Influence of Vegetable Oils on In Vitro Performance of Lutein-Loaded Lipid Carriers for Skin Delivery: Nanostructured Lipid Carriers vs. Nanoemulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Screening
2.3. Preparation of Lutein-Free and Lutein-Loaded Nanoparticles
2.4. Particle Size Analysis
2.5. Zeta Potential Analysis
2.6. Particle Morphology
2.7. Differential Scanning Calorimetry (DSC)
2.8. The Percentage of Entrapment Efficiency
2.9. Long-Term Stability
2.10. Skin Performance Studies
2.10.1. Occlusive Effect
2.10.2. In Vitro Release Study
2.10.3. Skin Permeation Study
Skin Preparation
In Vitro Skin Permeation Study
2.11. Statistical Analysis
3. Results
3.1. Lipid Screening
3.2. Particle Size snd Size Distribution of Lutein-Free and Lutein-Loaded Nanoparticles
3.3. Particle Morphology
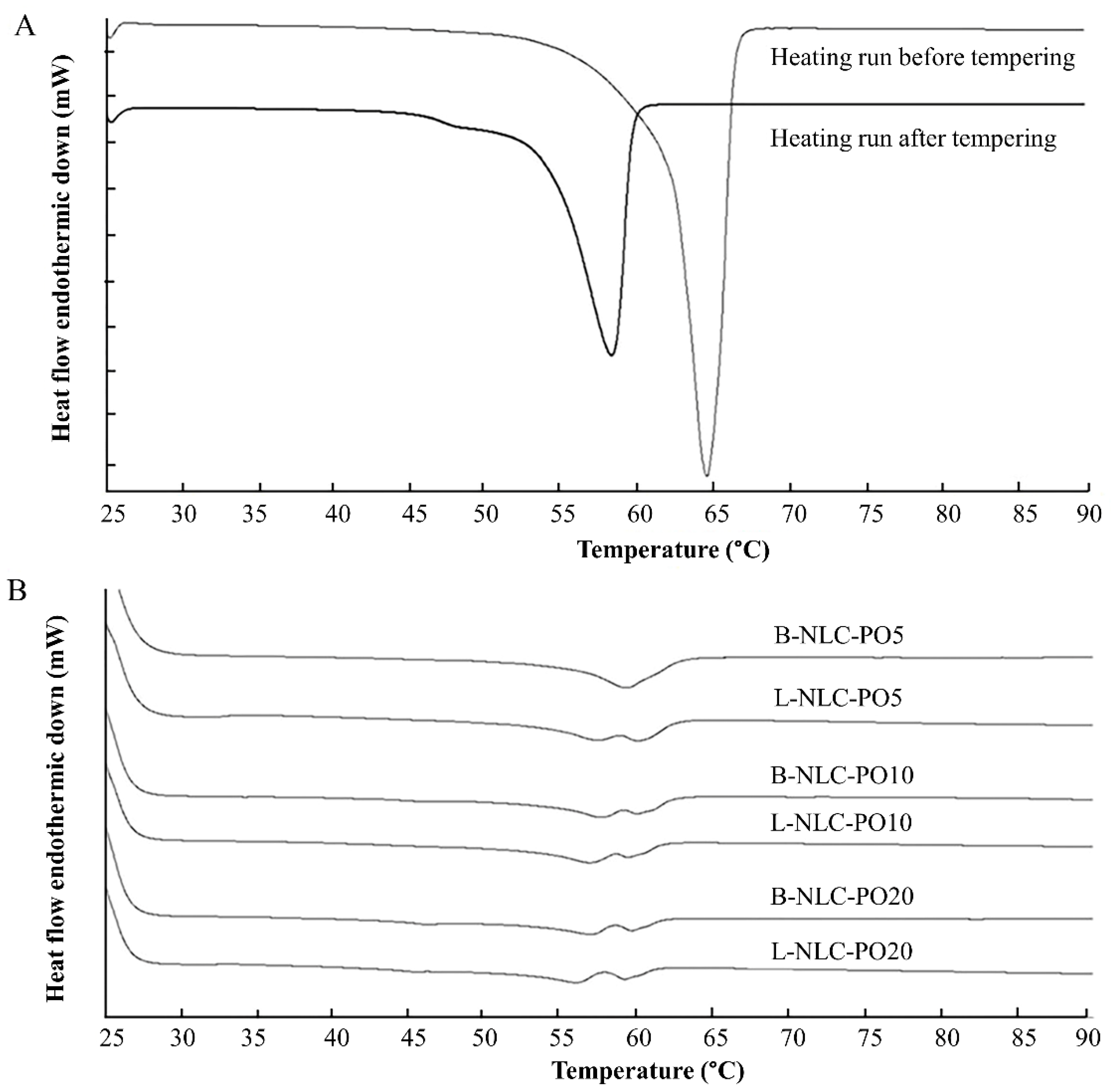
3.4. Differential Scanning Calorimetry (DSC)
3.5. The Percentage of Entrapment Efficiency (%E.E.)
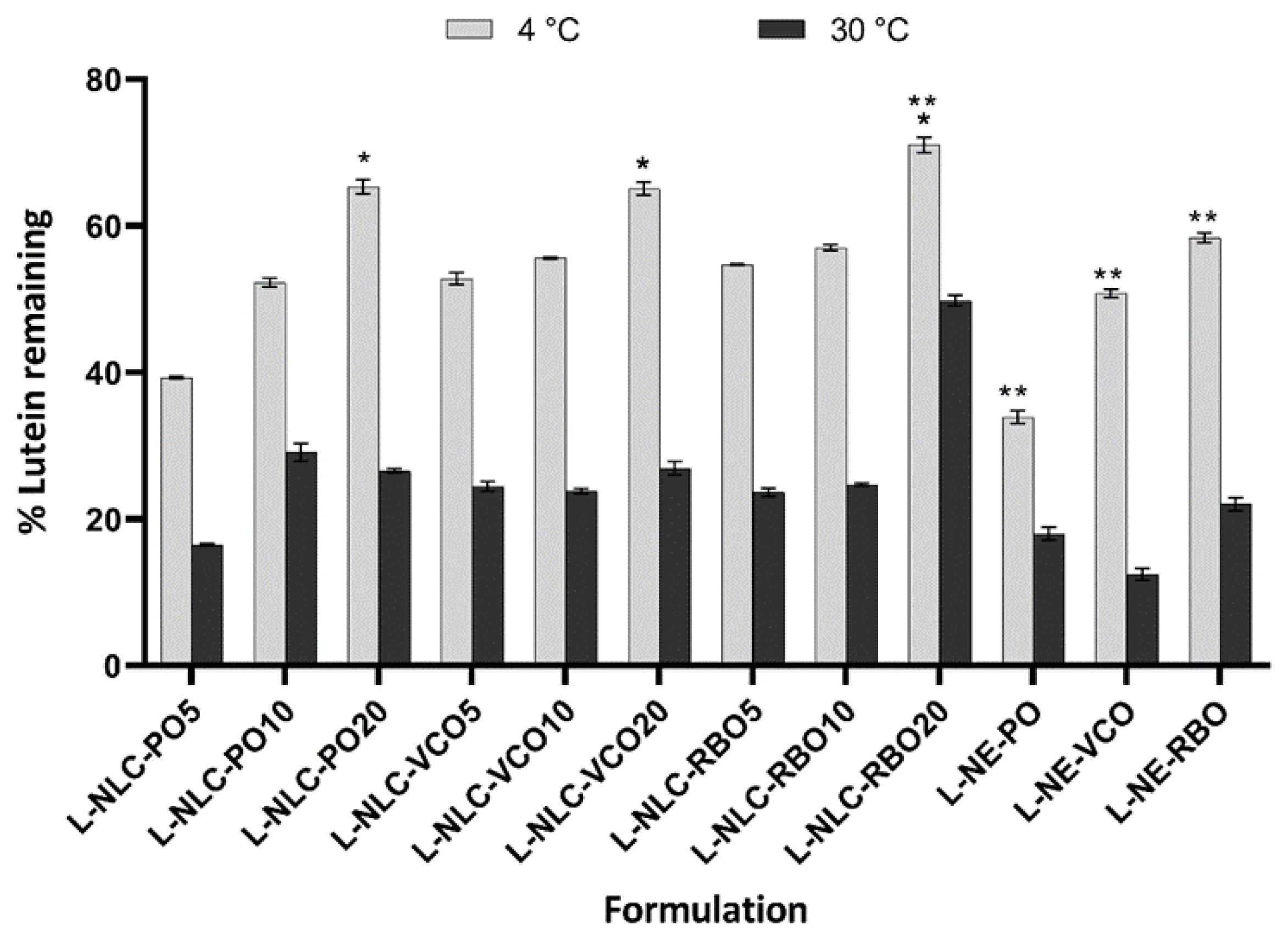
3.6. Long-Term Physical and Chemical Stability of L-NLC and L-NE
3.6.1. Physical Stability
3.6.2. Chemical Stability
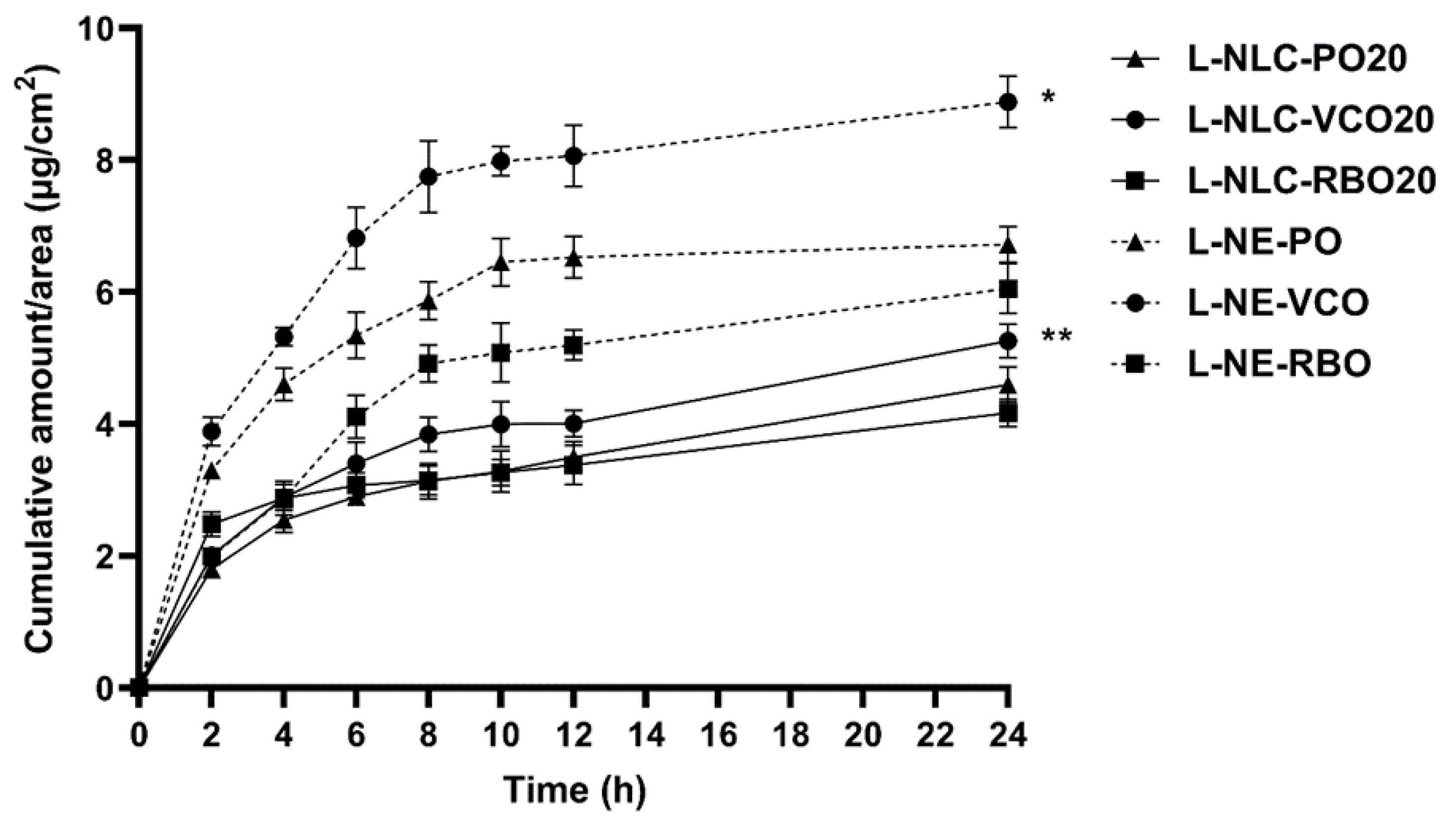
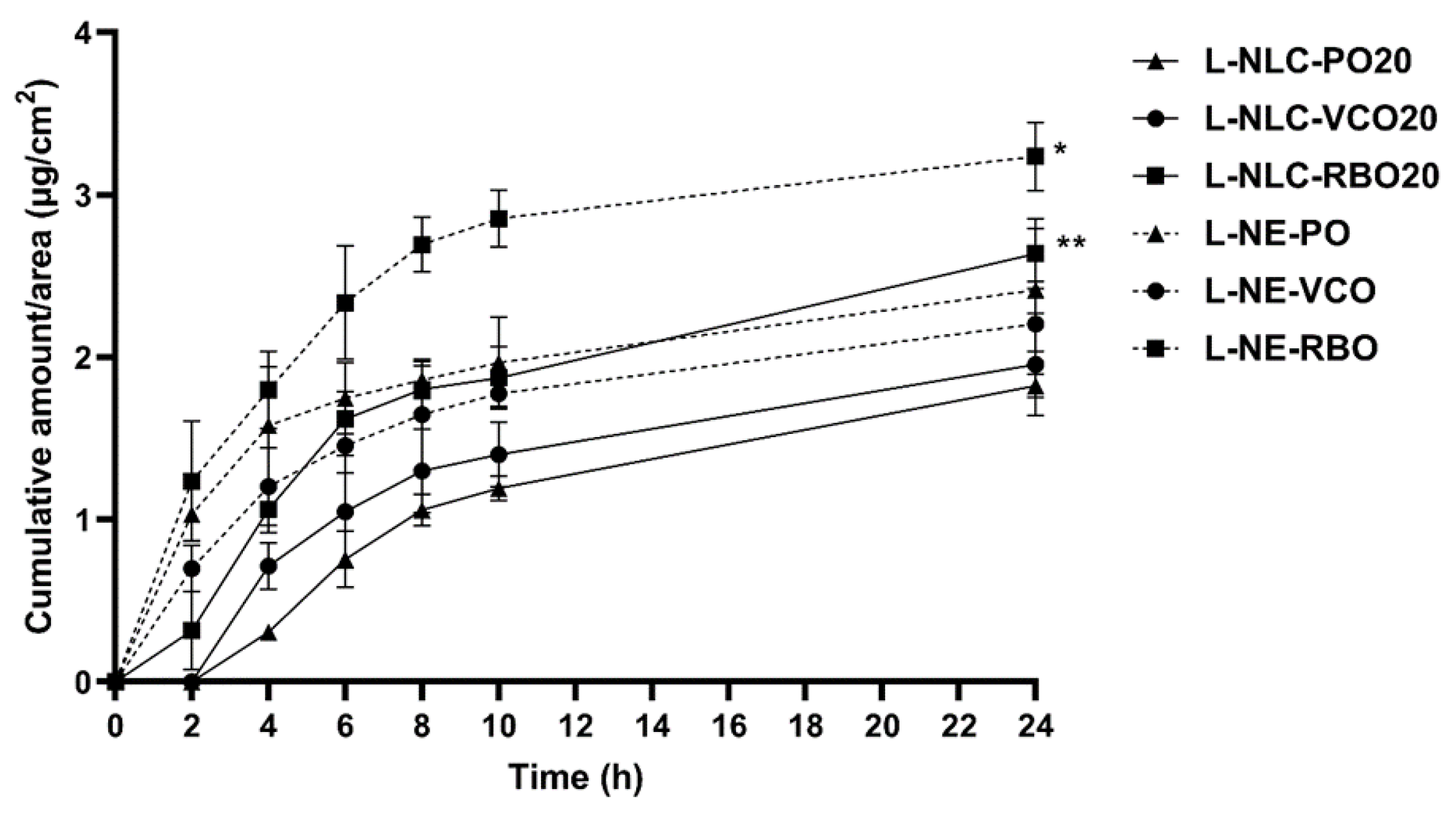
3.7. Skin Performance of Nanocarriers
3.7.1. Occlusive Effect
3.7.2. Release Study
3.7.3. Skin Permeation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Heinen, M.M.; Hughes, M.C.; Ibiebele, T.I.; Marks, G.C.; Green, A.C.; van der Pols, J.C. Intake of antioxidant nutrients and the risk of skin cancer. Eur. J. Cancer 2007, 43, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Turner, S.D.; Brautigan, D.L. Xanthophylls lutein and zeaxanthin modify gene expression and induce synthesis of hyaluronan in keratinocyte model of human skin. Biochem. Biophys. Rep. 2015, 4, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods. 2020, 66, 103771. [Google Scholar] [CrossRef]
- Shi, X.M.; Chen, F. Stability of lutein under various storage conditions. Nahrung Food 1997, 41, 38–41. [Google Scholar]
- Wang, Y.; Ye, H.; Zhou, C.; Lv, F.; Bie, X.; Lu, Z. Study on the spray-drying encapsulation of lutein in the porous starch and gelatin mixture. Eur. Food Res. Technol. 2012, 234, 157–163. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Muller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Tan, F.; Cui, H.; Bai, C.; Qin, C.; Xu, L.; Han, J. Preparation, optimization, and transcorneal permeability study of lutein-loaded solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 62, 102362. [Google Scholar] [CrossRef]
- Liu, C.-H.; Wu, C.-T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Alvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Jimenez Cartagena, C.; Alzate, L.M.; Londono-Londono, J. Microencapsulation of lutein by spray-drying: Characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.A.S.; Santos, P.P.d.; Silva, M.M.d.; Paese, K.; Guterres, S.S.; Costa, T.M.H.; Pohlmann, A.R.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Lutein-loaded lipid-core nanocapsules: Physicochemical characterization and stability evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 477–484. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Encapsulation of lutein in liposomes using supercritical carbon dioxide. Food Res. Int. (Ottawa Ont.) 2017, 100, 168–179. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed. Dermatol. 2020, 4, 12. [Google Scholar] [CrossRef]
- Masiero, J.F.; Barbosa, E.J.; de Oliveira Macedo, L.; de Souza, A.; Yukuyama, M.N.; Arantes, G.J.; Bou-Chacra, N.A. Vegetable oils in pharmaceutical and cosmetic lipid-based nanocarriers preparations. Ind. Crops. Prod. 2021, 170, 113838. [Google Scholar] [CrossRef]
- Sarkar, R.; Podder, I.; Gokhale, N.; Jagadeesan, S.; Garg, V.K. Use of vegetable oils in dermatology: An overview. Int. J. Dermatol. 2017, 56, 1080–1086. [Google Scholar] [CrossRef]
- Hewlings, S. Coconuts and health: Different chain lengths of saturated fats require different consideration. J. Cardiovasc. Dev. Dis. 2020, 7, 59. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-audom, M.; Ruksiriwanich, W.; Prom-u-Thai, C.; Sringarm, K. Comparative analysis of nutritional components and phytochemical attributes of selected Thai rice bran. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orru, S.; Buono, P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ali, J.; Baboota, S. Silymarin loaded nanostructured lipid carrier: From design and dermatokinetic study to mechanistic analysis of epidermal drug deposition enhancement. J. Mol. Liq. 2018, 255, 513–529. [Google Scholar] [CrossRef]
- Amasya, G.; Aksu, B.; Badilli, U.; Onay-Besikci, A.; Tarimci, N. QbD guided early pharmaceutical development study: Production of lipid nanoparticles by high pressure homogenization for skin cancer treatment. Int. J. Pharm. 2019, 563, 110–121. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Chantaburanan, T.; Teeranachaideekul, V.; Chantasart, D.; Jintapattanakit, A.; Junyaprasert, V.B. Effect of binary solid lipid matrix of wax and triglyceride on lipid crystallinity, drug-lipid interaction and drug release of ibuprofen-loaded solid lipid nanoparticles (SLN) for dermal delivery. J. Colloid Interface Sci. 2017, 504, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fischer, M.; Kirby, C.; Liu, R.; Zhu, H.; Zhang, H.; Chen, Y.; Sun, Y.; Zhang, L.; Tsao, R. Bioaccessibility, cellular uptake and transport of luteins and assessment of their antioxidant activities. Food Chem. 2018, 249, 66–67. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Zaera, A.M.; Isaac, V.L.B.; Corrêa, M.A.; Salgado, H.R.N. Release and permeation profiles of spray-dried chitosan microparticles containing caffeic acid. Saudi Pharm. J. 2018, 26, 410–415. [Google Scholar] [CrossRef]
- Pornputtapitak, W.; Pantakitcharoenkul, J.; Teeranachaideekul, V.; Sinthiptharakoon, K.; Sapcharoenkun, C.; Meemuk, B. Effect of oil content on physiochemical characteristics of gamma-oryzanol-loaded nanostructured lipid carriers. J. Oleo Sci. 2019, 68, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Attafi, I.M.; Ahmed, R.A.; Syed, N.K.; Sultan, M.H.; Al-Bratty, M.; Alhazmi, H.A.; Safhi, M.M.; et al. Gefitinib loaded nanostructured lipid carriers: Characterization, evaluation and anti-human colon cancer activity in vitro. Drug Deliv. 2020, 27, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Makoni, P.A.; Kasongo, K.W.; Walker, R.B. Preformulation studies of efavirenz with lipid excipients using thermal and spectroscopic techniques. Pharmazie 2020, 75, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Teeranachaideekul, V.; Muller, R.H.; Junyaprasert, V.B. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)-effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007, 340, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Souto, E.B.; Muller, R.H. Physicochemical investigations on the structure of drug-free and drug-loaded solid lipid nanoparticles (SLN) by means of DSC and 1H NMR. Pharmazie 2005, 60, 508–513. [Google Scholar] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and comparison of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as vectors to develop hydrochlorothiazide effective and safe pediatric oral liquid formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Borges, G.; Prazeres, P.; Malachias, A.; Yoshida, M.; Vilela, J.; Silva, A.; Oliveira, M.; Gomes, D.; Andrade, M.; Souza-Fagundes, E.; et al. Nanostructured lipid carriers as a novel tool to deliver sclareol: Physicochemical characterisation and evaluation in human cancer cell lines. Braz. J. Pharm. Sci. 2021, 57, 1–15. [Google Scholar] [CrossRef]
- Haila, K.M.; Lievonen, S.M.; Heinonen, M.I. Effects of lutein, lycopene, annatto, and γ-tocopherol on autoxidation of triglycerides. J. Agric. Food Chem. 1996, 44, 2096–2100. [Google Scholar] [CrossRef]
- Ong, A.S.; Goh, S.H. Palm oil: A healthful and cost-effective dietary component. Food Nutr. Bull. 2002, 23, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Krishna, A.G.G.; Gaurav, R.; Singh, B.A.; Kumar, P.K.P.; Chandrashekar, P. Coconut oil: Chemistry, production and its applications—A review. Indian Cocon. J. 2010, 53, 15–27. [Google Scholar]
- Lavecchia, R.; Zuorro, A. Shelf stability of lutein from marigold (Tagetes erecta L.) flowers in vegetable oils. Chem. Eng. Trans. 2008, 14, 199–204. [Google Scholar]
- Teeranachaideekul, V.; Chantaburanan, T.; Junyaprasert, V.B. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J. Drug Deliv. Sci. Technol. 2017, 39, 300–307. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Chellian, J. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5067–5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif Makhmal Zadeh, B.; Niro, H.; Rahim, F.; Esfahani, G. Ocular delivery system for propranolol hydrochloride based on nanostructured lipid carrier. Sci. Pharm. 2018, 86, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.A.; Li, S.K. Efficiency of fatty acids as chemical penetration enhancers: Mechanisms and structure enhancement relationship. Pharm. Res. 2010, 27, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Tavazzi, S.; Rossi, A.; Picarazzi, S.; Ascagni, M.; Farris, S.; Borghesi, A. Polymer-interaction driven diffusion of eyeshadow in soft contact lenses. Cont. Lens Anterior Eye 2017, 40, 335–339. [Google Scholar] [CrossRef]




| Formulation | GMS | PO | VCO | RBO | P188 | 20% Lutein | Water q.s. |
|---|---|---|---|---|---|---|---|
| B-NLC-PO5 | 9.5 | 0.5 | - | - | 5.0 | - | 100.0 |
| B-NLC-PO10 | 9.0 | 1.0 | - | - | 5.0 | - | 100.0 |
| B-NLC-PO20 | 8.0 | 2.0 | - | - | 5.0 | - | 100.0 |
| B-NLC-VCO5 | 9.5 | - | 0.5 | - | 5.0 | - | 100.0 |
| B-NLC-VCO10 | 9.0 | - | 1.0 | - | 5.0 | - | 100.0 |
| B-NLC-VCO20 | 8.0 | - | 2.0 | - | 5.0 | - | 100.0 |
| B-NLC-RBO5 | 9.5 | - | - | 0.5 | 5.0 | - | 100.0 |
| B-NLC-RBO10 | 9.0 | - | - | 1.0 | 5.0 | - | 100.0 |
| B-NLC-RBO20 | 8.0 | - | - | 2.0 | 5.0 | - | 100.0 |
| B-NE-PO | - | 10.0 | - | - | 5.0 | - | 100.0 |
| B-NE-VCO | - | - | 10.0 | - | 5.0 | - | 100.0 |
| B-NE-RBO | - | - | - | 10.0 | 5.0 | - | 100.0 |
| L-NLC-PO5 | 9.025 | 0.475 | - | - | 5.0 | 0.5 | 100.0 |
| L-NLC-PO10 | 8.550 | 0.950 | - | - | 5.0 | 0.5 | 100.0 |
| L-NLC-PO20 | 7.600 | 1.900 | - | - | 5.0 | 0.5 | 100.0 |
| L-NLC-VCO5 | 9.025 | - | 0.475 | - | 5.0 | 0.5 | 100.0 |
| L-NLC-VCO10 | 8.550 | - | 0.950 | - | 5.0 | 0.5 | 100.0 |
| L-NLC-VCO20 | 7.600 | - | 1.900 | - | 5.0 | 0.5 | 100.0 |
| L-NLC-RBO5 | 9.025 | - | - | 0.475 | 5.0 | 0.5 | 100.0 |
| L-NLC-RBO10 | 8.550 | - | - | 0.950 | 5.0 | 0.5 | 100.0 |
| L-NLC-RBO20 | 7.600 | - | - | 1.900 | 5.0 | 0.5 | 100.0 |
| L-NE-PO | - | 9.5 | - | - | 5.0 | 0.5 | 100.0 |
| L-NE-VCO | - | - | 9.5 | - | 5.0 | 0.5 | 100.0 |
| L-NE-RBO | - | - | - | 9.5 | 5.0 | 0.5 | 100.0 |
| Formulations | d10% | d50% | d90% | Span | Formulations | d10% | d50% | d90% | Span |
|---|---|---|---|---|---|---|---|---|---|
| B-NLC-PO5 | 0.132 | 0.171 | 0.232 | 0.587 | L-NLC-PO5 | 0.132 | 0.171 | 0.233 | 0.590 |
| B-NLC-PO10 | 0.132 | 0.171 | 0.232 | 0.584 | L-NLC-PO10 | 0.132 | 0.171 | 0.232 | 0.586 |
| B-NLC-PO20 | 0.132 | 0.170 | 0.231 | 0.582 | L-NLC-PO20 | 0.132 | 0.170 | 0.231 | 0.583 |
| B-NLC-VCO5 | 0.135 | 0.178 | 0.244 | 0.613 | L-NLC-VCO5 | 0.138 | 0.184 | 0.253 | 0.626 |
| B-NLC-VCO10 | 0.132 | 0.170 | 0.230 | 0.578 | L-NLC-VCO10 | 0.137 | 0.182 | 0.250 | 0.624 |
| B-NLC-VCO20 | 0.132 | 0.170 | 0.231 | 0.581 | L-NLC-VCO20 | 0.132 | 0.170 | 0.231 | 0.580 |
| B-NLC-RBO5 | 0.132 | 0.171 | 0.232 | 0.586 | L-NLC-RBO5 | 0.132 | 0.171 | 0.233 | 0.589 |
| B-NLC-RBO10 | 0.132 | 0.171 | 0.233 | 0.588 | L-NLC-RBO10 | 0.140 | 0.187 | 0.258 | 0.635 |
| B-NLC-RBO20 | 0.132 | 0.170 | 0.231 | 0.584 | L-NLC-RBO20 | 0.135 | 0.187 | 0.271 | 0.727 |
| B-NE-PO | 0.142 | 0.207 | 0.250 | 0.761 | L-NE-PO | 0.145 | 0.198 | 0.280 | 0.682 |
| B-NE-VCO | 0.135 | 0.178 | 0.245 | 0.614 | L-NE-VCO | 0.133 | 0.172 | 0.233 | 0.583 |
| B-NE-RBO | 0.142 | 0.193 | 0.271 | 0.669 | L-NE-RBO | 0.138 | 0.185 | 0.259 | 0.652 |
| Physical Mixtures | Onset (°C) | MP (°C) | CI (%) | Formulations | Onset (°C) | MP (°C) | CI (%) | Formulations | Onset (°C) | MP (°C) | CI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GMS/PO (95:5) | 52.81 | 57.74 | 87.80 | B-NLC-PO5 | 54.73 | 58.98 | 85.64 | L-NLC-PO5 | 56.79 | 59.73 | 73.89 |
| GMS/PO (90:10) | 52.02 | 57.84 | 85.13 | B-NLC-PO10 | 53.16 | 57.29 | 80.47 | L-NLC-PO10 | 51.85 | 56.58 | 66.46 |
| GMS/PO (80:20) | 50.83 | 56.94 | 72.65 | B-NLC-PO20 | 51.56 | 56.68 | 60.81 | L-NLC-PO20 | 50.86 | 55.69 | 50.16 |
| GMS/VCO (95:5) | 52.77 | 57.70 | 92.87 | B-NLC-VCO5 | 53.59 | 58.05 | 88.32 | L-NLC-VCO5 | 51.84 | 56.25 | 62.79 |
| GMS/VCO (90:10) | 51.30 | 57.44 | 82.31 | B-NLC-VCO10 | 52.58 | 57.10 | 85.78 | L-NLC-VCO10 | 50.98 | 55.73 | 59.21 |
| GMS/VCO (80:20) | 49.93 | 56.05 | 79.12 | B-NLC-VCO20 | 51.17 | 55.84 | 61.96 | L-NLC-VCO20 | 50.47 | 55.42 | 47.41 |
| GMS/RBO (95:5) | 52.90 | 58.17 | 91.33 | B-NLC-RBO5 | 54.15 | 58.08 | 80.95 | L-NLC-RBO5 | 51.98 | 56.25 | 60.59 |
| GMS/RBO (90:10) | 51.66 | 57.44 | 85.01 | B-NLC-RBO10 | 52.52 | 56.84 | 78.90 | L-NLC-RBO10 | 51.57 | 55.82 | 54.87 |
| GMS/RBO (80:20) | 50.75 | 56.96 | 75.04 | B-NLC-RBO20 | 52.51 | 56.97 | 45.78 | L-NLC-RBO20 | 50.06 | 55.40 | 41.01 |
| Bulk GMS (tempering) | 54.06 | 58.14 | 100.00 |
| Formulations | Entrapment Efficiency (%) | Coefficient of Determination (r2) | ||
|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi’s Model | ||
| L-NLC-PO5 | 95.67 ± 1.37 | |||
| L-NLC-PO10 | 97.05 ± 1.11 | |||
| L-NLC-PO20 | 98.57 ± 2.21 | 0.8813 | 0.7494 | 0.9663 |
| L-NLC-VCO5 | 95.82 ± 1.04 | |||
| L-NLC-VCO10 | 97.39 ± 1.05 | |||
| L-NLC-VCO20 | 98.71 ± 2.16 | 0.8203 | 0.6797 | 0.9377 |
| L-NLC-RBO5 | 95.67 ± 1.47 | |||
| L-NLC-RBO10 | 97.44 ± 2.30 | |||
| L-NLC-RBO20 | 98.02 ± 2.36 | 0.9333 | 0.8798 | 0.9743 |
| L-NE-PO | 99.43 ± 3.05 | 0.5302 | 0.4670 | 0.7368 |
| L-NE-VCO | 97.57 ± 2.51 | 0.5857 | 0.5019 | 0.7831 |
| L-NE-RBO | 98.07 ± 1.32 | 0.6541 | 0.5358 | 0.8383 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teeranachaideekul, V.; Boribalnukul, P.; Morakul, B.; Junyaprasert, V.B. Influence of Vegetable Oils on In Vitro Performance of Lutein-Loaded Lipid Carriers for Skin Delivery: Nanostructured Lipid Carriers vs. Nanoemulsions. Pharmaceutics 2022, 14, 2160. https://doi.org/10.3390/pharmaceutics14102160
Teeranachaideekul V, Boribalnukul P, Morakul B, Junyaprasert VB. Influence of Vegetable Oils on In Vitro Performance of Lutein-Loaded Lipid Carriers for Skin Delivery: Nanostructured Lipid Carriers vs. Nanoemulsions. Pharmaceutics. 2022; 14(10):2160. https://doi.org/10.3390/pharmaceutics14102160
Chicago/Turabian StyleTeeranachaideekul, Veerawat, Putita Boribalnukul, Boontida Morakul, and Varaporn Buraphacheep Junyaprasert. 2022. "Influence of Vegetable Oils on In Vitro Performance of Lutein-Loaded Lipid Carriers for Skin Delivery: Nanostructured Lipid Carriers vs. Nanoemulsions" Pharmaceutics 14, no. 10: 2160. https://doi.org/10.3390/pharmaceutics14102160
APA StyleTeeranachaideekul, V., Boribalnukul, P., Morakul, B., & Junyaprasert, V. B. (2022). Influence of Vegetable Oils on In Vitro Performance of Lutein-Loaded Lipid Carriers for Skin Delivery: Nanostructured Lipid Carriers vs. Nanoemulsions. Pharmaceutics, 14(10), 2160. https://doi.org/10.3390/pharmaceutics14102160








