Enhanced Production of ECM Proteins for Pharmaceutical Applications Using Mammalian Cells and Sodium Heparin Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Cell Culture
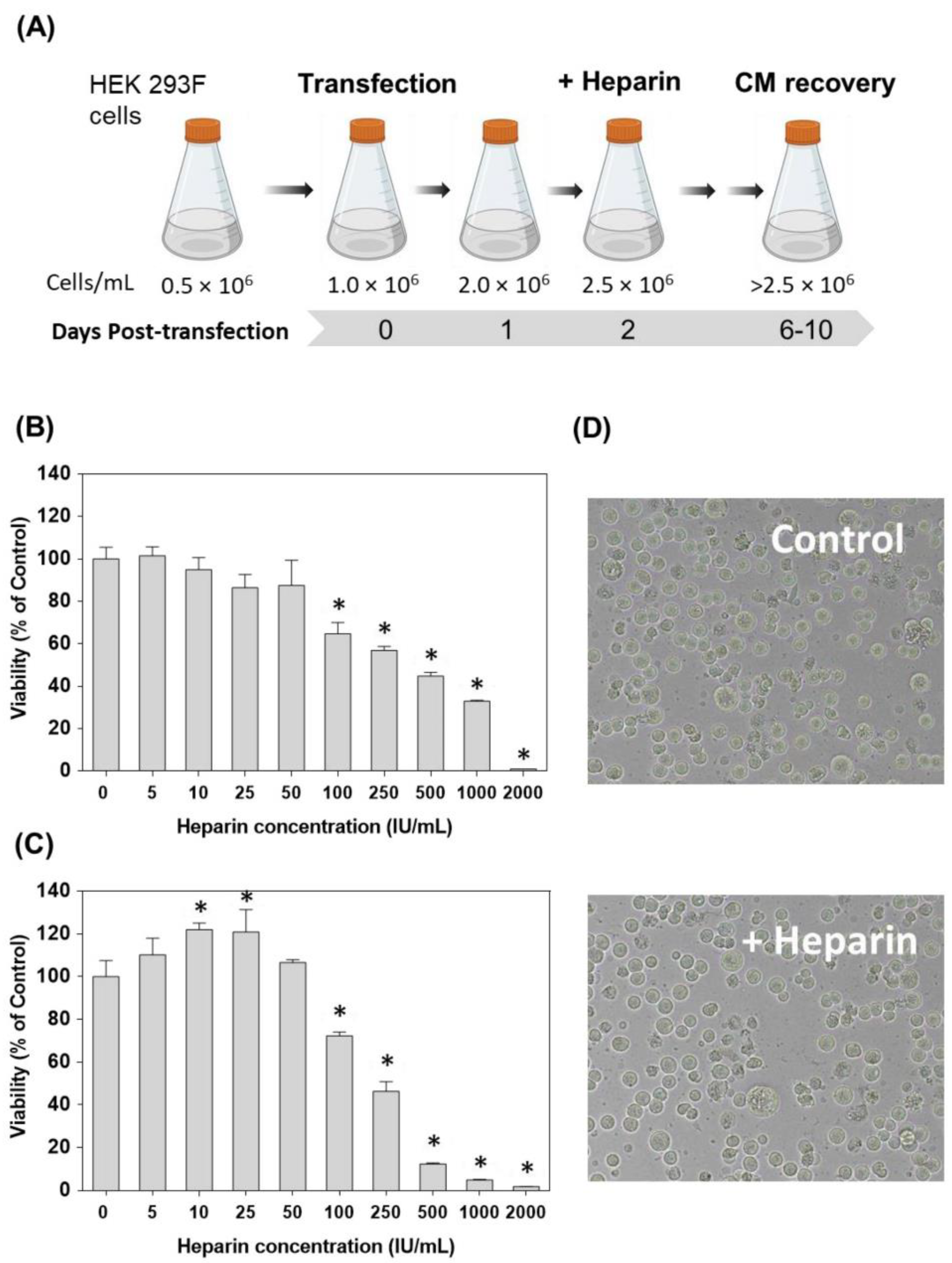
2.2. Step-by-Step Protein Expression Procedure
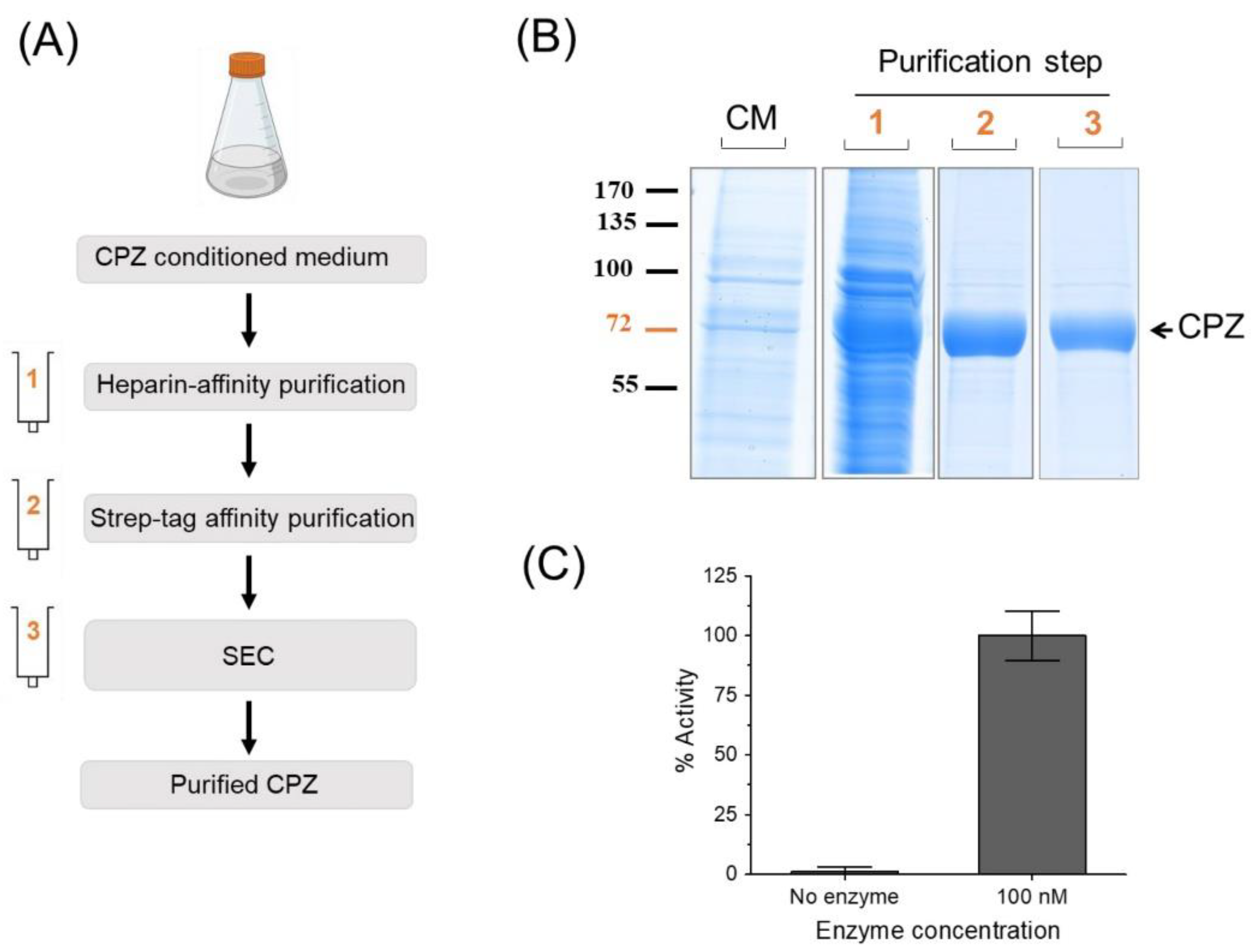
2.3. Optimized Protein Purification Protocol
2.3.1. Purification Step-1
2.3.2. Purification Step-2
2.3.3. Purification Step-3
2.4. Cytotoxicity Experiments
2.5. Enzyme Activity Assays
2.6. Statistical Analyses
3. Results
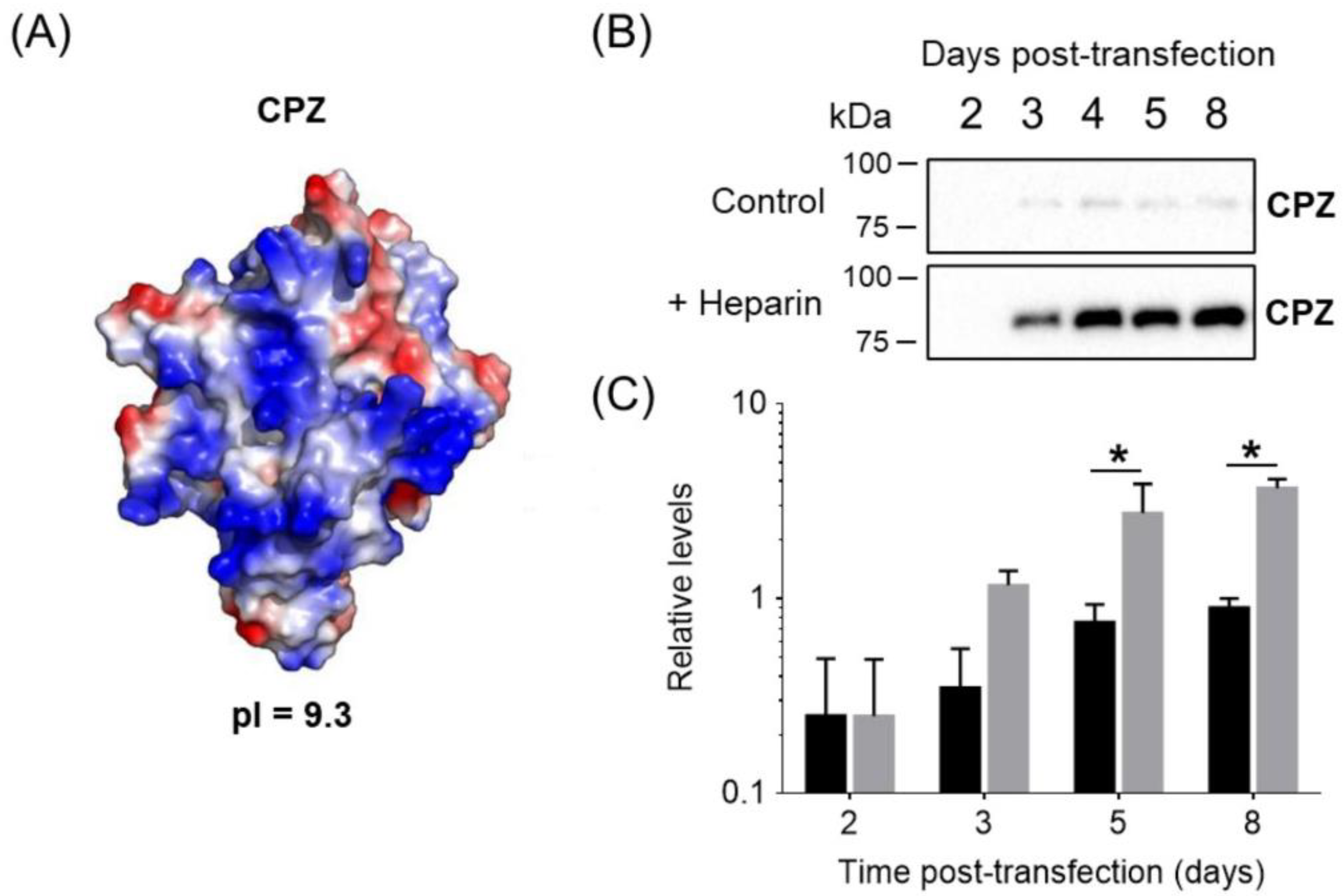
3.1. Heparin as an Additive to Enhance Recombinant Protein Expression
3.2. Enhanced Expression of Biomedically Relevant ECM/Heparin-Binding Proteins by Heparin Supplementation
3.3. Our Approach Is Compatible with a Straightforward Purification of ECM/Heparin-Binding Proteins
3.4. Considerations for Selecting ECM/Heparin-Binding Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.H. Gene Expression in Mammalian Cells and its Applications. Adv. Pharm. Bull. 2013, 3, 257–263. [Google Scholar] [CrossRef]
- Dumont, J.A.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R.R. Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Crit. Rev. Biotechnol. 2015, 36, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, F.R. Recombinant expression systems in the pharmaceutical industry. Appl. Microbiol. Biotechnol. 2004, 65, 363–372. [Google Scholar] [CrossRef]
- Li, Z.-M.; Fan, Z.-L.; Wang, X.-Y.; Wang, T.-Y. Factors Affecting the Expression of Recombinant Protein and Improvement Strategies in Chinese Hamster Ovary Cells. Front. Bioeng. Biotechnol. 2022, 10, 880155. [Google Scholar] [CrossRef]
- Portolano, N.; Watson, P.J.; Fairall, L.; Millard, C.; Milano, C.P.; Song, Y.; Cowley, S.; Schwabe, J.W.R. Recombinant Protein Expression for Structural Biology in HEK 293F Suspension Cells: A Novel and Accessible Approach. J. Vis. Exp. 2014, 92, e51897. [Google Scholar] [CrossRef] [Green Version]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef]
- Thoring, L.; Dondapati, S.K.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. High-yield production of “difficult-to-express” proteins in a continuous exchange cell-free system based on CHO cell lysates. Sci. Rep. 2017, 7, 11710. [Google Scholar] [CrossRef] [Green Version]
- Malm, M.; Kuo, C.-C.; Barzadd, M.M.; Mebrahtu, A.; Wistbacka, N.; Razavi, R.; Volk, A.-L.; Lundqvist, M.; Kotol, D.; Tegel, H.; et al. Harnessing secretory pathway differences between HEK293 and CHO to rescue production of difficult to express proteins. Metab. Eng. 2022, 72, 171–187. [Google Scholar] [CrossRef]
- Sohail, A.A.; Gaikwad, M.; Khadka, P.; Saaranen, M.J.; Ruddock, L.W. Production of Extracellular Matrix Proteins in the Cytoplasm of E. coli: Making Giants in Tiny Factories. Int. J. Mol. Sci. 2020, 21, 688. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, B. Biology of the Extracellular Matrix. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, P.; Callaway, M.B.; Fricker, L.D. Characterization of Carboxypeptidase A6, an Extracellular Matrix Peptidase. J. Biol. Chem. 2008, 283, 7054–7063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Capila, I.; Linhardt, R.J. Heparin-protein interactions. Angew. Chem. 2002, 41, 391–412. [Google Scholar] [CrossRef]
- Meneghetti, M.C.Z.; Hughes, A.; Rudd, T.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [Green Version]
- Wilgus, T.A. Growth Factor–Extracellular Matrix Interactions Regulate Wound Repair. Adv. Wound Care 2012, 1, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Clark, R.A. Fibronectin at Select Sites Binds Multiple Growth Factors and Enhances their Activity: Expansion of the Collaborative ECM-GF Paradigm. J. Investig. Dermatol. 2014, 134, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Camilleri, E.T.; Helledie, T.; Samsonraj, R.M.; Titmarsh, D.M.; Chua, R.J.; Dreesen, O.; Dombrowski, C.; Rider, D.A.; Galindo, M.; et al. Effect of heparin on the biological properties and molecular signature of human mesenchymal stem cells. Gene 2015, 576, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Dai, Y. Heparin: An intervenor in cell communication. J. Cell. Mol. Med. 2009, 14, 175–180. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pardo, J.; Tanco, S.; Díaz, L.; Dasgupta, S.; Fernandez-Recio, J.; Lorenzo, J.; Aviles, F.X.; Fricker, L.D. Substrate specificity of human metallocarboxypeptidase D: Comparison of the two active carboxypeptidase domains. PLoS ONE 2017, 12, e0187778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Pardo, J.; Tanco, S.; Garcia-Guerrero, M.C.; Dasgupta, S.; Avilés, F.X.; Lorenzo, J.; Fricker, L.D. Substrate Specificity and Structural Modeling of Human Carboxypeptidase Z: A Unique Protease with a Frizzled-Like Domain. Int. J. Mol. Sci. 2020, 21, 8687. [Google Scholar] [CrossRef]
- Garcia-Guerrero, M.C.; Garcia-Pardo, J.; Berenguer, E.; Fernandez-Alvarez, R.; Barfi, G.B.; Lyons, P.J.; Aviles, F.X.; Huber, R.; Lorenzo, J.; Reverter, D. Crystal structure and mechanism of human carboxypeptidase O: Insights into its specific activity for acidic residues. Proc. Natl. Acad. Sci. USA 2018, 115, 201803685–E3939. [Google Scholar] [CrossRef] [Green Version]
- Corchero, J.L.; Mendoza, R.; Lorenzo, J.; Rodríguez-Sureda, V.; Domínguez, C.; Vázquez, E.; Ferrer-Miralles, N.; Villaverde, A. Integrated approach to produce a recombinant, his-tagged human α-galactosidase a in mammalian cells. Biotechnol. Prog. 2011, 27, 1206–1217. [Google Scholar] [CrossRef]
- Núñez, C.; Oliveira, E.; García-Pardo, J.; Diniz, M.; Lorenzo, J.; Capelo, J.L.; Lodeiro, C. A novel quinoline molecular probe and the derived functionalized gold nanoparticles: Sensing properties and cytotoxicity studies in MCF-7 human breast cancer cells. J. Inorg. Biochem. 2014, 137, 115–122. [Google Scholar] [CrossRef]
- Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 1991, 142, 257–265. [Google Scholar] [CrossRef]
- GraphPad Prism, 6.01; GraphPad Software: San Diego, CA, USA, 2012.
- Novikova, E.G.; Reznik, S.E.; Varlamov, O.; Fricker, L.D. Carboxypeptidase Z Is Present in the Regulated Secretory Pathway and Extracellular Matrix in Cultured Cells and in Human Tissues. J. Biol. Chem. 2000, 275, 4865–4870. [Google Scholar] [CrossRef] [Green Version]
- Sanglas, L.; Valnickova, Z.; Arolas, J.L.; Pallares, I.; Guevara, T.; Solà, M.; Kristensen, T.; Enghild, J.J.; Aviles, F.X.; Gomis-Ruth, F.X. Structure of Activated Thrombin-Activatable Fibrinolysis Inhibitor, a Molecular Link between Coagulation and Fibrinolysis. Mol. Cell 2008, 31, 598–606. [Google Scholar] [CrossRef]
- Lyons, P.J.; Fricker, L.D. Substrate Specificity of Human Carboxypeptidase A6. J. Biol. Chem. 2010, 285, 38234–38242. [Google Scholar] [CrossRef]
- Lyons, P.J.; Ma, L.-H.; Baker, R.; Fricker, L.D. Carboxypeptidase A6 in Zebrafish Development and Implications for VIth Cranial Nerve Pathfinding. PLoS ONE 2010, 5, e12967. [Google Scholar] [CrossRef] [Green Version]
- Salzmann, A.; Guipponi, M.; Lyons, P.J.; Fricker, L.D.; Sapio, M.; Lambercy, C.; Buresi, C.; Bencheikh, B.O.A.; Lahjouji, F.; Ouazzani, R.; et al. Carboxypeptidase A6 gene (CPA6) mutations in a recessive familial form of febrile seizures and temporal lobe epilepsy and in sporadic temporal lobe epilepsy. Hum. Mutat. 2011, 33, 124–135. [Google Scholar] [CrossRef]
- Sanglas, L.; Arolas, J.L.; Valnickova, Z.; Aviles, F.X.; Enghild, J.J.; Gomis-Rüth, F.X. Insights into the molecular inactivation mechanism of human activated thrombin-activatable fibrinolysis inhibitor, TAFIa. J. Thromb. Haemost. 2010, 8, 1056–1065. [Google Scholar] [CrossRef]
- Schwarting, G.A.; Gajewski, A.; Carroll, P.; Dewolf, W.C. Inhibition of ganglioside sialyltransferase activity and stimulation of neutral glycolipid exocytosis by heparin. Arch. Biochem. Biophys. 1987, 256, 69–77. [Google Scholar] [CrossRef]
- Schrödinger, L. The PyMOL Molecular Graphics System, 2.0. Available online: http://www.pymol.org/pymol (accessed on 1 June 2022).
- Weiss, R.J.; Esko, J.D.; Tor, Y. Targeting heparin and heparan sulfate protein interactions. Org. Biomol. Chem. 2017, 15, 5656–5668. [Google Scholar] [CrossRef]
- Novikova, E.G.; Fricker, L.D. Purification and Characterization of Human Metallocarboxypeptidase Z. Biochem. Biophys. Res. Commun. 1999, 256, 564–568. [Google Scholar] [CrossRef]
- Kapiloff, M.; Strittmatter, S.M.; Fricker, L.D.; Snyder, S.H. A fluorometric assay for angiotensin-converting enzyme activity. Anal. Biochem. 1984, 140, 293–302. [Google Scholar] [CrossRef]
- Xu, D.; Esko, J.D. Demystifying Heparan Sulfate–Protein Interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef]
- Arolas, J.; Vendrell, J.; Aviles, F.; Fricker, L. Metallocarboxypeptidases: Emerging Drug Targets in Biomedicine. Curr. Pharm. Des. 2007, 13, 349–366. [Google Scholar] [CrossRef]
- Anand, K.; Pallares, I.; Valnickova, Z.; Christensen, T.; Vendrell, J.; Wendt, K.U.; Schreuder, H.A.; Enghild, J.J.; Aviles, F.X. The Crystal Structure of Thrombin-activable Fibrinolysis Inhibitor (TAFI) Provides the Structural Basis for Its Intrinsic Activity and the Short Half-life of TAFIa. J. Biol. Chem. 2008, 283, 29416–29423. [Google Scholar] [CrossRef]
- Garnham, R.; Scott, E.; Livermore, K.E.; Munkley, J. ST6GAL1: A key player in cancer (Review). Oncol. Lett. 2019, 18, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiselli, G. Heparin Binding Proteins as Therapeutic Target: An Historical Account and Current Trends. Medicines 2019, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Bolten, S.N.; Rinas, U.; Scheper, T. Heparin: Role in protein purification and substitution with animal-component free material. Appl. Microbiol. Biotechnol. 2018, 102, 8647–8660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Zhang, L.; He, Q.-Y. Fractionation of Proteins by Heparin Chromatography. 2D PAGE Sample Prep. Fractionation 2008, 424, 213–221. [Google Scholar] [CrossRef]
- Song, L.; Fricker, L.D. Cloning and Expression of Human Carboxypeptidase Z, a Novel Metallocarboxypeptidase. J. Biol. Chem. 1997, 272, 10543–10550. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Pardo, J.; Montané, S.; Avilés, F.X.; Tanco, S.; Lorenzo, J. Enhanced Production of ECM Proteins for Pharmaceutical Applications Using Mammalian Cells and Sodium Heparin Supplementation. Pharmaceutics 2022, 14, 2138. https://doi.org/10.3390/pharmaceutics14102138
Garcia-Pardo J, Montané S, Avilés FX, Tanco S, Lorenzo J. Enhanced Production of ECM Proteins for Pharmaceutical Applications Using Mammalian Cells and Sodium Heparin Supplementation. Pharmaceutics. 2022; 14(10):2138. https://doi.org/10.3390/pharmaceutics14102138
Chicago/Turabian StyleGarcia-Pardo, Javier, Sergi Montané, Francesc Xavier Avilés, Sebastian Tanco, and Julia Lorenzo. 2022. "Enhanced Production of ECM Proteins for Pharmaceutical Applications Using Mammalian Cells and Sodium Heparin Supplementation" Pharmaceutics 14, no. 10: 2138. https://doi.org/10.3390/pharmaceutics14102138







