Influence of the Hydrophobicity of Pluronic Micelles Encapsulating Curcumin on the Membrane Permeability and Enhancement of Photoinduced Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Micelles Preparation
2.2.2. Characterization of Micelles
2.2.3. Optical Properties of Curcumin-Loaded Polymeric Micelles
2.2.4. Drug Solubility and Entrapment Efficiency
2.2.5. In Vitro Drug Release
2.2.6. Microbial Membrane Permeability Assessment
2.2.7. Antimicrobial Activity and Photoinactivation
2.2.8. Statistical Analysis
3. Results
3.1. Critical Micelle Concentration
3.2. Polymeric Micelles Characterization
3.3. Drug Loading and Entrapment Efficiency
3.4. In Vitro CURC Release
3.5. Photophysical Properties of Curcumin Encapsulated in Pluronic Micelles
3.5.1. Absorption and Fluorescence Spectra
3.5.2. Stability of Encapsulated CURC under Visible Irradiation
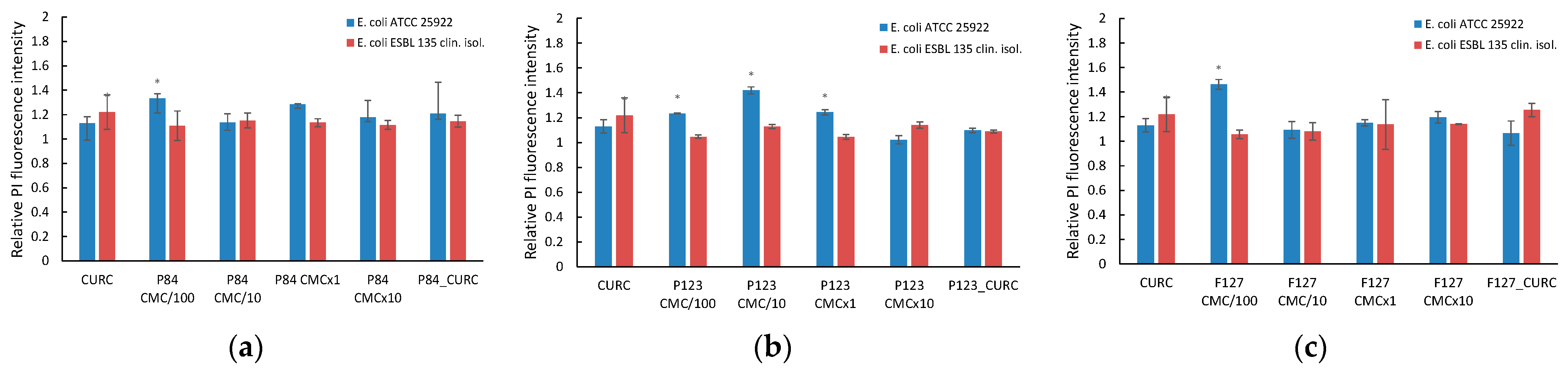
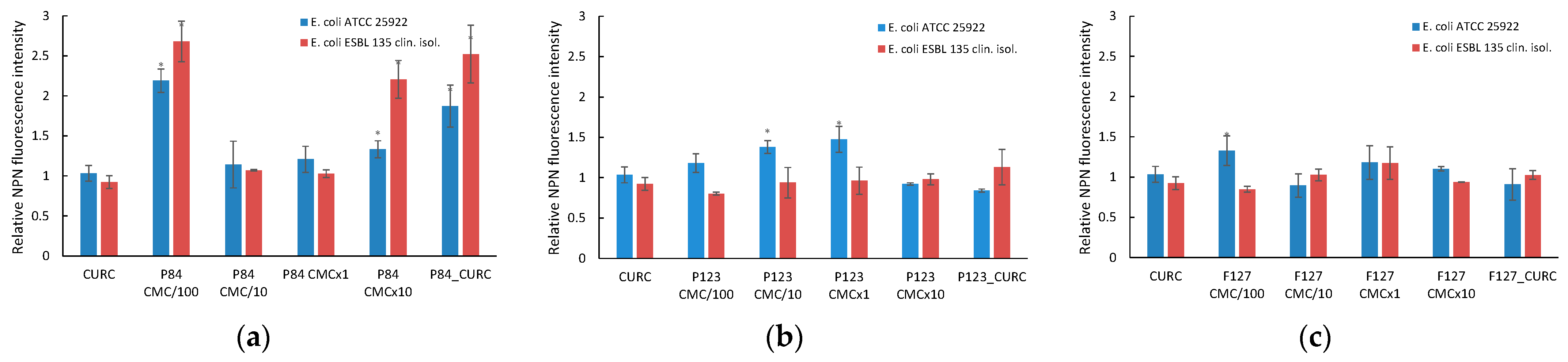
3.6. Influence of Curcumin-Loaded Pluronic Micelles on Microbial Membrane Permeability
3.6.1. Microbial Membrane Permeability
3.6.2. Outer Membrane Permeabilization
3.7. Photoinduced Antimicrobial Activity of Curcumin-Loaded Pluronic Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Huang, S.-Y.; Zhou, D.-D.; Xiong, R.-G.; Zhao, C.-N.; Fang, A.-P.; Zhang, Y.-J.; Li, H.-B.; Zhu, H.-L. Effects and Mechanisms of Curcumin for the Prevention and Management of Cancers: An Updated Review. Antioxidants 2022, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Shome, S.; Das Talukdar, A.; Upadhyaya, H. Antibacterial activity of curcumin and its essential nanoformulations against some clinically important bacterial pathogens: A comprehensive review. Biotechnol. Appl. Biochem. 2021, 1–30. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Huang, Z.; Chen, X.; Yan, X.; Zhai, J.; Ma, Y. Evaluation of curcumin-mediated photodynamic therapy on the reverse of multidrug resistance in tumor cells. RSC Adv. 2020, 10, 298–306. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and release performance of curcumin-loaded liposomes with varying content of hydrogenated phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Na, Q.; Xiyou, D.; Ji, J.; Zhai, G. A review of stimuli-responsive polymeric micelles for tumor-targeted delivery of curcumin. Drug Dev. Ind. Pharm. 2021, 47, 839–856. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef]
- Scomoroscenco, C.; Teodorescu, M.; Raducan, A.; Stan, M.; Voicu, S.; Trica, B.; Ninciuleanu, C.; Nistor, C.; Mihaescu, C.; Petcu, C.; et al. Novel Gel Microemulsion as Topical Drug Delivery System for Curcumin in Dermatocosmetics. Pharmaceutics 2021, 13, 505. [Google Scholar] [CrossRef]
- Mirzahosseinipour, M.; Khorsandi, K.; Hosseinzadeh, R.; Ghazaeian, M.; Shahidi, F.K. Antimicrobial photodynamic and wound healing activity of curcumin encapsulated in silica nanoparticles. Photodiagnosis Photodyn. Ther. 2019, 29, 101639. [Google Scholar] [CrossRef]
- Tiwari, S.; Kansara, V.; Bahadur, P. Targeting anticancer drugs with pluronic aggregates: Recent updates. Int. J. Pharm. 2020, 586, 119544. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric Drug Delivery System Based on Pluronics for Cancer Treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Kwon, G.S. Polymeric Micelles for Delivery of Poorly Water-Soluble Compounds. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 357–403. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. The effects of Pluronic block copolymers on the aggregation state of nystatin. J. Control. Release 2004, 95, 161–171. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sharma, R.; Shaikh, S.; Ray, D.; Aswal, V.K.; Pathak, C. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2018, 2, e1133. [Google Scholar] [CrossRef]
- dos Santos, D.D.L.; Besegato, J.F.; de Melo, P.B.G.; Junior, J.A.O.; Chorilli, M.; Deng, D.; Bagnato, V.S.; de Souza Rastelli, A.N. Curcumin-loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Dias, V.H.C.; Malacrida, A.M.; dos Santos, A.R.; Batista, A.F.P.; Campanerut-Sá, P.A.Z.; Braga, G.; Bona, E.; Caetano, W.; Mikcha, J.M.G. pH interferes in photoinhibitory activity of curcumin nanoencapsulated with pluronic® P123 against Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2021, 33, 102085. [Google Scholar] [CrossRef]
- Thakur, V.; Uniyal, A.; Tiwari, V. A comprehensive review on pharmacology of efflux pumps and their inhibitors in antibiotic resistance. Eur. J. Pharmacol. 2021, 903, 174151. [Google Scholar] [CrossRef]
- Purro, M.; Qiao, J.; Liu, Z.; Ashcraft, M.; Xiong, M.P. Desferrioxamine:gallium-pluronic micelles increase outer membrane permeability and potentiate antibiotic activity againstPseudomonas aeruginosa. Chem. Commun. 2018, 54, 13929–13932. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2007, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scirè, A.; Armeni, T. Side Effects of Curcumin: Epigenetic and Antiproliferative Implications for Normal Dermal Fibroblast and Breast Cancer Cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Pellosi, D.; Tessaro, A.L.; Moret, F.; Gaio, E.; Reddi, E.; Caetano, W.; Quaglia, F.; Hioka, N. Pluronic® mixed micelles as efficient nanocarriers for benzoporphyrin derivatives applied to photodynamic therapy in cancer cells. J. Photochem. Photobiol. A Chem. 2016, 314, 143–154. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, Y.T.; Youn, Y.S.; Nam, M.; Park, B.; Yun, J.; Kim, J.H.; Song, H.-T.; Oh, K.T. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf. B Biointerfaces 2011, 82, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mashreghi, M.; Saremi, S.S.; Jaafari, M.R. Spectrofluorometric Method Development and Validation for the Determination of Curcumin in Nanoliposomes and Plasma. J. Fluoresc. 2020, 30, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Fang, C.; Zhang, J.; Wang, M.; Luo, X.; Hou, Z. Contemporaneous Measurement of Outer and Inner Membrane Permeability in Gram-negative Bacteria. Bio-Protoc. 2020, 10, e3548. [Google Scholar] [CrossRef]
- Bucuresteanu, R.; Ditu, L.-M.; Ionita, M.; Calinescu, I.; Raditoiu, V.; Cojocaru, B.; Cinteza, L.O.; Curutiu, C.; Holban, A.M.; Enachescu, M.; et al. Preliminary Study on Light-Activated Antimicrobial Agents as Photocatalytic Method for Protection of Surfaces with Increased Risk of Infections. Materials 2021, 14, 5307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Y.; Zhang, W.-M. Recent progress in drug delivery of pluronic P123: Pharmaceutical perspectives. J. Drug Target. 2017, 25, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Tănase, M.; Raducan, A.; Oancea, P.; Diţu, L.; Stan, M.; Petcu, C.; Scomoroşcenco, C.; Ninciuleanu, C.; Nistor, C.; Cinteza, L. Mixed Pluronic—Cremophor Polymeric Micelles as Nanocarriers for Poorly Soluble Antibiotics—The Influence on the Antibacterial Activity. Pharmaceutics 2021, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Sobczyński, J.; Kristensen, S.; Berg, K. The influence of Pluronics nanovehicles on dark cytotoxicity, photocytotoxicity and localization of four model photosensitizers in cancer cells. Photochem. Photobiol. Sci. 2013, 13, 8–22. [Google Scholar] [CrossRef]
- Moghimi, S. Mechanisms regulating body distribution of nanospheres conditioned with pluronic and tetronic block co-polymers. Adv. Drug Deliv. Rev. 1995, 16, 183–193. [Google Scholar] [CrossRef]
- Rezvan, G.; Pircheraghi, G.; Bagheri, R. Curcumin incorporated PVA-borax dual delivery hydrogels as potential wound dressing materials-Correlation between viscoelastic properties and curcumin release rate. J. Appl. Polym. Sci. 2018, 135, 46734. [Google Scholar] [CrossRef]
- Nagy, N.Z.; Varga, Z.; Mihály, J.; Domján, A.; Fenyvesi, É; Kiss, É. Highly Enhanced Curcumin Delivery Applying Association Type Nanostructures of Block Copolymers, Cyclodextrins and Polycyclodextrins. Polymers 2020, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Pham, D.-T.; Leung, M.H.M.; Ngo, H.T.; Lincoln, S.F.; Easton, C.J.; Kee, T.W. Cooperative Binding and Stabilization of the Medicinal Pigment Curcumin by Diamide Linked γ-Cyclodextrin Dimers: A Spectroscopic Characterization. J. Phys. Chem. B 2010, 115, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Bagchi, D.; Pal, U.; Kumari, M.; Sharma, M.; Bera, A.; Shabir, J.; Pal, S.K.; Saha-Dasgupta, T.; Mozumdar, S. The Role of Imidazolium-Based Surface-Active Ionic Liquid to Restrain the Excited-State Intramolecular H-Atom Transfer Dynamics of Medicinal Pigment Curcumin: A Theoretical and Experimental Approach. ACS Omega 2020, 5, 25582–25592. [Google Scholar] [CrossRef]
- Alakhov, V.Y.; Moskaleva, E.Y.; Batrakova, A.E.V.; Kabanov, A.V. Hypersensitization of Multidrug Resistant Human Ovarian Carcinoma Cells by Pluronic P85 Block Copolymer. Bioconjugate Chem. 1996, 7, 209–216. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef]
- Hong, W.; Shi, H.; Qiao, M.; Zhang, Z.; Yang, W.; Dong, L.; Xie, F.; Zhao, C.; Kang, L. pH-sensitive micelles for the intracellular co-delivery of curcumin and Pluronic L61 unimers for synergistic reversal effect of multidrug resistance. Sci. Rep. 2017, 7, 42465. [Google Scholar] [CrossRef]
- Neto, J.B.D.A.; Cabral, V.P.D.F.; Nogueira, L.F.B.; da Silva, C.R.; Sá, L.G.D.A.V.; da Silva, A.R.; da Silva, W.M.B.; Silva, J.; Marinho, E.S.; Cavalcanti, B.C.; et al. Anti-MRSA activity of curcumin in planktonic cells and biofilms and determination of possible action mechanisms. Microb. Pathog. 2021, 155, 104892. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic Action of LED-Activated Curcumin against Staphylococcus aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Bondar, O.; Sagitova, A.; Badeev, Y.; Shtyrlin, Y.; Abdullin, T. Conjugation of succinic acid to non-ionogenic amphiphilic polymers modulates their interaction with cell plasma membrane and reduces cytotoxic activity. Colloids Surf. B Biointerfaces 2013, 109, 204–211. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.; Vega-Chacón, Y.; Soares, A.; Mima, E. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Holden, B.S.; Taylor, M.F.; Wilson, J.; Coburn, J.; Hilton, B.; Nance, T.; Gubler, S.; Genberg, C.; Deng, S.; et al. Antibacterial and Antifungal Activities of Poloxamer Micelles Containing Ceragenin CSA-131 on Ciliated Tissues. Molecules 2018, 23, 596. [Google Scholar] [CrossRef] [PubMed]
- Ryu, V.; Ruiz-Ramirez, S.; Chuesiang, P.; McLandsborough, L.; McClements, D.; Corradini, M. Use of Micellar Delivery Systems to Enhance Curcumin’s Stability and Microbial Photoinactivation Capacity. Foods 2021, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.D.L.; Besegato, J.F.; de Melo, P.B.G.; Junior, J.A.O.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.D.S. Effect of curcumin-encapsulated Pluronic® F-127 over duo-species biofilm of Streptococcus mutans and Candida albicans. Lasers Med. Sci. 2021, 37, 1775–1786. [Google Scholar] [CrossRef] [PubMed]

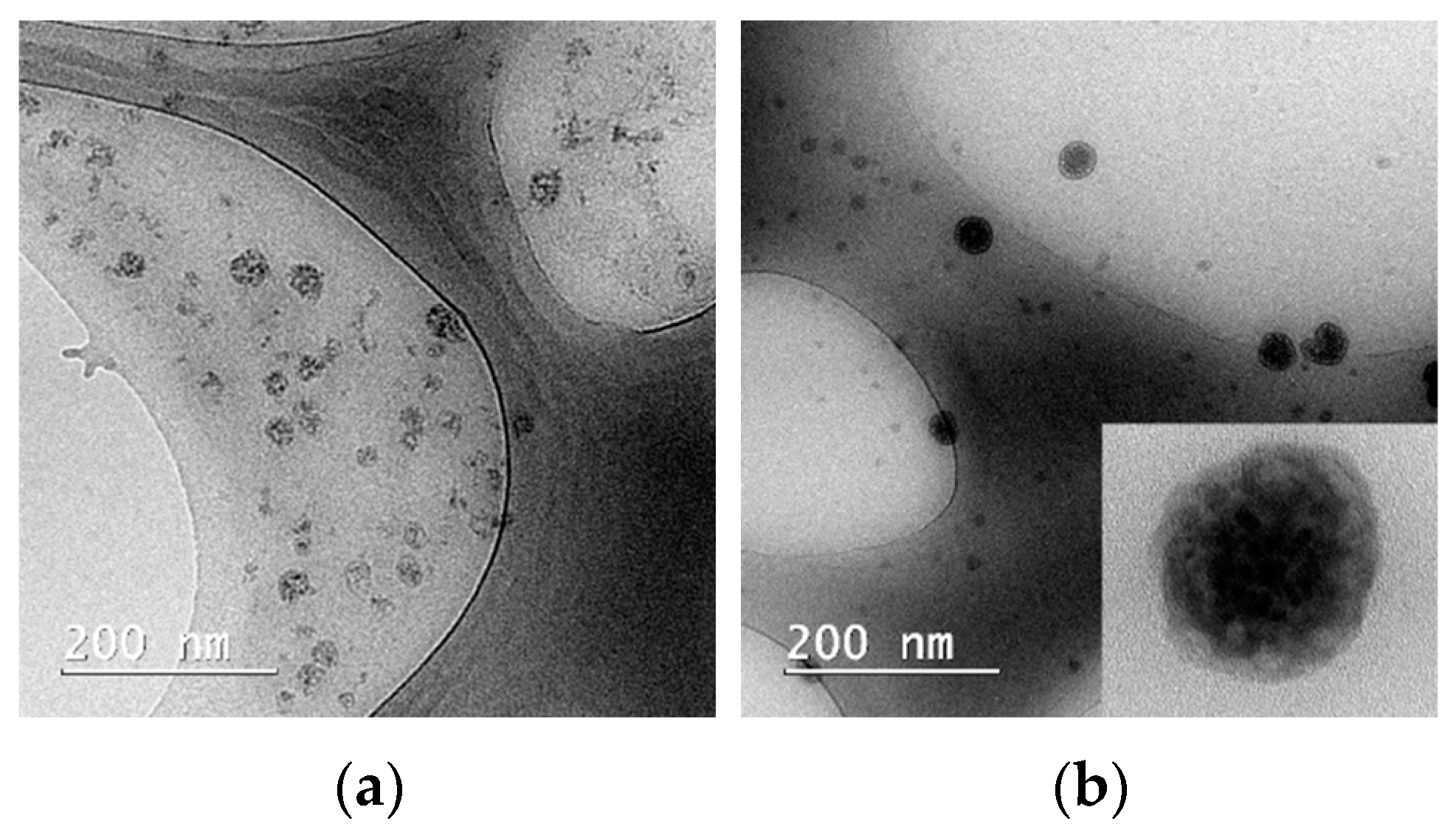

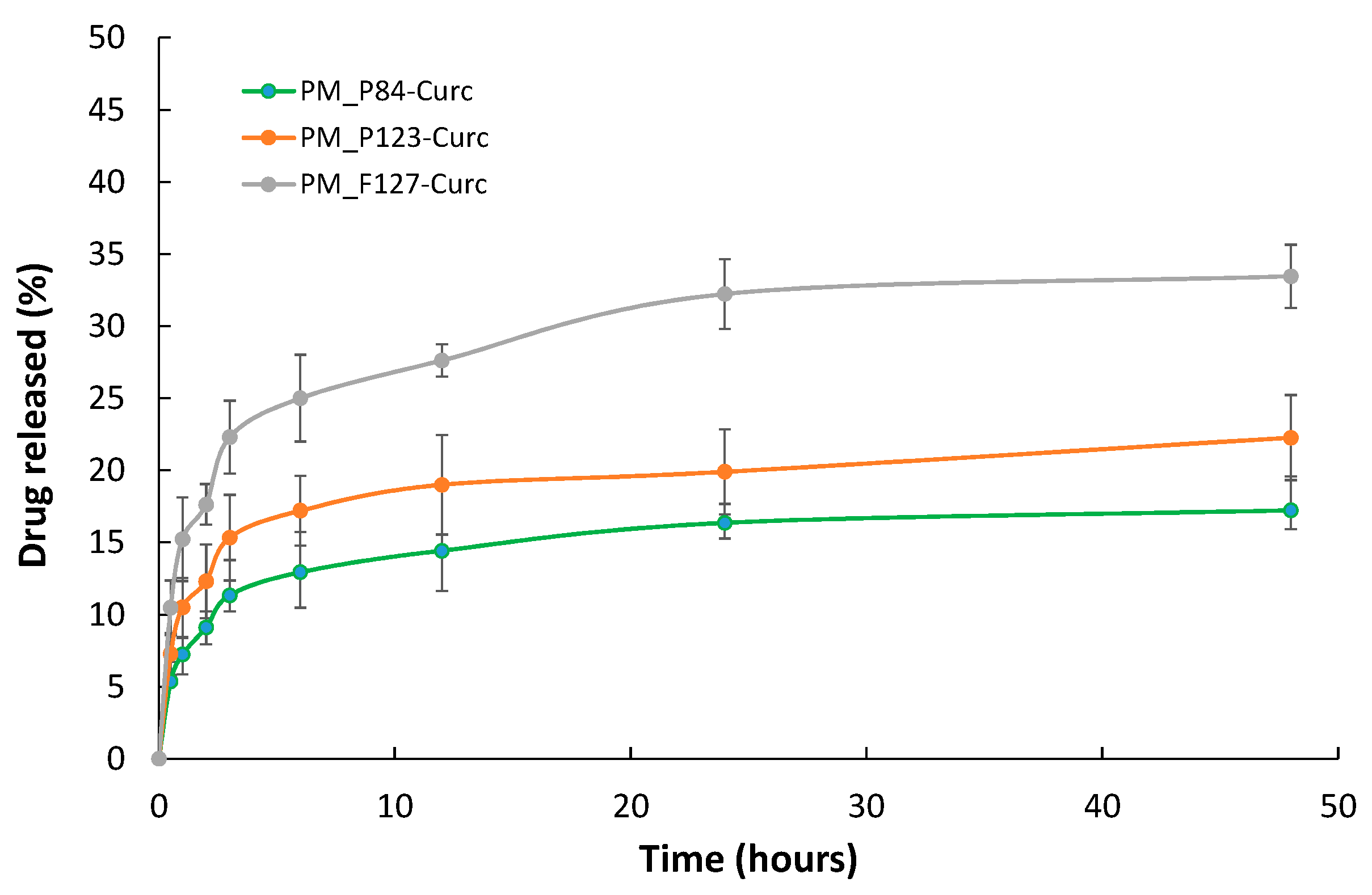
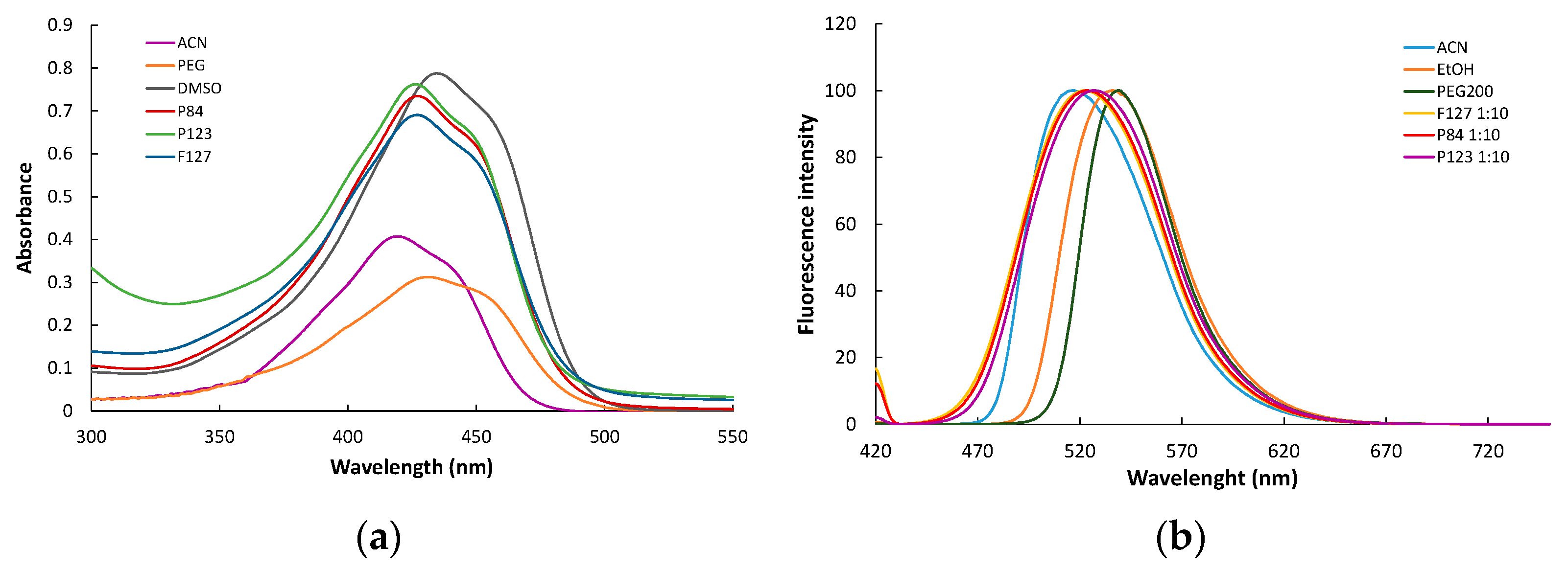
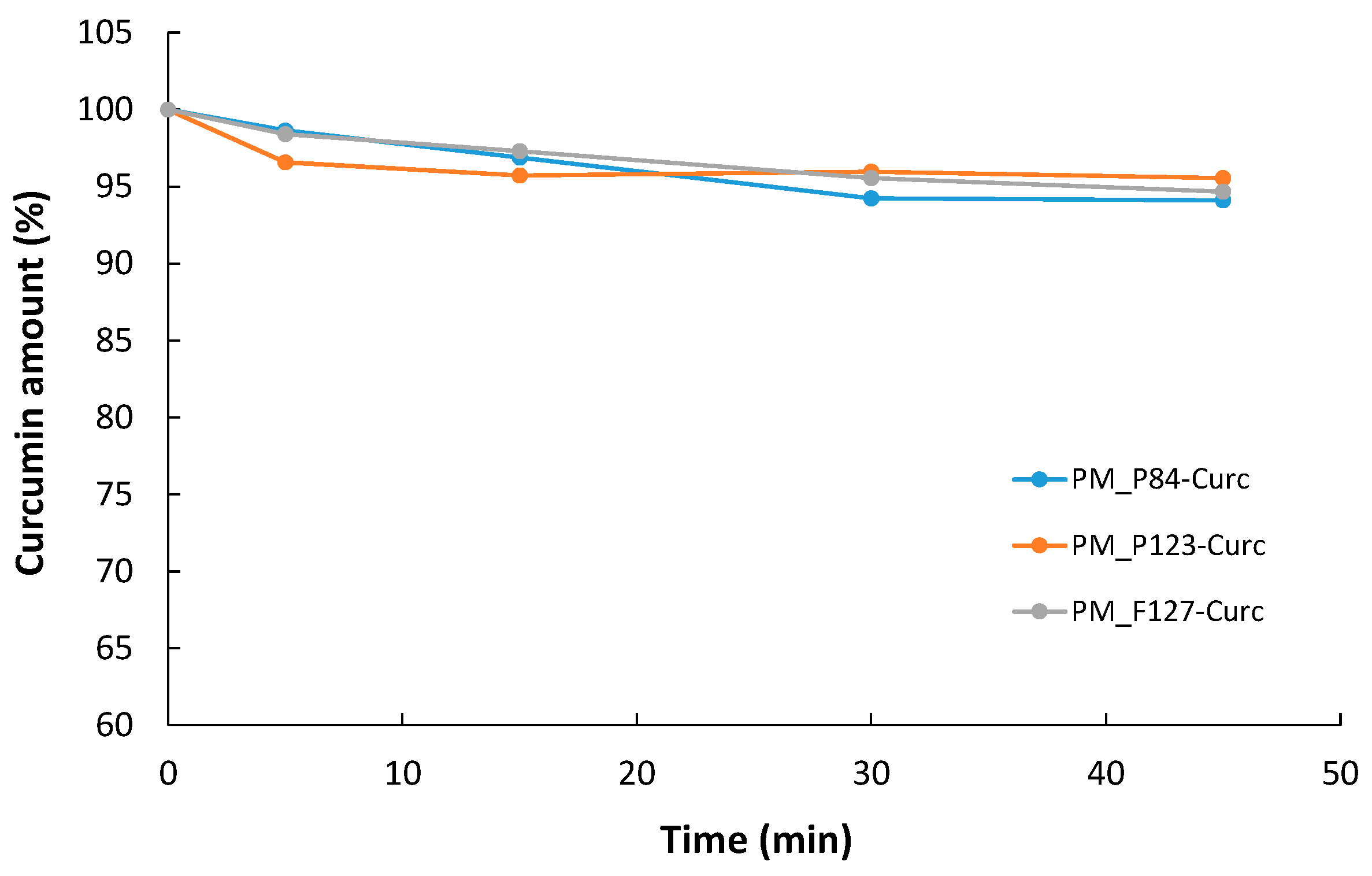


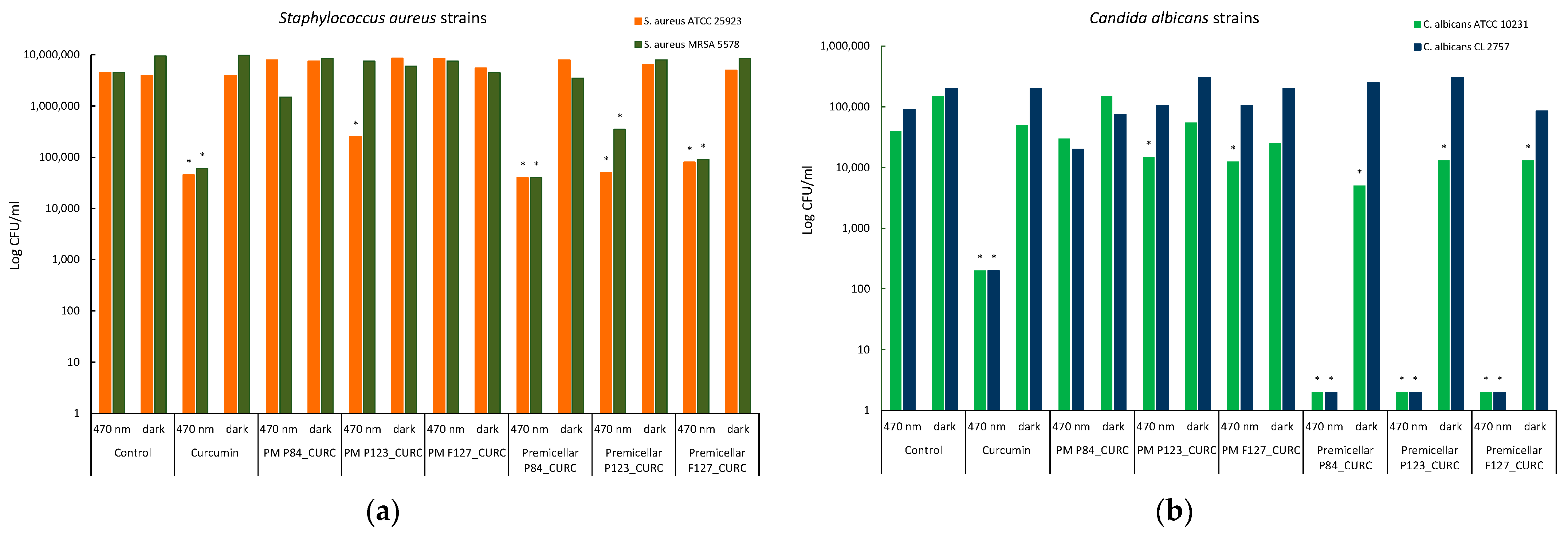

| Sample Code | Curcumin Concentration (μM) | Pluronic Concentration (μM) | Solvent |
|---|---|---|---|
| P84_CCMx10 | - | 1900 | PBS |
| P84_CCM | - | 190 | PBS |
| P84_CCM/10 | - | 19 | PBS |
| P84_CCM/100 | - | 1.9 | PBS |
| P123_CCMx10 | - | 88 | PBS |
| P123_CCM | - | 8.8 | PBS |
| P123_CCM/10 | - | 0.88 | PBS |
| P123_CCM/100 | - | 0.088 | PBS |
| F127_CCMx10 | - | 79 | PBS |
| F127_CCM | - | 7.9 | PBS |
| F127_CCM/10 | - | 0.79 | PBS |
| F127_CCM/100 | - | 0.079 | PBS |
| CURC_P84 | 100 | 3.57 | PBS |
| CURC_P123 | 100 | 2.60 | PBS |
| CURC_F127 | 100 | 1.19 | PBS |
| CURC_DMSO | 100 | - | DMSO |
| Sample Code | Content |
|---|---|
| DMSO | DMSO (Dimethyl sulfoxide) |
| CURC | 100 μM Curcumin in DMSO |
| PM_P84 | P84 1.5% |
| PM P84_CURC | 100 μM Curcumin in P84 1.5% |
| PM_P123 | P123 1.5% |
| PM P123_CURC | 100 μM Curcumin in P123 1.5% |
| PM F127 | F127 1.5% |
| PM F127_CURC | 100 μM Curcumin in F127 1.5% |
| DMSO-premicellar P84 | 1 mL DMSO + 1 mL P84 4 × 10−4 M |
| Premicellar P84_CURC | 2 mL 200 μM Curcumin in DMSO + 2 mL P84 4 × 10−4 M |
| DMSO-premicellar P84 | 1 mL DMSO + 1 mL P123 1.8 × 10−5 M |
| Premicellar P123_CURC | 2 mL 200 μM Curcumin in DMSO + 2 mL P123 1.8 × 10−5 M |
| DMSO-premicellar P84 | 1 mL DMSO + 1 mL F127 1.6 × 10−5 M |
| Premicellar F127_CURC | 2 mL 200 μM Curcumin in DMSO + 2 mL F127 1.6 × 10−5 M |
| Pluronic Derivative | Structural Formula | MW | HLB | CMC 1 |
|---|---|---|---|---|
| Pluronic P84 | (PEO)19-(PPO)43-(PEO)19 | 4200 | 14 | 1.9 × 10−4 M |
| Pluronic P123 | (PEO)18-(PPO)62-(PEO)18 | 5750 | 8 | 8.8 × 10−6 M |
| Pluronic F127 | (PEO)95-(PPO)62-(PEO)95 | 12600 | 22 | 7.9 × 10−6 M |
| Sample | Composition | Size (nm) | PdI |
|---|---|---|---|
| M1 | Micelles Pluronic P84 in PBS | 18.00 ± 0.38 | 0.380 ± 0.017 |
| M2 | Micelles Pluronic P123 in PBS | 19.69 ± 0.43 | 0.056 ± 0.010 |
| M3 | Micelles Pluronic F127 in PBS | 25.08 ± 1.83 | 0.164 ± 0.035 |
| P1 | CURC-loaded Micelles Pluronic P84 in PBS | 19.27 ± 2.04 | 0.323 ± 0.097 |
| P2 | CURC-loaded Micelles Pluronic P123 in PBS | 25.50 ± 0.57 | 0.156 ± 0.011 |
| P3 | CURC-loaded Micelles Pluronic F127 in PBS | 28.06 ± 0.77 | 0.268 ± 0.063 |
| Samples Code | Incubation Conditions | Inhibition Zone Diameters (mm) | |||||
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus MRSA 5578 | E. coli ATCC 25922 | E. coli ESBL 135 | C. albicans ATCC 10231 | C. albicans CL 2757 | ||
| DMSO control | 470 | 0 | 0 | 0 | 0 | 0 | 0 |
| darkness | 0 | 0 | 0 | 0 | 0 | 0 | |
| CURC control | 470 | 10 | 12 | 9 | 10 | 15 | 15 |
| darkness | 8 | 11 | 8 | 9 | 10 | 12 | |
| PM P84_CURC | 470 | 13 | 14 | 10 | 15 | 12 | 12 |
| darkness | 12 | 13 | 10 | 12 | 10 | 10 | |
| PM P123_CURC | 470 | 10 | 12 | 10 | 14 | 10 | 10 |
| darkness | 0 | 10 | 10 | 0 | 0 | 11 | |
| PM F127_CURC | 470 | 10 | 10 | 10 | 10 | 8 | 15 |
| darkness | 9 | 8 | 10 | 10 | 8 | 10 | |
| Premicellar P84_CURC | 470 | 10 | 10 | 10 | 10 | 20 | 13 |
| darkness | 9 | 10 | 10 | 10 | 10 | 10 | |
| Premicellar P123_CURC | 470 | 10 | 10 | 10 | 10 | 20 | 15 |
| darkness | 9 | 10 | 10 | 10 | 10 | 10 | |
| Premicellar F127_CURC | 470 | 10 | 12 | 10 | 10 | 15 | 13 |
| darkness | 10 | 10 | 8 | 8 | 10 | 10 | |
| Samples Code | Incubation Conditions | Minimal Inhibitory Concentration (µM) | |||||
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus MRSA 5578 | E. coli ATCC 25922 | E. coli ESBL 135 | C. albicans ATCC 10231 | C. albicans CL 2757 | ||
| Curcumin control | 470 | 50 | 50 | 100 | 100 | 25 | 25 |
| darkness | 100 | 100 | >100 | >100 | 100–50 | 100–50 | |
| PM P84_CURC | 470 | 100 | 100 | >100 | >100 | >100 | >100 |
| darkness | 100 | 100 | >100 | >100 | >100 | >100 | |
| PM P123_CURC | 470 | 100 | 100 | >100 | >100 | >100 | >100 |
| darkness | 100 | 100 | >100 | >100 | >100 | >100 | |
| PM F127_CURC | 470 | 75 | 100 | >100 | >100 | >100 | >100 |
| darkness | 100 | 100 | >100 | >100 | >100 | >100 | |
| Premicellar P84_CURC | 470 | 75 | 75 | >100 | >100 | 12.5 | 6.25 |
| darkness | 100 | 100 | >100 | >100 | >100 | >100 | |
| Premicellar P123_CURC | 470 | 75 | 75 | >100 | >100 | 12.5 | 12.5 |
| darkness | 100 | 100 | >100 | >100 | >100 | >100 | |
| Premicellar F127_CURC | 470 | 75 | 75 | >100 | >100 | 12.5 | 12.5 |
| darkness | 100 | 100 | >100 | >100 | >100 | >100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tănase, M.A.; Soare, A.C.; Diţu, L.M.; Nistor, C.L.; Mihaescu, C.I.; Gifu, I.C.; Petcu, C.; Cinteza, L.O. Influence of the Hydrophobicity of Pluronic Micelles Encapsulating Curcumin on the Membrane Permeability and Enhancement of Photoinduced Antibacterial Activity. Pharmaceutics 2022, 14, 2137. https://doi.org/10.3390/pharmaceutics14102137
Tănase MA, Soare AC, Diţu LM, Nistor CL, Mihaescu CI, Gifu IC, Petcu C, Cinteza LO. Influence of the Hydrophobicity of Pluronic Micelles Encapsulating Curcumin on the Membrane Permeability and Enhancement of Photoinduced Antibacterial Activity. Pharmaceutics. 2022; 14(10):2137. https://doi.org/10.3390/pharmaceutics14102137
Chicago/Turabian StyleTănase, Maria Antonia, Andreia Cristina Soare, Lia Mara Diţu, Cristina Lavinia Nistor, Catalin Ionut Mihaescu, Ioana Catalina Gifu, Cristian Petcu, and Ludmila Otilia Cinteza. 2022. "Influence of the Hydrophobicity of Pluronic Micelles Encapsulating Curcumin on the Membrane Permeability and Enhancement of Photoinduced Antibacterial Activity" Pharmaceutics 14, no. 10: 2137. https://doi.org/10.3390/pharmaceutics14102137
APA StyleTănase, M. A., Soare, A. C., Diţu, L. M., Nistor, C. L., Mihaescu, C. I., Gifu, I. C., Petcu, C., & Cinteza, L. O. (2022). Influence of the Hydrophobicity of Pluronic Micelles Encapsulating Curcumin on the Membrane Permeability and Enhancement of Photoinduced Antibacterial Activity. Pharmaceutics, 14(10), 2137. https://doi.org/10.3390/pharmaceutics14102137









