AuNP/Chitosan Nanocomposites Synthesized through Plasma Induced Liquid Chemistry and Their Applications in Photothermal Induced Bacteria Eradication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AuNP/CS Nanocomposites
2.3. Characterization
2.4. Antibacterial Testing
2.5. Statistical Analysis
3. Results and Discussion
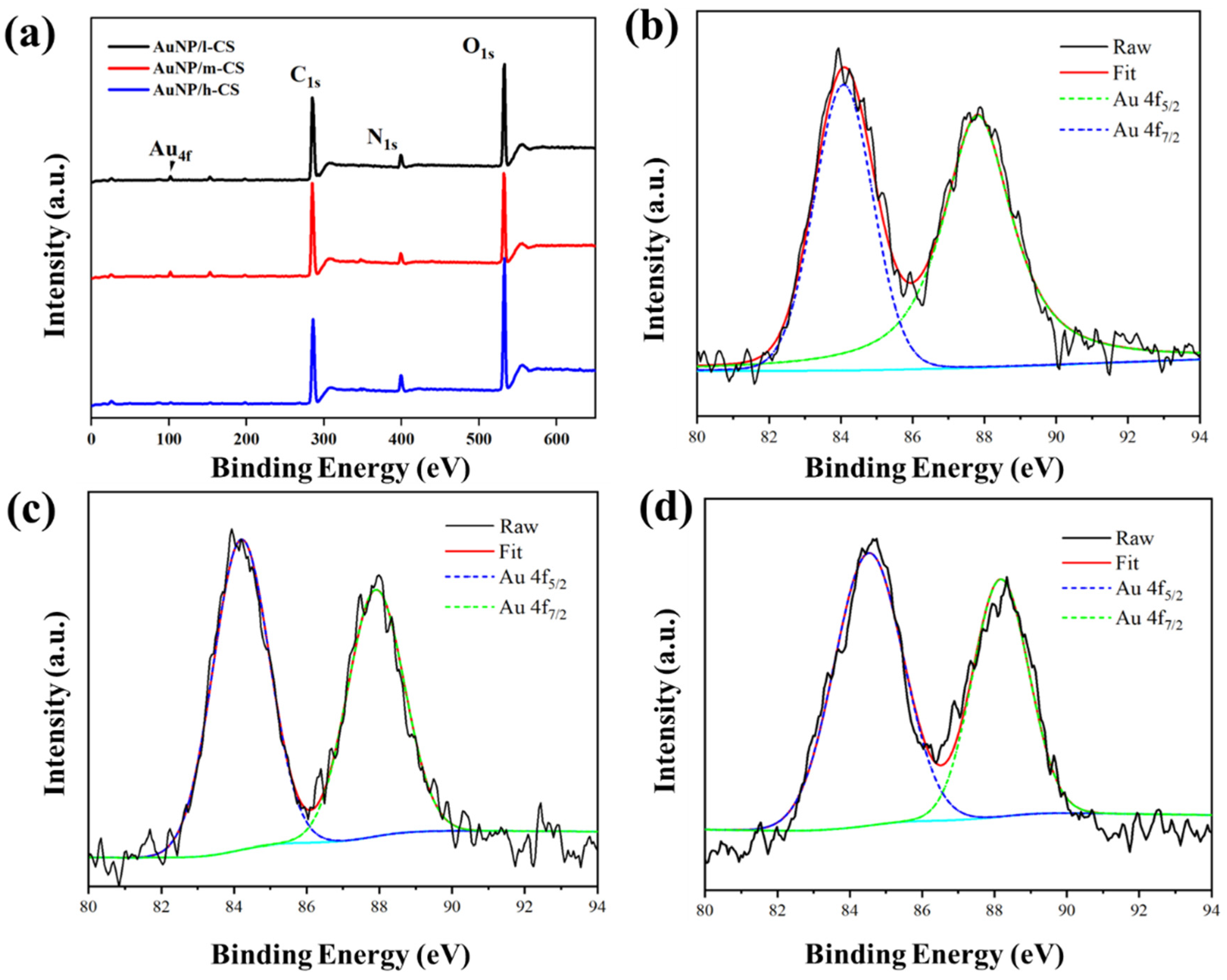
3.1. Physical Properties of AuNP/CS Nanocomposites
3.2. AuNP/CS Nanocomposite Formation Mechanisms
3.3. Thermal and Swelling Properties of AuNP/CS Nanocomposite Film
3.4. Photothermal Conversion of Anti-Bacterial Properties of AuNP/CS Nanocomposite Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asnag, G.M.; Oraby, A.H.; Abdelghany, A.M. Green synthesis of gold nanoparticles and its effect on the optical, thermal and electrical properties of carboxymethyl cellulose. Compos. Part B Eng. 2019, 172, 436–446. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape effects of gold nanoparticles in photothermal cancer therapy. Mater. Today Sustain. 2021, 2021, 100078. [Google Scholar]
- Sun, D.; Tang, M.; Zhang, L.; Falzon, B.G.; Padmanaban, D.B.; Mariotti, D.; Maguire, P.; Xu, H.; Chen, M.; Sun, D. Microplasma assisted synthesis of gold nanoparticle/graphene oxide nanocomposites and their potential application in SERS sensing. Nanotechnology 2019, 30, 455603. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McLaughlan, J.; Zhang, L.; Falzon, B.G.; Mariotti, D.; Maguire, P.; Sun, D. Atmospheric Pressure Plasma-Synthesized Gold Nanoparticle/Carbon Nanotube Hybrids for Photothermal Conversion. Langmuir 2019, 35, 4577–4588. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tsai, P.-J.; Chen, Y.-C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, F.R.; Fornasier, F.; Carvalho, A.S.; Silva, B.M.; Lima, M.C.; Pimentel, A.S. Polymer-coated gold nanoparticles and polymeric nanoparticles as nanocarrier of the BP100 antimicrobial peptide through a lung surfactant model. J. Mol. Liq. 2020, 314, 113661. [Google Scholar] [CrossRef]
- Kolya, H.; Pal, S.; Pandey, A.; Tripathy, T. Preparation of gold nanoparticles by a novel biodegradable graft copolymer sodium alginate-g-poly (N,N-dimethylacrylamide-co-acrylic acid) with anti micro bacterial application. Eur. Polym. J. 2015, 66, 139–148. [Google Scholar] [CrossRef]
- Kleszcz, K.; Hebda, M.; Kyzioł, A.; Krawiec, H.; Kyzioł, K. Towards prevention of biofilm formation: Ti6Al7Nb modified with nanocomposite layers of chitosan and Ag/Au nanoparticles. Appl. Surf. Sci. 2021, 557, 149795. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, N.; Moore, J.; McCoy, C.P.; Ziminska, M.; Rafferty, C.; Sarri, G.; Hamilton, A.R.; Li, Y.; Zhang, L.; et al. Nanoscale Hybrid Coating Enables Multifunctional Tissue Scaffold for Potential Multimodal Therapeutic Applications. ACS Appl. Mater. Interfaces 2019, 11, 27269–27278. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Vilsinski, B.H.; Bonafé, E.G.; Monteiro, J.P.; Kipper, M.J.; Martins, A.F. Chitosan content modulates durability and structural homogeneity of chitosan-gellan gum assemblies. Int. J. Biol. Macromol. 2019, 128, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Brahman, P.K.; Reddy, K.R.; Bondili, J.S. Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzym. Microb. Technol. 2018, 112, 43–51. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Monti, S.; Jose, J.; Sahajan, A.; Kalarikkal, N.; Thomas, S. Structure and dynamics of gold nanoparticles decorated with chitosan–gentamicin conjugates: ReaxFF molecular dynamics simulations to disclose drug delivery. Phys. Chem. Chem. Phys. 2019, 21, 13099–13108. [Google Scholar] [CrossRef]
- Dube, E.; Oluwole, D.O.; Prinsloo, E.; Nyokong, T. A gold–chitosan composite with low symmetry zinc phthalocyanine for enhanced singlet oxygen generation and improved photodynamic therapy activity. New J. Chem. 2018, 42, 10214–10225. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, X.; Jasinski, J.; Patel, D.; Gobin, A.M. Gold/Chitosan Nanocomposites with Specific Near Infrared Absorption for Photothermal Therapy Applications. J. Nanomater. 2012, 2012, 853416. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.B.; Rufato, K.B.; de Oliveira, A.C.; Souza, P.R.; da Silva, E.P.; Muniz, E.C.; Vilsinski, B.H.; Martins, A.F. Composite materials based on chitosan/gold nanoparticles: From synthesis to biomedical applications. Int. J. Biol. Macromol. 2020, 161, 977–998. [Google Scholar] [CrossRef]
- Patel, N.G.; Kumar, A.; Jayawardana, V.N.; Woodworth, C.D.; Yuya, P.A. Fabrication, nanomechanical characterization, and cytocompatibility of gold-reinforced chitosan bio-nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Piątkowski, M.; Radwan-Pragłowska, J.; Janus, Ł.; Bogdał, D.; Matysek, D.; Cablik, V. Microwave-assisted synthesis and characterization of chitosan aerogels doped with Au-NPs for skin regeneration. Polym. Test. 2019, 73, 366–376. [Google Scholar] [CrossRef]
- Han, L.; Zhao, J.; Zhang, X.; Cao, W.; Hu, X.; Zou, G.; Duan, X.; Liang, X. Enhanced siRNA Delivery and Silencing GoldChitosan Nanosystem with Surface Charge-Reversal Polymer Assembly and Good Biocompatibility. ACS Nano 2012, 6, 7340–7351. [Google Scholar] [CrossRef] [PubMed]
- Labala, S.; Jose, A.; Venugati, V.V.K. Transcutaneous iontophoretic delivery of STAT3 siRNA using layer-by-layer chitosan coated gold nanoparticles to treat melanoma. Colloids Surf. B Biointerfaces 2016, 146, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, E.; Sharifiaghdam, M.; Keersmaecker, H.D.; Rycke, R.D.; Smedt, S.D.; Faridi-Majidi, R.; Braeckmans, K.; Fraire, J.C. Layer by Layer Assembled Chitosan-Coated Gold Nanoparticles for Enhanced siRNA Delivery and Silencing. Int. J. Mol. Sci. 2021, 22, 831. [Google Scholar] [CrossRef]
- Sun, L.; Pu, S.; Li, J.; Cai, J.; Zhou, B.; Ren, G.; Ma, Q.; Zhong, L. Size controllable one step synthesis of gold nanoparticles using carboxymethyl chitosan. Int. J. Biol. Macromol. 2019, 122, 770–783. [Google Scholar] [CrossRef]
- Ma, H.; Sun, J.; Zhang, Y.; Bian, C.; Xia, S.; Zhen, T. Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens. Bioelectron. 2016, 80, 222–229. [Google Scholar] [CrossRef]
- Leiva, A.; Bonardd, S.; Pino, M.; Saldías, C.; Kortaberria, G.; Radić, D. Improving the performance of chitosan in the synthesis and stabilization of gold nanoparticles. Eur. Polym. J. 2015, 68, 419–431. [Google Scholar] [CrossRef]
- Caldera-Villalobos, M.; García-Serrano, J.; Peláez-Cid, A.A.; Herrera-González, A.M. Polyelectrolytes with sulfonate groups obtained by chemical modification of chitosan useful in green synthesis of Au and Ag nanoparticles. J. Appl. Polym. Sci. 2017, 134, 45240. [Google Scholar] [CrossRef]
- Pestov, A.; Nazirov, A.; Privar, Y.; Modin, E.; Bratskaya, S. Role of Au(III) coordination by polymer in green synthesis of gold nanoparticles using chitosan derivatives. Int. J. Biol. Macromol. 2016, 91, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Cai, J.; Zhong, L.; Ren, G.; Ma, Q. One pot synthesis of gold nanoparticles using chitosan with varying degree of deacetylation and molecular weight. Carbohydr. Polym. 2017, 178, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Turner, J.; Jiang, N.; Zhu, S.; Zhang, L.; Falzon, B.G.; McCoy, C.P.; Maguire, P.; Mariotti, D.; Sun, D. Atmospheric pressure microplasma for antibacterial silver nanoparticle/chitosan nanocomposites with tailored properties. Compos. Sci. Technol. 2020, 186, 107911. [Google Scholar] [CrossRef]
- Sun, D.; Maddi, C.; Rafferty, C.; Tang, M.; Chen, M.; Falzon, B.G.; Sarri, G.; Mariotti, D.; Maguire, P.; Sun, D. Effect of precursor pH on AuNP/MWCNT nanocomposites synthesized by plasma-induced non-equilibrium electrochemistry. J. Phys. D Appl. Phys. 2020, 53, 425207. [Google Scholar] [CrossRef]
- Nolan, H.; Sun, D.; Falzon, B.G.; Chakrabarti, S.; Padmanaba, D.B.; Maguire, P.; Mariotti, D.; Yu, T.; Jones, D.; Andrews, G.; et al. Metal nanoparticle-hydrogel nanocomposites for biomedical applications—An atmospheric pressure plasma synthesis approach. Plasma Processes Polym. 2018, 15, 1800112. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Němcová, L.; Maguire, P.; Graham, W.G.; Mariotti, D. Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-induced liquid chemistry. Nanotechnology 2013, 24, 245604. [Google Scholar] [CrossRef] [Green Version]
- Marisca, O.; Leopold, N. Anisotropic Gold Nanoparticle-Cell Interactions Mediated by Collagen. Materials 2019, 12, 1131. [Google Scholar] [CrossRef] [Green Version]
- Caballero, R.; D’Olivo, J.C.; Medina-Tanco, G.; Nellen, L.; Sánchez, F.A.; Valdés-Galicia, J.F. Contribution of Atmospheric Scattering of Light to Shower Signal in a Fluorescence Detector-NASA/ADS; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2008; Volume 4, pp. 515–518. [Google Scholar]
- Ortiz-Csstillo, J.E.; Gallo-Villanueva, R.C.; Madou, M.J.; Perez-Gonzalez, V.H.P. Anisotropic gold nanoparticles: A survey of recent synthetic methodologies. Coord. Chem. Rev. 2020, 425, 213489. [Google Scholar] [CrossRef]
- Molina, R.; Jovancic, P.; Vilchez, S.; Tzanov, T.; Solans, C. In situ chitosan gelation initiated by atmospheric plasma treatment. Carbohydr. Polym. 2014, 103, 472–479. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tai, M.-C.; Cheng, F.-H. Kinetics and Products of the Degradation of Chitosan by Hydrogen Peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. Npj Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Boyen, H.G.; Kästle, G.; Weigl, F.; Koslowski, B.; Dietrich, C.; Ziemann, P.; Spatz, J.P.; Riethmüller, S.; Hartmann, C.; Möller, M.; et al. Oxidation-Resistant Gold-55 Clusters. Science 2002, 297, 1533. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, B.; Jia, F.; Song, S. Reduction mechanism of Au metal ions into Au nanoparticles on molybdenum disulfide. Nanoscale 2019, 11, 9488–9497. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Oh, S.-K.; Crooks, R.M. Preparation and Characterization of 1−2 nm Dendrimer-Encapsulated Gold Nanoparticles Having Very Narrow Size Distributions. Chem. Mater. 2004, 16, 167–172. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mohamed, P.A.; Kadir, M.F.Z.; Hamsan, M.H.; Abdulwahid, R.T.; Woo, H.J. Increase of metallic silver nanoparticles in Chitosan:AgNt based polymer electrolytes incorporated with alumina filler. Results Phys. 2019, 13, 102326. [Google Scholar] [CrossRef]
- Su, Z.; Sun, D.; Zhang, L.; He, M.; Jiang, Y.; Millar, B.; Douglas, P.; Mariotti, D.; Maguire, P.; Sun, D. Chitosan/Silver Nanoparticle/Graphene Oxide Nanocomposites with Multi-Drug Release, Antimicrobial, and Photothermal Conversion Functions. Materials 2021, 14, 2351. [Google Scholar] [CrossRef]
- Chen, A.M.; Taratula, O.; Wei, D.; Yen, H.-I.; Thomas, T.; Thomas, T.J.; Minko, T.; He, H. Labile Catalytic Packaging of DNA/siRNA: Control of Gold Nanoparticles “out” of DNA/siRNA Complexes. ACS Nano 2010, 4, 3679–3688. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Wang, D.; Wang, Y.; He, S. Multifunctional Gold Nanorods with Ultrahigh Stability and Tunability for In Vivo Fluorescence Imaging, SERS Detection, and Photodynamic Therapy. Angew. Chem. Int. Ed. 2013, 52, 1148–1151. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma–Liquid Interactions at Atmospheric Pressure for Nanomaterials Synthesis and Surface Engineering. Plasma Processes Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, Z.-G.; Chen, X.; Meng, F.-L.; Liu, J.-H.; Huang, X.-J. UV irradiation synthesis of an Au–graphene nanocomposite with enhanced electrochemical sensing properties. J. Mater. Chem. A 2013, 1, 9189–9195. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef] [PubMed]
- Saber-Samandari, S.; Yilmaz, O.; Yilmaz, E. Photoinduced Graft Copolymerization onto Chitosan Under Heterogeneous Conditions. J. Macromol. Sci. Part A 2012, 49, 591–598. [Google Scholar] [CrossRef]
- Vara, J.A.; Dave, P.N.; Chaturvedi, S. The catalytic activity of transition metal oxide nanoparticles on thermal decomposition of ammonium perchlorate. Def. Technol. 2019, 15, 629–635. [Google Scholar] [CrossRef]
- Lavorgna, M.; Attianese, I.; Buonocore, G.G.; Conte, A.; Del Nobile, M.A.; Tescione, F.; Amendola, E. MMT-supported Ag nanoparticles for chitosan nanocomposites: Structural properties and antibacterial activity. Carbohydr. Polym. 2014, 102, 385–392. [Google Scholar] [CrossRef]
- Gorjanc, M.; Bukošek, V.; Gorenšek, M.; Mozetič, M. CF4 plasma and silver functionalized cotton. Text. Res. J. 2010, 80, 2204–2213. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.; et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Sun, I.C.; Yun, W.S.; Kim, J.; Lim, D.K.; Ahn, C.H.; Kim, K. Thiol-Responsive Gold Nanodot Swarm with Glycol Chitosan for Photothermal Cancer Therapy. Molecules 2021, 26, 5980. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.S.; Hegazy, S.F.; Obayya, S.S.A. Nanogold-loaded chitosan nanocomposites for pH/light-responsive drug release and synergistic chemo-photothermal cancer therapy. Colloid Interface Sci. Commun. 2021, 41, 100361. [Google Scholar] [CrossRef]
- Zakaria, H.; Abdelaziz, W.S.; Youssef, T. Effect of size, concentration, and type of spherical gold nanoparticles on heat evolution following laser irradiation using tissue-simulating phantoms. Lasers Med. Sci. 2016, 31, 625–634. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; EI-Sayed, I.H.; EI-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si-Au and Fe-Au core-shell on photothermal cancer treatment. Photodiagnosis Photodyn. Ther. 2021, 33, 102144. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.G.; Montalbán, M.G.; Carissimi, G.; Lima, B.; Feresin, G.E.; Cano, M.; Giner-Casares, J.J.; López-Cascales, J.J.; Enriz, R.D.; Víllora, G. Antibacterial Effect of Chitosan–Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model. Nanomaterials 2020, 10, 2340. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Mahmoud, N.N. Photothermal-Induced Antibacterial Activity of Gold Nanorods Loaded into Polymeric Hydrogel against Pseudomonas aeruginosa Biofilm. Molecules 2019, 24, 2661. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Khan, F.; Hoang, G.; Mondal, S.; Kim, H.; Hoang Minh Doan, V.; Kim, Y.-M.; Oh, J. Thiol chitosan-wrapped gold nanoshells for near-infrared laser-induced photothermal destruction of antibiotic-resistant bacteria. Carbohydr. Polym. 2019, 225, 115228. [Google Scholar] [CrossRef] [PubMed]










| Sample Acronym | CS Viscosity | HAuCl4 Concentration | APM |
|---|---|---|---|
| l-CS | 50–100 mPa·s | none | no |
| m-CS | 200–400 mPa·s | none | no |
| h-CS | >400 mPa·s | none | no |
| 2.5 µM AuNP/m-CS | 200–400 mPa·s | 2.5 µM | yes |
| 0.1 mM AuNP/m-CS | 200–400 mPa·s | 0.1 mM | yes |
| 0.2 mM AuNP/m-CS | 200–400 mPa·s | 0.2 mM | yes |
| 1.0 mM AuNP/m-CS | 200–400 mPa·s | 1.0 mM | yes |
| 2.0 mM AuNP/m-CS | 200–400 mPa·s | 1.0 mM | yes |
| 0.5 mM AuNP/l-CS | 50–100 mPa·s | 0.5 mM | yes |
| 0.5 mM AuNP/m-CS | 200–400 mPa·s | 0.5 mM | yes |
| 0.5 mM AuNP/h-CS | >400 mPa·s | 0.5 mM | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Sun, D.; Zhou, X.; Xu, H.; Huang, Y.; Chu, C.; Shen, B. AuNP/Chitosan Nanocomposites Synthesized through Plasma Induced Liquid Chemistry and Their Applications in Photothermal Induced Bacteria Eradication. Pharmaceutics 2022, 14, 2147. https://doi.org/10.3390/pharmaceutics14102147
Guo Z, Sun D, Zhou X, Xu H, Huang Y, Chu C, Shen B. AuNP/Chitosan Nanocomposites Synthesized through Plasma Induced Liquid Chemistry and Their Applications in Photothermal Induced Bacteria Eradication. Pharmaceutics. 2022; 14(10):2147. https://doi.org/10.3390/pharmaceutics14102147
Chicago/Turabian StyleGuo, Zhijun, Dan Sun, Xian Zhou, Huan Xu, Yizhou Huang, Chenglin Chu, and Baolong Shen. 2022. "AuNP/Chitosan Nanocomposites Synthesized through Plasma Induced Liquid Chemistry and Their Applications in Photothermal Induced Bacteria Eradication" Pharmaceutics 14, no. 10: 2147. https://doi.org/10.3390/pharmaceutics14102147
APA StyleGuo, Z., Sun, D., Zhou, X., Xu, H., Huang, Y., Chu, C., & Shen, B. (2022). AuNP/Chitosan Nanocomposites Synthesized through Plasma Induced Liquid Chemistry and Their Applications in Photothermal Induced Bacteria Eradication. Pharmaceutics, 14(10), 2147. https://doi.org/10.3390/pharmaceutics14102147






