. [Google Scholar] [CrossRef]
- Gao, S.; Feng, W.; Sun, H.; Zong, L.; Li, X.; Zhao, L.; Ye, F.; Fu, Y. Fabrication and Characterization of Antifungal Hydroxypropyl-β-Cyclodextrin/Pyrimethanil Inclusion Compound Nanofibers Based on Electrospinning. J. Agric. Food Chem. 2022, 70, 7911–7920. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Electrospun Polymer-Free Nanofibers Incorporating Hydroxypropyl-β-Cyclodextrin/Difenoconazole via Supramolecular Assembly for Antifungal Activity. J. Agric. Food Chem. 2021, 69, 5871–5881. [Google Scholar] [CrossRef]
- Li, S.; Yuan, L.; Zhang, B.; Zhou, W.; Wang, X.; Bai, D. Photostability and Antioxidant Activity Studies on the Inclusion Complexes of Trans-Polydatin with β-Cyclodextrin and Derivatives. RSC Adv. 2018, 8, 25941–25948. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An Investigation into the Supramolecular Structure, Solubility, Stability and Antioxidant Activity of Rutin/Cyclodextrin Inclusion Complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic Acid—From Bench to Valuable Applications in Food Industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan–Rosmarinic Acid Conjugates with Antioxidant, Anti-Inflammatory and Photoprotective Properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef] [PubMed]
- Abdul Aziz, A.H.; Mohd Idrus, N.F.; Putra, N.R.; Awang, M.A.; Idham, Z.; Mamat, H.; Che Yunus, M.A. Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon Stamineus Leaves. ChemEngineering 2022, 6, 59. [Google Scholar] [CrossRef]
- Sethi, B.; Sahdev, A.K.; Purwar, S. Development, Optimization Characterization and In Vitro Study of Rosmarinic Acid Phytovesicles. Res. J. Pharm. Technol. 2019, 12, 5231–5239. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Adomavičiūtė, E.; Jankauskaite, V.; Marksa, M.; Barsteigienė, Z.; Bernatoniene, J. Formation and Investigation of Electrospun Eudragit E100/Oregano Mats. Molecules 2019, 24, 628. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial Transport of Rosmarinic Acid in Intestinal Caco-2 Cell Monolayers. Biosci. Biotechnol. Biochem. 2005, 69, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Bel-Rhlid, R.; Crespy, V.; Pagé-Zoerkler, N.; Nagy, K.; Raab, T.; Hansen, C.-E. Hydrolysis of Rosmarinic Acid from Rosemary Extract with Esterases and Lactobacillus Johnsonii in Vitro and in a Gastrointestinal Model. J. Agric. Food Chem. 2009, 57, 7700–7705. [Google Scholar] [CrossRef] [PubMed]
- Özevren, H.; Deveci, E.; Tuncer, M.C. The Effect of Rosmarinic Acid on Deformities Occurring in Brain Tissue by Craniectomy Method. Histopathological Evaluation of IBA-1 and GFAP Expressions 1. Acta Cirúrgica Bras. 2020, 35, e202000406. [Google Scholar] [CrossRef]
- Khamse, S.; Sadr, S.S.; Roghani, M.; Hasanzadeh, G.; Mohammadian, M. Rosmarinic Acid Exerts a Neuroprotective Effect in the Kainate Rat Model of Temporal Lobe Epilepsy: Underlying Mechanisms. Pharm. Biol. 2015, 53, 1818–1825. [Google Scholar] [CrossRef]
- Taram, F.; Ignowski, E.; Duval, N.; Linseman, D.A. Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons. Molecules 2018, 23, 2956. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effects of Rosmarinic Acid on Nervous System Disorders: An Updated Review. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1779–1795. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Scozzafava, A.; Supuran, C.T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S.H. Rosmarinic Acid Inhibits Some Metabolic Enzymes Including Glutathione S-Transferase, Lactoperoxidase, Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase Isoenzymes. J. Enzyme Inhib. Med. Chem. 2016, 31, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Design of Polymer-Free Vitamin-A Acetate/Cyclodextrin Nanofibrous Webs: Antioxidant and Fast-Dissolving Properties. Food Funct. 2020, 11, 7626–7637. [Google Scholar] [CrossRef]
- Inoue, Y.; Yoshida, M.; Ezawa, T.; Tanikawa, T.; Arce, F.; See, G.L.; Tomita, J.; Suzuki, M.; Oguchi, T. Inclusion Complexes of Daidzein with Cyclodextrin-Based Metal-Organic Framework-1 Enhance Its Solubility and Antioxidant Capacity. AAPS PharmSciTech 2021, 23, 2. [Google Scholar] [CrossRef]
- Shiozawa, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of Antioxidant Activity of Caffeic Acid with Cyclodextrins Using Ground Mixture Method. Asian J. Pharm. Sci. 2018, 13, 24–33. [Google Scholar] [CrossRef]
- Cavdar, H.; Senturk, M.; Guney, M.; Durdagi, S.; Kayik, G.; Supuran, C.T.; Ekinci, D. Inhibition of Acetylcholinesterase and Butyrylcholinesterase with Uracil Derivatives: Kinetic and Computational Studies. J. Enzyme Inhib. Med. Chem. 2019, 34, 429–437. [Google Scholar] [CrossRef] [Green Version]




| Guest Molecule: | Binding Energy [kJ/mol] | |
|---|---|---|
| Substitution Pattern * | Substitution Pattern # | |
| 2-HP-α-CD | −5.0–−4.8 | −5.4–−5.2 |
| 2-HP-β-CD2-HP-γ-CD | −6.2–−5.9−6.5–−6.1 | −7.2–−6.9−7.0–−6.9 |
| DPPH | ABTS | CUPRAC | FRAP | |
|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | IC0.5 (µg/mL) | IC0.5 (µg/mL) | |
| RA | 59.483 ± 0.041 | 102.578 ± 3.427 | 13.677 ± 0.993 | 10.558 ± 0.203 |
| RA–HP-α-CD s.e. | 59.796 ± 0.042 | 106.940 ± 3.479 | 14.140 ± 0.106 | 10.435 ± 0.216 |
| RA–HP-β-CD s.e. | 58.490 ± 0.884 | 98.028 ± 4.223 | 14.331 ± 0.136 | 10.316 ± 0.152 |
| RA–HP-γ-CD s.e. | 57.398 ± 0.762 * | 87.766 ± 1.802 * | 13.823 ± 0.027 | 10.126 ± 0.132 * |
| Trolox | 93.640 ± 1.072 | 120.188 ± 2.726 | 56.564 ± 0.664 | 41.941 ± 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiłowicz-Krzemień, A.; Rosiak, N.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Tykarska, E.; Cielecka-Piontek, J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics 2022, 14, 2098. https://doi.org/10.3390/pharmaceutics14102098
Stasiłowicz-Krzemień A, Rosiak N, Płazińska A, Płaziński W, Miklaszewski A, Tykarska E, Cielecka-Piontek J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics. 2022; 14(10):2098. https://doi.org/10.3390/pharmaceutics14102098
Chicago/Turabian StyleStasiłowicz-Krzemień, Anna, Natalia Rosiak, Anita Płazińska, Wojciech Płaziński, Andrzej Miklaszewski, Ewa Tykarska, and Judyta Cielecka-Piontek. 2022. "Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid" Pharmaceutics 14, no. 10: 2098. https://doi.org/10.3390/pharmaceutics14102098
APA StyleStasiłowicz-Krzemień, A., Rosiak, N., Płazińska, A., Płaziński, W., Miklaszewski, A., Tykarska, E., & Cielecka-Piontek, J. (2022). Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics, 14(10), 2098. https://doi.org/10.3390/pharmaceutics14102098








