Therapeutic Application of an Ag-Nanoparticle-PNIPAAm-Modified Eggshell Membrane Construct for Dermal Regeneration and Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
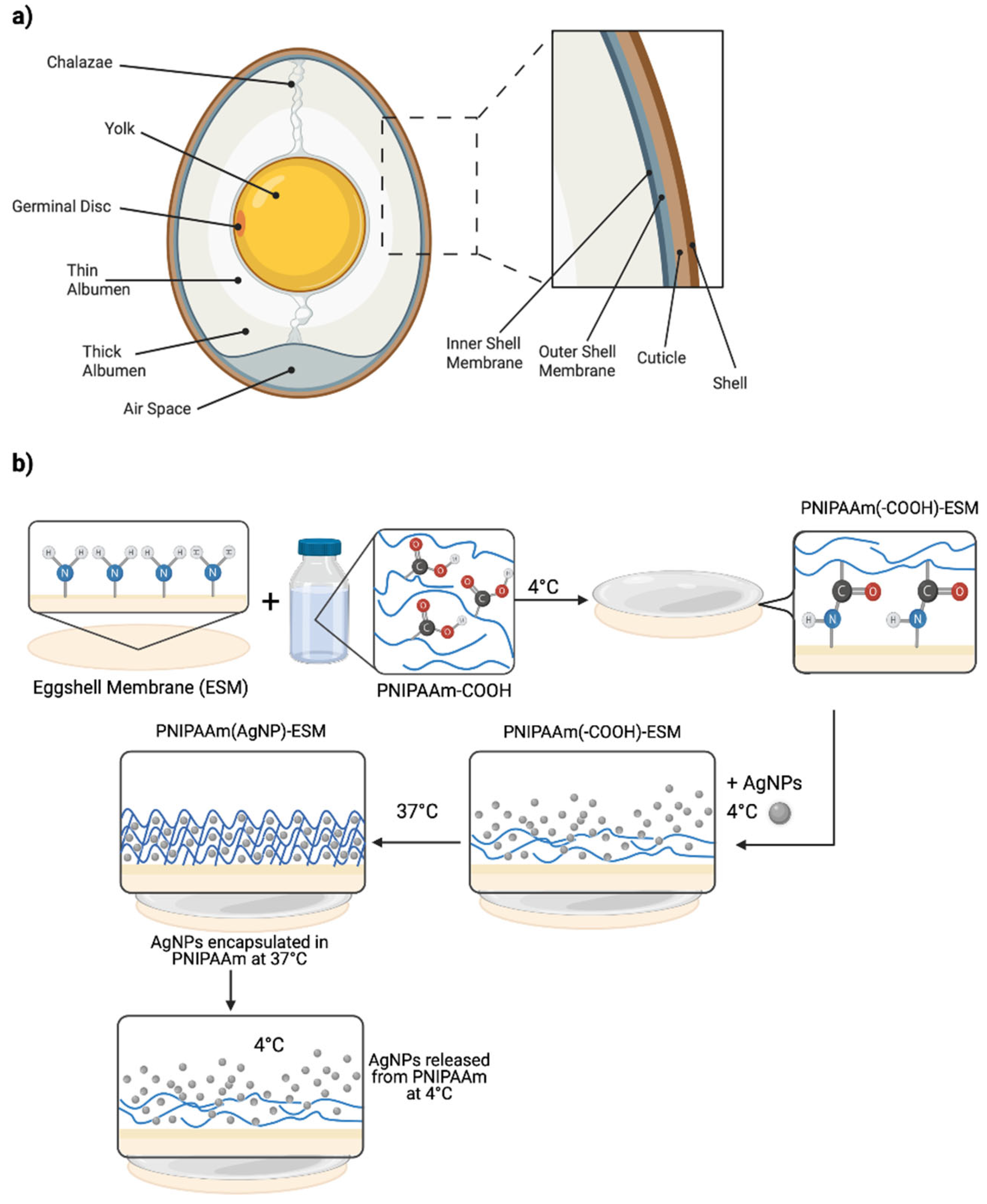
2.1. Membrane Isolation
2.2. Membrane Modification
2.3. Material Characterisation
2.4. Biological Characterisation
2.5. Statistical Analysis
3. Results and Discussion
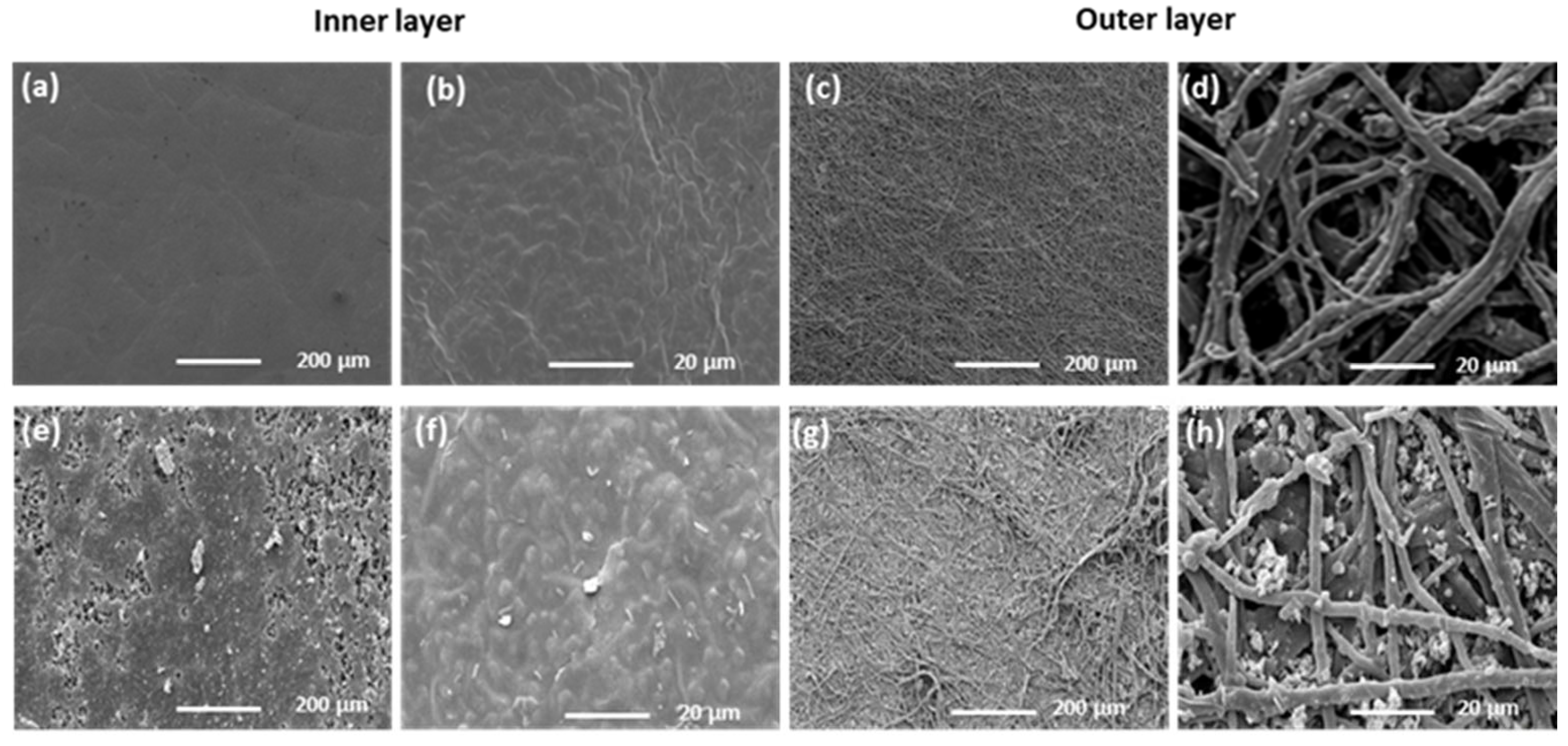
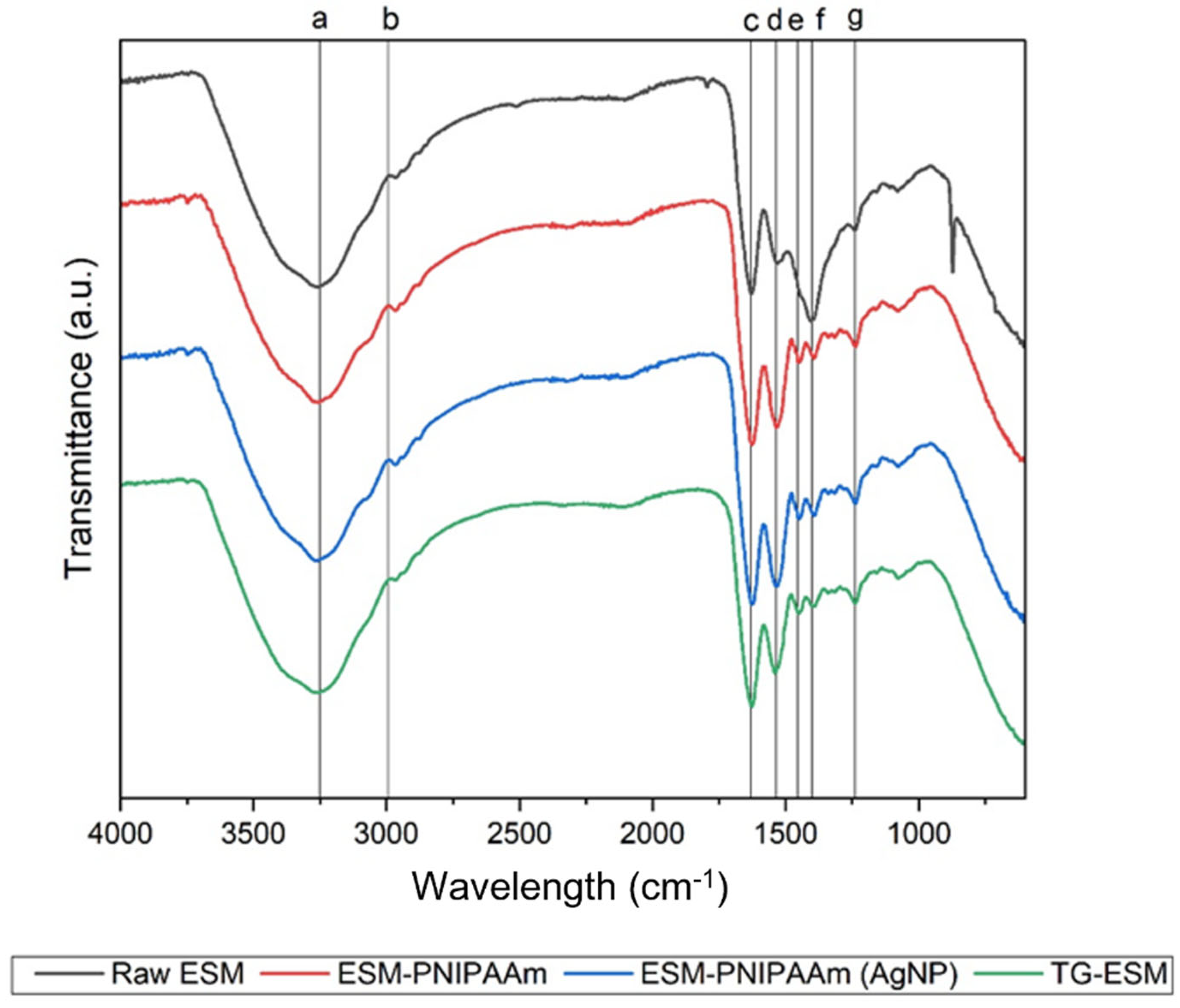
3.1. Material Characterisation
3.2. Thermal Properties
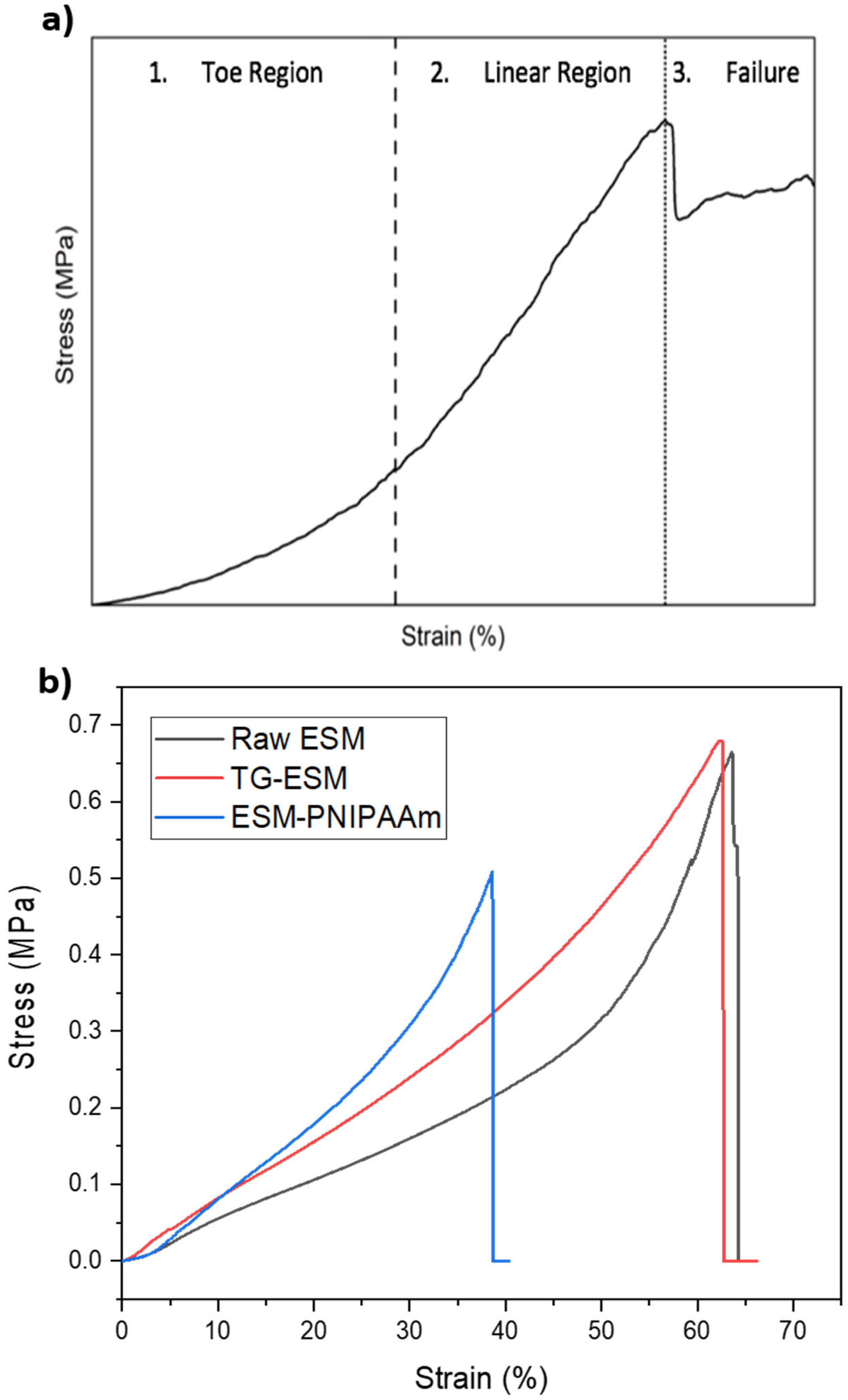
3.3. Mechanical Properties
3.4. Drug Release Profile—ESM-PNIPAAm
3.5. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffcoate, W.J.; Harding, K.G. Diabetic foot ulcers. Lancet 2003, 361, 1545–1551. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2017, 14, 322–330. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Collagen-based Biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 335–382. [Google Scholar] [CrossRef] [Green Version]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.; She, Z.; Fan, K.; Xu, C.; Chu, B.; Chen, C.; Shi, S.; Tan, R. Collagen/chitosan based two-compartment and bi-functional dermal scaffolds for skin regeneration. Mater. Sci. Eng. C 2015, 52, 155–162. [Google Scholar] [CrossRef]
- Williams, C. Algosteril calcium alginate dressing for moderate/high exudate. Br. J. Nurs. 1999, 8, 313–317. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jo SBin Kim, H.; Park, S.-M.; Patel, K.D.; Cho, K.J.; Cook, M.T.; Kirton, S.B.; Hutter, V.; Sidney, E.L.; Alves-Lima, D. The eggshell membrane: A potential biomaterial for corneal wound healing. J. Biomater. Appl. 2021, 36, 912–929. [Google Scholar] [CrossRef]
- Yang, J.Y.; Chuang, S.-S.; Yang, W.-G.; Tsay, P.-K. Egg membrane as a new biological dressing in split-thickness skin graft donor sites: A preliminary clinical evaluation. Chang Gung Med. J. 2003, 26, 153–159. [Google Scholar]
- Jun, H.J.; Oh, K.-H.; Yoo, J.; Han, W.-G.; Chang, J.; Jung, H.H.; Choi, J. A new patch material for tympanic membrane perforation by trauma: The membrane of a hen egg shell. Acta Oto-Laryngologica 2013, 134, 250–254. [Google Scholar] [CrossRef]
- Guarderas, F.; Leavell, Y.; Sengupta, T.; Zhukova, M.; Megraw, T.L. Assessment of Chicken-Egg Membrane as a Dressing for Wound Healing. Adv. Ski. Wound Care 2016, 29, 131–134. [Google Scholar] [CrossRef]
- Adamia, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Brand, F.; Dautzenberg, H. Structural Analysis in Interpolyelectrolyte Complex Formation of Sodium Poly(styrenesulfonate) and Diallyldimethylammonium Chloride−Acrylamide Copolymers by Viscometry. Langmuir 1997, 13, 2905–2910. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Kumashiro, Y.; Yamato, M.; Okano, T. Cell Attachment–Detachment Control on Temperature-Responsive Thin Surfaces for Novel Tissue Engineering. Ann. Biomed. Eng. 2010, 38, 1977–1988. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): A model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Nguyen, L.T.B.; Odeleye, A.O.O.; Chui, C.; Baudequin, T.; Cui, Z.; Ye, H. Development of thermo-responsive polycaprolactone macrocarriers conjugated with Poly(N-isopropyl acrylamide) for cell culture. Sci. Rep. 2019, 9, 3477. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.E. The eggshell: Strength, structure and function. Br. Poult. Sci. 2010, 51 (Suppl. S1), 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, F.G.; Troncoso, O.P.; Piaggio, F.; Hijar, A. Structure-property relationships of a biopolymer network: The eggshell membrane. Acta Biomater. 2010, 6, 3687–3693. [Google Scholar] [CrossRef]
- Baláž, M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef]
- Bellairs, R.; Boyde, A. Scanning electron microscopy of the shell membranes of the hen’s egg. Z. Zellforsch Mikrosk. Anat 1969, 96, 237–249. [Google Scholar] [CrossRef]
- Gupta, K.C.; Khandekar, K. Temperature-responsive cellulose by ceric(IV) ion-initiated graft copolymerization of N-isopropylacrylamide. Biomacromolecules 2003, 4, 758–765. [Google Scholar] [CrossRef]
- Buehler, M.J.; Wong, S.Y. Entropic elasticity controls nanomechanics of single tropocollagen molecules. Biophys. J. 2007, 93, 37–43. [Google Scholar] [CrossRef]
- Daxer, A.; Fratzl, P. Erratum: Collagen fibril orientation in the human corneal stroma and its implication in keratoconus (Investigate Ophthalmology and Visual Science. Investig. Ophthalmol. Vis. Sci. 1997, 38, 121–129. [Google Scholar]
- Duboeuf, F.; Liebgott, H.; Basarab, A.; Brusseau, E.; Delachartre, P.; Vray, D. Static mechanical assessment of elastic Young’s modulus of tissue mimicking materials used for medical imaging. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Lyon, France, 22–26 August 2007. [Google Scholar]
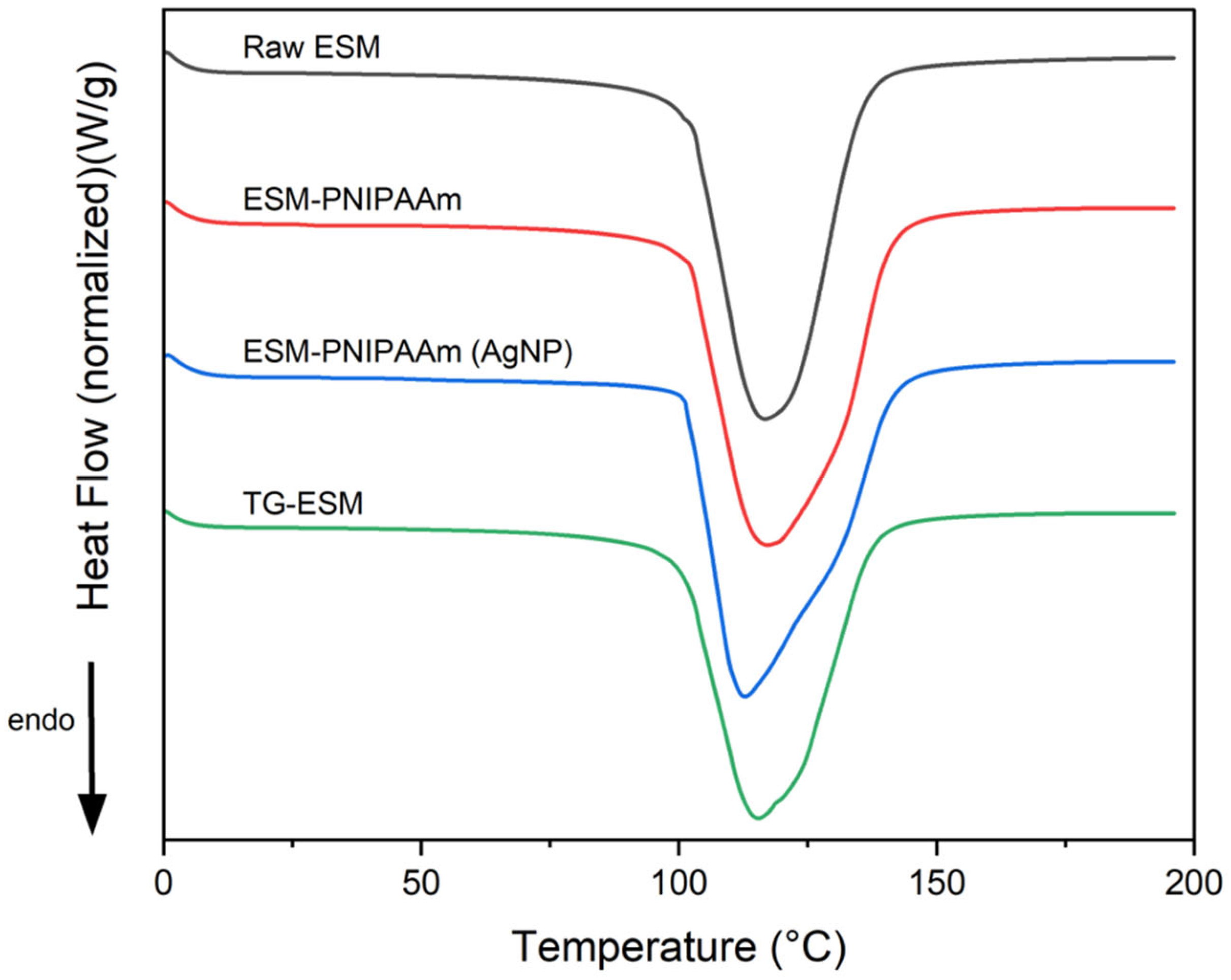


| Sample Type | % Mass Loss | Onset Temp (°C) | Peak Temp (°C) | Enthalpy (J/g) |
|---|---|---|---|---|
| Raw ESM | 73.607 ± 7.624 | 100.67 ± 0.707 | 113.817 ± 2.586 | 1838.167 ± 145.858 |
| ESM-PNIPAAm | 62.724 ± 8.874 | 99.544 ± 1.221 | 114.122 ± 2.102 | 1750.52 ± 176.214 |
| ESM-PNIPAAm (AgNP) | 67.9 ± 1.718 | 101.41 ± 2 | 111.493 ± 1.44 | 2041.933 ± 72.403 |
| TG-ESM | 73.64 ± 6.399 | 100.592 ± 1.253 | 112.2 ± 2.032 | 1745.067 ± 342.617 |
| Sample Type | % Elongation | UTS (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|
| Raw ESM | 15.248 ± 2.819 | 0.419 ± 0.035 | 2.242 ± 0.309 |
| ESM-PNIPAAm | 27.045 ± 8.352 (*) | 0.348 ± 0.124 | 2.854 ± 0.192 (*) |
| TG-ESM | 37.678 ± 1.739 (**) | 0.612 ± 0.051 (**) | 3.892 ± 0.190 (**) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briggs, E.; Mensah, R.A.; Patel, K.D.; Mandakhbayar, N.-E.; Sharifulden, N.S.; Erdogan, Z.K.; Silva, L.V.B.; Salim, K.; Kim, H.-W.; Nguyen, L.T.B.; et al. Therapeutic Application of an Ag-Nanoparticle-PNIPAAm-Modified Eggshell Membrane Construct for Dermal Regeneration and Reconstruction. Pharmaceutics 2022, 14, 2162. https://doi.org/10.3390/pharmaceutics14102162
Briggs E, Mensah RA, Patel KD, Mandakhbayar N-E, Sharifulden NS, Erdogan ZK, Silva LVB, Salim K, Kim H-W, Nguyen LTB, et al. Therapeutic Application of an Ag-Nanoparticle-PNIPAAm-Modified Eggshell Membrane Construct for Dermal Regeneration and Reconstruction. Pharmaceutics. 2022; 14(10):2162. https://doi.org/10.3390/pharmaceutics14102162
Chicago/Turabian StyleBriggs, Emily, Rosemond A. Mensah, Kapil D. Patel, Nandin-Erdene Mandakhbayar, Nik San Sharifulden, Zalike Keskin Erdogan, Lady V. Barrios Silva, Kawther Salim, Hae-Won Kim, Linh T. B. Nguyen, and et al. 2022. "Therapeutic Application of an Ag-Nanoparticle-PNIPAAm-Modified Eggshell Membrane Construct for Dermal Regeneration and Reconstruction" Pharmaceutics 14, no. 10: 2162. https://doi.org/10.3390/pharmaceutics14102162
APA StyleBriggs, E., Mensah, R. A., Patel, K. D., Mandakhbayar, N.-E., Sharifulden, N. S., Erdogan, Z. K., Silva, L. V. B., Salim, K., Kim, H.-W., Nguyen, L. T. B., & Chau, D. Y. S. (2022). Therapeutic Application of an Ag-Nanoparticle-PNIPAAm-Modified Eggshell Membrane Construct for Dermal Regeneration and Reconstruction. Pharmaceutics, 14(10), 2162. https://doi.org/10.3390/pharmaceutics14102162









