Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells
Abstract
1. Introduction
2. Heparin and Its Immunomodulatory and Antitumor Effects
- -
- Binding of free growth factors or angiogenesis;
- -
- Suppression of lymphatic vessel formation;
- -
- Inhibition of tumor cell attachment to vascular endothelium;
- -
- Control of multidrug resistance (MDR) of tumor cells.
3. Multiple Drug Resistance
- (1)
- Increase in drug release from cells by membrane carriers, the major carriers being ATP-binding cassettes (ABC) [44];
- (2)
- Reducing drug uptake by influx vectors such as solute transporters [45];
- (3)
- Acceleration of drug metabolism, including elimination by glutathione S-transferase and cytochrome P450 enzymes [46];
- (4)
- Blockage of apoptotic pathways by increased expression of B-cell lymphoma family Bcl proteins or mutations in the p53 pathway [47];
- (5)
- Increased adaptability through epigenetic and miRNA regulation [48];
- (6)
- Mutation of targets or feedback activation of other targets and signaling pathways [49];
- (7)
- Chemoresistance caused by changes in the microenvironment, such as response to hypoxia and regulation of cancer stem cells [50].
4. MDR Genes and Membrane Carriers
4.1. MDR1/P-gp/ABCB1
4.2. MRP1/ABCC1
4.3. MRP7/ABCC10
4.4. BCRP/ABCG2
5. Immune Cells of the Tumor Microenvironment in the Osteosarcoma Infiltrate
5.1. Macrophages
5.2. CD8+ T Cells
5.3. CD4+ T Lymphocytes
5.4. Treg Cells
5.5. Th1.17 Cells
5.6. Tfh Cells (T Follicular Helper Cells)
5.7. NK Cells
5.8. γδ-T Cells
- -
- They produce angiogenic factors that promote tumor growth;
- -
5.9. B Lymphocytes
5.10. Mast Cells
5.11. Dendritic Cells
5.12. Cancer Stem Cells (CSCs)
5.13. ABC-Transporters in CSCs
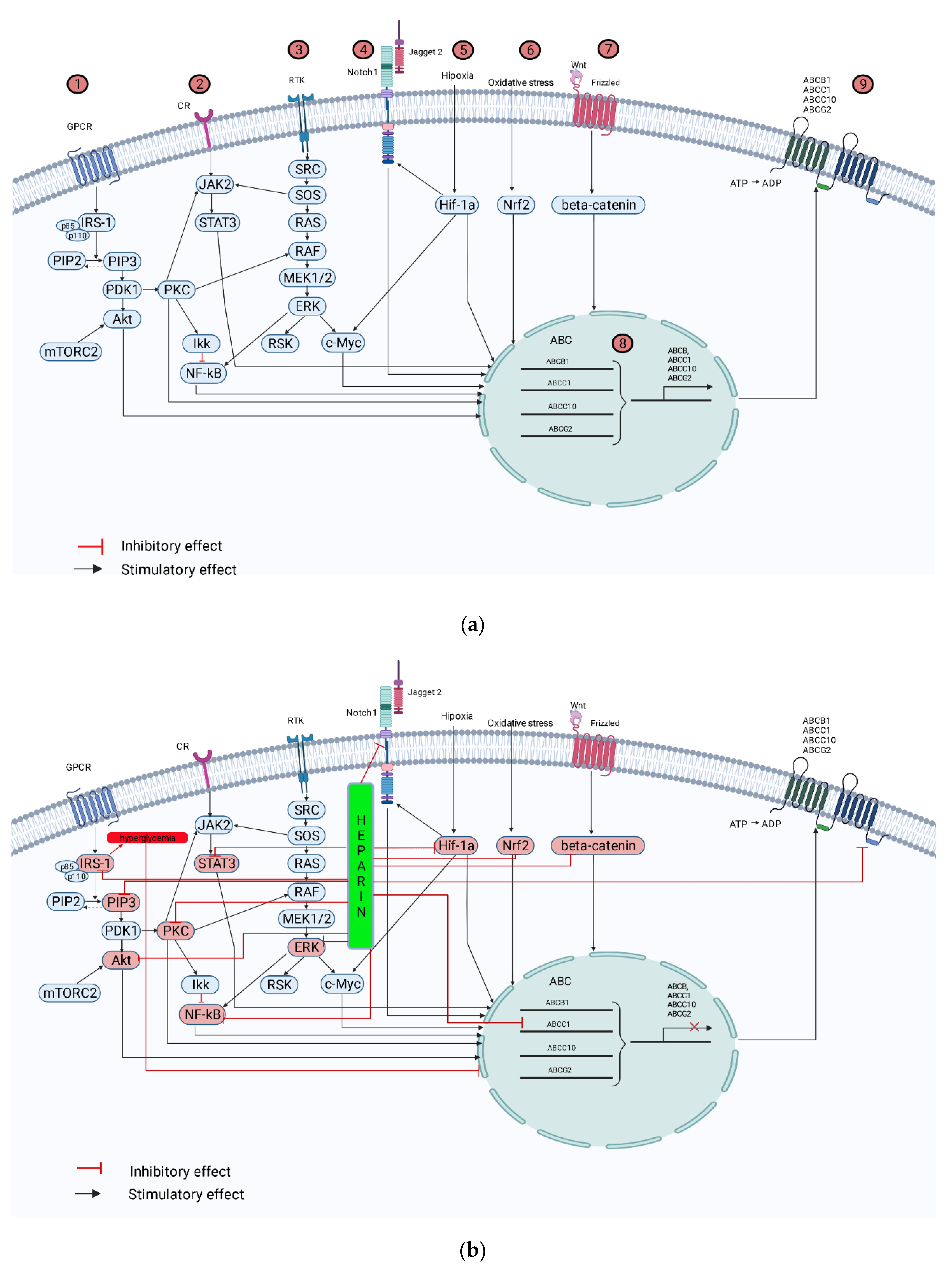
6. Molecular Mechanisms of MDR Development and Heparin Effects in OS Microenvironment
6.1. MDR of OS and Heparin
- -
- Promotes proliferation of OS cells, induces G0/G1-S-G2/M phase transition, and increases resistance of human OS cells to drug therapy;
- -
- Increases the expression of Notch1 and MRP1 proteins in human OS cells.
6.2. HIF-1α
6.3. Notch1
6.4. TGF-β Family
6.5. Nrf2
6.6. NF-kB
6.7. STAT3
6.8. Wnt/β-Catenin Signaling Pathway
6.9. CCN2 Gene
6.10. Other Signal Transduction Pathways
7. Cells of the Immunological Microenvironment of OS and Heparin
7.1. Macrophages and Heparin
7.2. T Lymphocytes and Heparin
7.3. NK Cells and Heparin
8. Prospects for Heparin-Containing Medical Constructs
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litvinova, L.S.; Yurova, K.A.; Khaziakhmatova, O.G.; Khlusova, M.Y.; Malashchenko, V.V.; Shunkin, E.O.; Todosenko, N.M.; Norkin, I.K.; Ivanov, P.A.; Khlusov, I.A. Osteogenic and Angiogenic Properties of Heparin as a System for Delivery of Biomolecules for Bone Bioengineering: A Brief Critical Review. Biochem. Mosc. Suppl. Ser. B 2021, 15, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Atallah, J.; Khachfe, H.H.; Berro, J.; Assi, H.I. The use of heparin and heparin-like molecules in cancer treatment: A review. Cancer Treat. Res. Commun. 2020, 24, 100192. [Google Scholar] [CrossRef] [PubMed]
- Khachfe, H.H.; Salhab, H.A.; Fares, M.Y.; Khachfe, H.M. Probing the Colorectal Cancer Incidence in Lebanon: An 11-Year Epidemiological Study. J. Gastrointest. Canc. 2020, 51, 805–812. [Google Scholar] [CrossRef]
- Jo, V.Y.; Fletcher, C.D.M. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.F.; Brown, H.K.; Heymann, D. Drugs in early clinical development for the treatment of osteosarcoma. Expert Opin. Investig. Drugs 2016, 25, 1265–1280. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex but Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef]
- Haddox, C.L.; Han, G.; Anijar, L.; Binitie, O.; Letson, G.D.; Bui, M.M.; Reed, D.R. Osteosarcoma in Pediatric Patients and Young Adults: A Single Institution Retrospective Review of Presentation, Therapy, and Outcome. Sarcoma 2014, 2014, 402509. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, S.; Bennett, S.; Tang, H.; Song, D.; Wood, D.; Zhan, X.; Xu, J. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021, 54. [Google Scholar] [CrossRef]
- Liu, R.; Hu, Y.; Liu, T.; Wang, Y. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of osteosarcoma cancer. BMC Cancer 2021, 21, 1345. [Google Scholar] [CrossRef]
- Xu, S.; Gong, Y.; Yin, Y.; Xing, H.; Zhang, N. The multiple function of long noncoding RNAs in osteosarcoma progression, drug resistance and prognosis. Biomed. Pharmacother. 2020, 127, 110141. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Federman, N.; Eilber, F.R.; Nelson, S.D.; Eckardt, J.J.; Eilber, F.C.; Tap, W.D. Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. Cancer 2012, 118, 5888–5893. [Google Scholar] [CrossRef] [PubMed]
- Kuijjer, M.L.; Hogendoorn, P.C.W.; Cleton-Jansen, A.M. Genome-wide analyses on high-grade osteosarcoma: Making sense of a genomically most unstable tumor: Systems biology to study osteosarcoma. Int. J. Cancer 2013, 133, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, J.; Jiang, S.; Zhao, G.; Lu, J.; Jiang, B. The Effect of miR-138 on the Function of Follicular Helper T Cells and the Differentiation of B Cells in Osteosarcoma. Comput. Math. Methods Med. 2021, 2021, 2057782. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Song, J.; Lin, K.; Chen, F.; Ma, X.; Jiang, J.; Li, F. Survival analysis of patients with metastatic osteosarcoma: A Surveillance, Epidemiology, and End Results population-based study. Int. Orthop. (SICOT) 2019, 43, 1983–1991. [Google Scholar] [CrossRef]
- Hegyi, M.; Arany, A.; Semsei, A.F.; Csordas, K.; Eipel, O.; Gezsi, A.; Kutszegi, N.; Csoka, M.; Muller, J.; Erdelyi, D.J.; et al. Pharmacogenetic analysis of high-dose methotrexate treatment in children with osteosarcoma. Oncotarget 2017, 8, 9388–9398. [Google Scholar] [CrossRef]
- Yu, W.; Tang, L.; Lin, F.; Li, D.; Wang, J.; Yang, Y.; Shen, Z. Stereotactic radiosurgery, a potential alternative treatment for pulmonary metastases from osteosarcoma. Int. J. Oncol. 2014, 44, 1091–1098. [Google Scholar] [CrossRef]
- Daw, N.C.; Chou, A.J.; Jaffe, N.; Rao, B.N.; Billups, C.A.; Rodriguez-Galindo, C.; Meyers, P.A.; Huh, W.W. Recurrent osteosarcoma with a single pulmonary metastasis: A multi-institutional review. Br. J. Cancer 2015, 112, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, J.Y.; Choi, S.; Lee, H.; Morales, L.D.; Koh, J.T.; Kim, S.H.; Choi, Y.D.; Choi, C.; Slaga, T.J.; et al. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy 2017, 13, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Fantoni, L.; Casotti, C.; Riganti, C.; Serra, M. Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers 2021, 13, 2878. [Google Scholar] [CrossRef] [PubMed]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Le, T.; Su, S.; Shahriyari, L. Immune classification of osteosarcoma. Math. Biosci. Eng. 2021, 18, 1879–1897. [Google Scholar] [CrossRef]
- Beurskens, D.M.H.; Huckriede, J.P.; Schrijver, R.; Hemker, H.C.; Reutelingsperger, C.P.; Nicolaes, G.A.F. The Anticoagulant and Nonanticoagulant Properties of Heparin. Thromb. Haemost. 2020, 120, 1371–1383. [Google Scholar] [CrossRef]
- Bounameaux, H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc. Med. 1998, 3, 41–46. [Google Scholar] [CrossRef]
- Meneghetti, M.C.Z.; Hughes, A.J.; Rudd, T.R.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal Structure of a Ternary FGF-FGFR-Heparin Complex Reveals a Dual Role for Heparin in FGFR Binding and Dimerization. Mol. Cell 2000, 6, 743–750. [Google Scholar] [CrossRef]
- Dudás, J.; Ramadori, G.; Knittel, T.; Neubauer, K.; Raddatz, D.; Egedy, K.; Kovalszky, I. Effect of heparin and liver heparan sulphate on interaction of HepG2-derived transcription factors and their cis-acting elements: Altered potential of hepatocellular carcinoma heparan sulphate. Biochem. J. 2000, 350, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Laner-Plamberger, S.; Oeller, M.; Rohde, E.; Schallmoser, K.; Strunk, D. Heparin and Derivatives for Advanced Cell Therapies. Int. J. Mol. Sci. 2021, 22, 12041. [Google Scholar] [CrossRef] [PubMed]
- Holley, R.J.; Meade, K.A.; Merry, C.L.R. Using embryonic stem cells to understand how glycosaminoglycans regulate differentiation. Biochem. Soc. Trans. 2014, 42, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Simann, M.; Schneider, V.; Le Blanc, S.; Dotterweich, J.; Zehe, V.; Krug, M.; Jakob, F.; Schilling, T.; Schütze, N. Heparin affects human bone marrow stromal cell fate: Promoting osteogenic and reducing adipogenic differentiation and conversion. Bone 2015, 78, 102–113. [Google Scholar] [CrossRef]
- Li, B.; Lin, Z.; Mitsi, M.; Zhang, Y.; Vogel, V. Heparin-induced conformational changes of fibronectin within the extracellular matrix promote hMSC osteogenic differentiation. Biomater. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.; Kim, M.Y.; Shin, H.I.; Jeong, D. Suppressive effect of syndecan ectodomains and N-desulfated heparins on osteoclastogenesis via direct binding to macrophage-colony stimulating factor. Cell Death Dis. 2018, 9, 1119. [Google Scholar] [CrossRef]
- Ma, S.N.; Mao, Z.X.; Wu, Y.; Liang, M.X.; Wang, D.D.; Chen, X.; Chang, P.A.; Zhang, W.; Tang, J.H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and insights into drug resistance in cancer. Front. Pharm. 2013, 4, 28. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef] [PubMed]
- Lippert, T.H.; Ruoff, H.J.; Volm, M. Current Status of Methods to Assess Cancer Drug Resistance. Int. J. Med. Sci. 2011, 8, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Chun, S.Y.; Kwon, Y.S.; Nam, K.S.; Kim, S. Lapatinib enhances the cytotoxic effects of doxorubicin in MCF-7 tumorspheres by inhibiting the drug efflux function of ABC transporters. Biomed. Pharmacother. 2015, 72, 37–43. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.M.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; Hoffmaster, K.A.; et al. The International Transporter Consortium. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Turella, P.; Dupuis, M.L.; Forini, O.; Ciriolo, M.R.; Cianfriglia, M.; Pezzola, S.; Federici, G.; Caccuri, A.M. 6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol, a specific glutathione S -transferase inhibitor, overcomes the multidrug resistance (MDR)-associated protein 1–mediated MDR in small cell lung cancer. Mol. Cancer Ther. 2008, 7, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.S.; Medeiros, L.J.; Lai, R.; Butch, A.W.; McCourty, A.; Kelly, K.; Brynes, R.K. DNA Topoisomerase IIα in Multiple Myeloma: A Marker of Cell Proliferation and Not Drug Resistance. Mod. Pathol. 2001, 14, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, B.B. Friend or foe: The role of microRNA in chemotherapy resistance. Acta Pharm. Sin. 2013, 34, 870–879. [Google Scholar] [CrossRef]
- Camidge, D.R.; Pao, W.; Sequist, L.V. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat. Rev. Clin. Oncol. 2014, 11, 473–481. [Google Scholar] [CrossRef]
- Milane, L.; Duan, Z.; Amiji, M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011, 11, 3. [Google Scholar] [CrossRef]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Zhang, D.M.; Degenhardt, K.; Chen, Z.S. Autophagy and Transporter-Based Multi-Drug Resistance. Cells 2012, 1, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Bossennec, M.; Di Roio, A.; Caux, C.; Ménétrier-Caux, C. MDR1 in immunity: Friend or foe? OncoImmunology 2018, 7, e1499388. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Noguchi, K.; Sugimoto, Y. Regulations of P-Glycoprotein/ABCB1/ MDR1 in Human Cancer Cells. New J. Sci. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Bakos, É.; Homolya, L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Pflug. Arch-Eur. J. Physiol. 2007, 453, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Drach, J.; Zhao, S.; Drach, D.; Körbling, M.; Engel, H.; Andreeff, M. Expression of MDR1 by Normal Bone Marrow Cells and its Implication for Leukemic Hematopoiesis. Leuk. Lymphoma 1995, 16, 419–424. [Google Scholar] [CrossRef]
- Zhang, G.; Miao, F.; Xu, J.; Wang, R. Mesenchymal stem cells from bone marrow regulate invasion and drug resistance of multiple myeloma cells by secreting chemokine CXCL13. Bosn. J. Basic Med. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Sugiu, K.; Tazawa, H.; Hasei, J.; Yamakawa, Y.; Omori, T.; Komatsubara, T.; Mochizuki, Y.; Kondo, H.; Osaki, S.; Fujiwara, T.; et al. Oncolytic virotherapy reverses chemoresistance in osteosarcoma by suppressing MDR1 expression. Cancer Chemother. Pharm. 2021, 88, 513–524. [Google Scholar] [CrossRef]
- Fan, G.T.; Ling, Z.H.; He, Z.W.; Wu, S.J.; Zhou, G.X. Suppressing CHD1L reduces the proliferation and chemoresistance in osteosarcoma. Biochem. Biophys. Res. Commun. 2021, 554, 214–221. [Google Scholar] [CrossRef]
- Chen, M.L.; Sun, A.; Cao, W.; Eliason, A.; Mendez, K.M.; Getzler, A.J.; Tsuda, S.; Diao, H.; Mukori, C.; Bruno, N.E.; et al. Physiological expression and function of the MDR1 transporter in cytotoxic T lymphocytes. J. Exp. Med. 2020, 217, e20191388. [Google Scholar] [CrossRef]
- Molina-Ortiz, D.; Torres-Zárate, C.; Cárdenas-Cardós, R.; Palacios-Acosta, J.M.; Hernández-Arrazola, D.; Shalkow-Klincovstein, J.; Díaz-Díaz, E.; Vences-Mejía, A. MDR1 not CYP3A4 gene expression is the predominant mechanism of innate drug resistance in pediatric soft tissue sarcoma patients. Cancer Biomark. 2018, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, X.; Pei, Y.; Wang, W.; Zheng, K.; Qiu, E.; Zhang, X. PTHR1 May Be Involved in Progression of Osteosarcoma by Regulating miR-124-3p- AR-Tgfb1i1, miR-27a-3p- PPARG-Abca1, and miR-103/590-3p- AXIN2 Axes. DNA Cell Biol. 2019, 38, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Fanelli, M.; Tavanti, E.; Vella, S.; Ferrari, S.; Picci, P.; Serra, M. Advances in emerging drugs for osteosarcoma. Expert Opin. Emerg. Drugs 2015, 20, 495–514. [Google Scholar] [CrossRef] [PubMed]
- Belisario, D.C.; Akman, M.; Godel, M.; Campani, V.; Patrizio, M.P.; Scotti, L.; Hattinger, C.M.; De Rosa, G.; Donadelli, M.; Serra, M.; et al. ABCA1/ABCB1 Ratio Determines Chemo- and Immune-Sensitivity in Human Osteosarcoma. Cells 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Hall, M.D.; Gottesman, M.M.; Boumendjel, A.; Kachadourian, R.; Day, B.J.; Baubichon-Cortay, H.; Di Pietro, A. Targeting the Achilles Heel of Multidrug-Resistant Cancer by Exploiting the Fitness Cost of Resistance. Chem. Rev. 2014, 114, 5753–5774. [Google Scholar] [CrossRef] [PubMed]
- Anreddy, N.; Gupta, P.; Kathawala, R.; Patel, A.; Wurpel, J.; Chen, Z.S. Tyrosine Kinase Inhibitors as Reversal Agents for ABC Transporter Mediated Drug Resistance. Molecules 2014, 19, 13848–13877. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.K.; Kathawala, R.; Chen, Z.S. Repositioning of Tyrosine Kinase Inhibitors as Antagonists of ATP-Binding Cassette Transporters in Anticancer Drug Resistance. Cancers 2014, 6, 1925–1952. [Google Scholar] [CrossRef]
- Fanelli, M.; Hattinger, C.M.; Vella, S.; Tavanti, E.; Michelacci, F.; Gudeman, B.; Barnett, D.; Picci, P.; Serra, M. Targeting ABCB1 and ABCC1 with their Specific Inhibitor CBT-1 ® can Overcome Drug Resistance in Osteosarcoma. Curr. Cancer Drug Targets 2016, 16, 261–274. [Google Scholar] [CrossRef]
- Sone, K.; Oguri, T.; Uemura, T.; Takeuchi, A.; Fukuda, S.; Takakuwa, O.; Maeno, K.; Fukumitsu, K.; Kanemitsu, Y.; Ohkubo, H.; et al. Genetic variation in the ATP binding cassette transporter ABCC10 is associated with neutropenia for docetaxel in Japanese lung cancer patients cohort. BMC Cancer 2019, 19, 246. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; Shi, J.; Dai, Y.; Wu, L.; Zhou, H. ABCC10 Plays a Significant Role in the Transport of Gefitinib and Contributes to Acquired Resistance to Gefitinib in NSCLC. Front. Pharmacol. 2018, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Q.; Lei, Z.; Teng, Q.X.; Ji, N.; Lin, L.; Liu, Z.; Chen, Z.S. Insights on the structure–function relationship of human multidrug resistance protein 7 (MRP7/ABCC10) from molecular dynamics simulations and docking studies. MedComm 2021, 2, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, V.; Dubova, M.; Susova, S.; Vycital, O.; Bruha, J.; Skala, M.; Liska, V.; Daum, O.; Soucek, P. Protein expression of ATP-binding cassette transporters ABCC10 and ABCC11 associates with survival of colorectal cancer patients. Cancer Chemother. Pharmacol. 2016, 78, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Wong, Z.W.; Sandanaraj, E.; Xiang, X.; Ang, P.C.; Lee, E.J.; Chowbay, B. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008, 99, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Bonito, C.A.; Cordeiro, M.N.D.S.; Ferreira, M.J.U.; dos Santos, D.J.V.A. Structure-function relationships in ABCG2: Insights from molecular dynamics simulations and molecular docking studies. Sci. Rep. 2017, 7, 15534. [Google Scholar] [CrossRef]
- Cole, S.P.C. Multidrug Resistance Protein 1 (MRP1, ABCC1), a “Multitasking” ATP-binding Cassette (ABC) Transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef]
- Wang, W.J.; Sui, H.; Qi, C.; Li, Q.; Zhang, J.; Wu, S.F.; Mei, M.Z.; Lu, Y.Y.; Wan, Y.T.; Chang, H.; et al. Ursolic acid inhibits proliferation and reverses drug resistance of ovarian cancer stem cells by downregulating ABCG2 through suppressing the expression of hypoxia-inducible factor-1α in vitro. Oncol. Rep. 2016, 36, 428–440. [Google Scholar] [CrossRef]
- Damiani, D.; Tiribelli, M.; Geromin, A.; Michelutti, A.; Cavallin, M.; Sperotto, A.; Fanin, R. ABCG2 overexpression in patients with acute myeloid leukemia: Impact on stem cell transplantation outcome: ABCG2 and Allogeneic SCT in AML. Am. J. Hematol. 2015, 90, 784–789. [Google Scholar] [CrossRef]
- Tsai, H.; Chang, A.; Tsai, C.; Huang, Y.L.; Gan, L.; Chen, C.K.; Liu, S.C.; Huang, T.Y.; Fong, Y.C.; Tang, C.H. CCN2 promotes drug resistance in osteosarcoma by enhancing ABCG2 expression. J. Cell. Physiol. 2019, 234, 9297–9307. [Google Scholar] [CrossRef]
- Kim, C.K.; Oh, S.; Kim, S.J.; Leem, S.H.; Heo, J.; Chung, S.H. Correlation of IGF1R expression with ABCG2 and CD44 expressions in human osteosarcoma. Genes Genom. 2018, 40, 381–388. [Google Scholar] [CrossRef]
- Shu, H.; Yuan, B.; Huang, Y.; Wang, L.; He, B.; Sun, Q.; Sun, L. High expression of ABCG2 is associated with chemotherapy resistance of osteosarcoma. J. Orthop. Surg. Res. 2021, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef] [PubMed]
- Cortini, M.; Avnet, S.; Baldini, N. Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett. 2017, 405, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, X.; Li, W.; Li, M.; Tu, T.; Ba, X.; Wu, Y.; Huang, Z.; Fan, G.; Zhou, G.; et al. Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway. Cancer Lett. 2017, 403, 271–279. [Google Scholar] [CrossRef]
- Edwardson, D.W.; Parissenti, A.M.; Kovala, A.T. Chemotherapy and Inflammatory Cytokine Signalling in Cancer Cells and the Tumour Microenvironment. Adv. Exp. Med. Biol. 2019, 1152, 173–215. [Google Scholar] [CrossRef]
- Foukakis, T.; Lövrot, J.; Matikas, A.; Zerdes, I.; Lorent, J.; Tobin, N.; Suzuki, C.; Brage, S.E.; Carlsson, L.; Einbeigi, Z.; et al. Immune gene expression and response to chemotherapy in advanced breast cancer. Br. J. Cancer 2018, 118, 480–488. [Google Scholar] [CrossRef]
- Sui, S.; An, X.; Xu, C.; Li, Z.; Hua, Y.; Huang, G.; Sui, S.; Long, Q.; Sui, Y.; Xiong, Y.; et al. An immune cell infiltration-based immune score model predicts prognosis and chemotherapy effects in breast cancer. Theranostics 2020, 10, 11938–11949. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, J.; Huang, W.; Chen, H.; Xi, S.; Han, Z.; Huang, L.; Lin, T.; Zhao, L.Y.; Hu, Y.F.; et al. Tumor Immune Microenvironment and Chemosensitivity Signature for Predicting Response to Chemotherapy in Gastric Cancer. Cancer Immunol. Res. 2019, 7, 2065–2073. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, J.H.; Lin, Z.H.; Lv, H.Y.; Ye, Z.M.; Chen, Y.P.; Zhang, X.Y. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of osteosarcoma. Aging 2020, 12, 3486–3501. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, Z.; Fu, Y.; Wang, J. CXCR3 from chemokine receptor family correlates with immune infiltration and predicts poor survival in osteosarcoma. Biosci. Rep. 2019, 39, BSR20192134. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Xu, P. NK cell and macrophages confer prognosis and reflect immune status in osteosarcoma. J. Cell. Biochem. 2019, 120, 8792–8797. [Google Scholar] [CrossRef]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.J.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-Infiltrating Macrophages Are Associated with Metastasis Suppression in High-Grade Osteosarcoma: A Rationale for Treatment with Macrophage Activating Agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Y.; Zhu, X.; Fu, J.; Deng, C.; Chen, H.; Xu, H.; Song, G.; Lu, J.; Tang, Q.; et al. Immune Landscape of the Tumor Microenvironment Identifies Prognostic Gene Signature CD4/CD68/CSF1R in Osteosarcoma. Front. Oncol. 2020, 10, 1198. [Google Scholar] [CrossRef]
- Zhao, G.; Liang, J.; Cao, J.; Jiang, S.; Lu, J.; Jiang, B. Abnormal Function of Circulating Follicular Helper T Cells Leads to Different Manifestations of B Cell Maturation and Differentiation in Patients with Osteosarcoma. J. Healthc. Eng. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Ren, T.; Huang, Y.; Liang, X.; Wang, W.; Niu, J.; Han, Y.; Guo, W. Development of a prognostic gene signature based on an immunogenomic infiltration analysis of osteosarcoma. J. Cell Mol. Med. 2020, 24, 11230–11242. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Zhang, L.H.; Zhang, Y.; Lu, X.C.; Zhang, Y.; Liu, Y.K.; Khader, M.A.; Jia, W.; Tao, L.; Li, J.Z. Construction of immune-related gene pairs signature to predict the overall survival of osteosarcoma patients. Aging 2020, 12, 22906–22926. [Google Scholar] [CrossRef] [PubMed]
- Biller, B.J.; Guth, A.; Burton, J.H.; Dow, S.W. Decreased Ratio of CD8+ T Cells to Regulatory T Cells Associated with Decreased Survival in Dogs with Osteosarcoma: CD8:Treg in Canine Osteosarcoma. J. Vet. Intern. Med. 2010, 24, 1118–1123. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Current and Emerging Targets in Immunotherapy for Osteosarcoma. J. Oncol. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef]
- Casanova, J.M.; Almeida, J.S.; Reith, J.D.; Sousa, L.M.; Fonseca, R.; Freitas-Tavares, P.; Santos-Rosa, M.; Rodrigues-Santos, P. Tumor-Infiltrating Lymphocytes and Cancer Markers in Osteosarcoma: Influence on Patient Survival. Cancers 2021, 13, 6075. [Google Scholar] [CrossRef] [PubMed]
- Maciel, T.T.; Moura, I.C.; Hermine, O. The role of mast cells in cancers. F1000Prime Rep. 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Todosenko, N.; Vulf, M.; Yurova, K.; Skuratovskaia, D.; Khaziakhmatova, O.; Gazatova, N.; Melashchenko, O.; Urazova, O.; Litvinova, L. The Pathogenic Subpopulation of Th17 Cells in Obesity. Curr. Pharm. Des. 2021, 27, 3924–3938. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xia, K.; Gao, T.; Chen, J.; Zhang, Z.; Sun, X.; Simões, B.M.; Eyre, R.; Fan, Z.; Guo, W.; et al. The Notch Pathway Promotes Osteosarcoma Progression through Activation of Ephrin Reverse Signaling. Mol. Cancer Res. 2019, 17, 2383–2394. [Google Scholar] [CrossRef]
- Silva-Santos, B.; Mensurado, S.; Coffelt, S.B. γδ T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019, 19, 392–404. [Google Scholar] [CrossRef]
- Wu, C.C.; Beird, H.C.; Andrew Livingston, J.; Advani, S.; Mitra, A.; Cao, S.; Reuben, A.; Ingram, D.; Wang, W.L.; Ju, Z.; et al. Immuno-genomic landscape of osteosarcoma. Nat. Commun. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Michalski, M.N.; McCauley, L.K. Macrophages and skeletal health. Pharmacol. Ther. 2017, 174, 43–54. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Ligon, J.A.; Choi, W.; Cojocaru, G.; Fu, W.; Hsiue, E.H.; Oke, T.F.; Siegel, N.; Fong, M.H.; Ladle, B.; Pratilas, C.A.; et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J. Immunother. Cancer 2021, 9, e001772. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients With Osteosarcoma. JAMA Oncol. 2020, 6, 724. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zhou, R.; Yu, Y.; Luo, Y.; Zhang, J.; Sun, H.; Bin, J.; Liao, Y.; Rao, J.; Zhang, Y.; et al. Gene expression profiles for a prognostic immunoscore in gastric cancer. Br. J. Surg. 2018, 105, 1338–1348. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Y.; Li, M.; Lu, T.; Xi, J.; Lin, Z.; Jiang, W.; Guo, W.; Zhan, C.; Wang, Q. Identification and validation of an immune cell infiltrating score predicting survival in patients with lung adenocarcinoma. J. Transl. Med. 2019, 17, 217. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. OncoImmunology 2017, 6, e1331193. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, X.; Zhang, J.; He, X.; Teng, G.; Yu, D. Tim3/Gal9 interactions between T cells and monocytes result in an immunosuppressive feedback loop that inhibits Th1 responses in osteosarcoma patients. Int. Immunopharmacol. 2017, 44, 153–159. [Google Scholar] [CrossRef]
- Han, Q.; Shi, H.; Liu, F. CD163 + M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016, 34, 101–106. [Google Scholar] [CrossRef]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef]
- Wolf-Dennen, K.; Gordon, N.; Kleinerman, E.S. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. OncoImmunology 2020, 9, 1747677. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Chen, Y.; Chen, H.; Yuan, W.; Wang, X. The Heterogeneity of Infiltrating Macrophages in Metastatic Osteosarcoma and Its Correlation with Immunotherapy. J. Oncol. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mahlbacher, G.; Curtis, L.T.; Lowengrub, J.; Frieboes, H.B. Mathematical modeling of tumor-associated macrophage interactions with the cancer microenvironment. J. Immunother. Cancer 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Dhupkar, P.; Gordon, N.; Stewart, J.; Kleinerman, E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018, 7, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, C.; Vicari, A.P.; Sangaletti, S.; Trinchieri, G.; Colombo, M.P. Redirecting In vivo Elicited Tumor Infiltrating Macrophages and Dendritic Cells towards Tumor Rejection. Cancer Res. 2005, 65, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- Cory, T.J.; He, H.; Winchester, L.C.; Kumar, S.; Fletcher, C.V. Alterations in P-Glycoprotein Expression and Function Between Macrophage Subsets. Pharm. Res. 2016, 33, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, F.; Chen, Q.; Kang, A.; Lu, M.; Liu, W.; Zang, X.; Wang, G.; Zhang, J. Chronic inflammation up-regulates P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice. Sci. Rep. 2015, 5, 13558. [Google Scholar] [CrossRef]
- Heymann, M.F.; Lézot, F.; Heymann, D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. 2019, 343, 103711. [Google Scholar] [CrossRef]
- Turtle, C.J.; Swanson, H.M.; Fujii, N.; Estey, E.H.; Riddell, S.R. A Distinct Subset of Self-Renewing Human Memory CD8+ T Cells Survives Cytotoxic Chemotherapy. Immunity 2009, 31, 834–844. [Google Scholar] [CrossRef]
- Murata, K.; Tsukahara, T.; Emori, M.; Shibayama, Y.; Mizushima, E.; Matsumiya, H.; Yamashita, K.; Kaya, M.; Hirohashi, Y.; Kanaseki, T.; et al. Identification of a novel human memory T-cell population with the characteristics of stem-like chemo-resistance. OncoImmunology 2016, 5, e1165376. [Google Scholar] [CrossRef]
- Tsukahara, T.; Emori, M.; Murata, K.; Mizushima, E.; Shibayama, Y.; Kubo, T.; Kanaseki, T.; Hirohashi, Y.; Yamashita, T.; Sato, N.; et al. The future of immunotherapy for sarcoma. Expert Opin. Biol. Ther. 2016, 16, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Zhang, Z.; Tao, L.; Xu, F.F.; Li, H.Y.; Zhang, H.Y.; Liu, W. T cell exhaustion drives osteosarcoma pathogenesis. Ann. Transl. Med. 2021, 9, 1447. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Su, S.; Kirshtein, A.; Shahriyari, L. Data-Driven Mathematical Model of Osteosarcoma. Cancers 2021, 13, 2367. [Google Scholar] [CrossRef]
- Fritzsching, B.; Fellenberg, J.; Moskovszky, L.; Sápi, Z.; Krenacs, T.; Machado, I.; Poeschl, J.; Lehner, B.; Szendrõi, M.; Bosch, A.L.; et al. CD8+/FOXP3+-ratio in osteosarcoma microenvironment separates survivors from non-survivors: A multicenter validated retrospective study. OncoImmunology 2015, 4, e990800. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Dimeloe, S.; Frick, C.; Fischer, M.; Gubser, P.M.; Razik, L.; Bantug, G.R.; Ravon, M.; Langenkamp, A.; Hess, C. Human regulatory T cells lack the cyclophosphamide-extruding transporter ABCB1 and are more susceptible to cyclophosphamide-induced apoptosis. Immunomodulation Eur, J. Immunol. 2014, 44, 3614–3620. [Google Scholar] [CrossRef]
- Ramesh, R.; Kozhaya, L.; McKevitt, K.; Djuretic, I.M.; Carlson, T.J.; Quintero, M.A.; McCauley, J.L.; Abreu, M.T.; Unutmaz, D.; Sundrud, M.S. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J. Exp. Med. 2014, 211, 89–104. [Google Scholar] [CrossRef]
- Gourdin, N.; Bossennec, M.; Rodriguez, C.; Vigano, S.; Machon, C.; Jandus, C.; Bauché, D.; Faget, J.; Durand, I.; Chopin, N.; et al. Autocrine Adenosine Regulates Tumor Polyfunctional CD73+CD4+ Effector T Cells Devoid of Immune Checkpoints. Cancer Res. 2018, 78, 3604–3618. [Google Scholar] [CrossRef]
- Alsuliman, A.; Muftuoglu, M.; Khoder, A.; Ahn, Y.O.; Basar, R.; Verneris, M.R.; Muranski, P.; Barrett, A.J.; Liu, E.; Li, L.; et al. A subset of virus-specific CD161+ T cells selectively express the multidrug transporter MDR1 and are resistant to chemotherapy in AML. Blood 2017, 129, 740–758. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells. Immunol. Rev. 2016, 271, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhou, J.; Ji, B. Evidence of Interleukin 21 Reduction in Osteosarcoma Patients Due to PD-1/PD-L1-Mediated Suppression of Follicular Helper T Cell Functionality. DNA Cell Biol. 2017, 36, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, G.; Zhang, J.; Wu, X.; Chen, X. The Dual Roles of Human γδ T Cells: Anti-Tumor or Tumor-Promoting. Front. Immunol. 2021, 11, 619954. [Google Scholar] [CrossRef]
- Lamora, A.; Talbot, J.; Mullard, M.; Brounais-Le Royer, B.; Redini, F.; Verrecchia, F. TGF-β Signaling in Bone Remodeling and Osteosarcoma Progression. J. Clin. Med. 2016, 5, 96. [Google Scholar] [CrossRef]
- Yagi, K.; Yamamoto, K.; Umeda, S.; Abe, S.; Suzuki, S.; Onishi, I.; Kirimura, S.; Fukayama, M.; Arai, A.; Kitagawa, M.; et al. Expression of multidrug resistance 1 gene in B-cell lymphomas: Association with follicular dendritic cells: MDR1 expression with FDC. Histopathology 2013, 62, 414–420. [Google Scholar] [CrossRef]
- Inagaki, Y.; Hookway, E.; Williams, K.A.; Hassan, A.B.; Oppermann, U.; Tanaka, Y.; Soilleux, E.; Athanasou, N.A. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin. Sarcoma Res. 2016, 6, 13. [Google Scholar] [CrossRef]
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017, 386, 189–195. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Skoda, J.; Nunukova, A.; Loja, T.; Zambo, I.; Neradil, J.; Mudry, P.; Zitterbart, K.; Hermanova, M.; Hampl, A.; Sterba, J.; et al. Cancer stem cell markers in pediatric sarcomas: Sox2 is associated with tumorigenicity in immunodeficient mice. Tumor Biol. 2016, 37, 9535–9548. [Google Scholar] [CrossRef]
- Milosevic, V.; Kopecka, J.; Salaroglio, I.C.; Libener, R.; Napoli, F.; Izzo, S.; Orecchia, S.; Ananthanarayanan, P.; Bironzo, P.; Grosso, F.; et al. Wnt/IL-1β/IL-8 autocrine circuitries control chemoresistance in mesothelioma initiating cells by inducing ABCB5. Int. J. Cancer 2020, 146, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neves, S.R.; Paiva-Oliveira, D.I.; Wijers-Koster, P.M.; Abrunhosa, A.J.; Fontes-Ribeiro, C.; Bovée, J.V.; Cleton-Jansen, A.M.; Gomes, C.M. Chemotherapy induces stemness in osteosarcoma cells through activation of Wnt/β-catenin signaling. Cancer Lett. 2016, 370, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Roundhill, E.A.; Jabri, S.; Burchill, S.A. ABCG1 and Pgp identify drug resistant, self-renewing osteosarcoma cells. Cancer Lett. 2019, 453, 142–157. [Google Scholar] [CrossRef]
- Milane, L.; Ganesh, S.; Shah, S.; Duan, Z.-F.; Amiji, M. Multi-modal strategies for overcoming tumor drug resistance: Hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J. Control. Release 2011, 155, 237–247. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, L.; Xue, X.; Xie, H.; Shi, H.; Hu, Y. A lncRNA coordinates with Ezh2 to inhibit HIF-1α transcription and suppress cancer cell adaption to hypoxia. Oncogene 2020, 39, 1860–1874. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Mi, B.; Xu, F.; Wen, J.; Zhao, L.; Liu, J.; Huang, G. Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1α axis. Theranostics 2020, 10, 4762–4778. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Fatal Alliance of Hypoxia-/HIF-1α-Driven Microenvironmental Traits Promoting Cancer Progression. In Oxygen Transport to Tissue XLI. Vol 1232. Advances in Experimental Medicine and Biology; Ryu, P.D., LaManna, J.C., Harrison, D.K., Lee, S.S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 169–176. [Google Scholar] [CrossRef]
- Li, C.; Guo, D.; Tang, B.; Zhang, Y.; Zhang, K.; Nie, L. Notch1 is associated with the multidrug resistance of hypoxic osteosarcoma by regulating MRP1 gene expression. Neoplasma 2016, 63, 734–742. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, H.; Zhu, D.; Zhi, H.; Wang, T.; Wang, J.; Jiang, B.; Shu, Y.; Liu, P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother. Pharmacol. 2012, 69, 723–731. [Google Scholar] [CrossRef]
- Ikram, M.; Lim, Y.; Baek, S.Y.; Jin, S.; Jeong, Y.H.; Kwak, J.Y.; Yoon, S. Co-targeting of Tiam1/Rac1 and Notch ameliorates chemoresistance against doxorubicin in a biomimetic 3D lymphoma model. Oncotarget 2018, 9, 2058–2075. [Google Scholar] [CrossRef]
- Ding, Z.; Yang, L.; Xie, X.; Xie, F.; Pan, F.; Li, J.; He, J.; Liang, H. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J. Cancer Res. Clin. Oncol. 2010, 136, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Roncuzzi, L.; Pancotti, F.; Baldini, N. Involvement of HIF-1α activation in the doxorubicin resistance of human osteosarcoma cells. Oncol. Rep. 2014, 32, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J.; Jang, H. Oncogenic Ras Isoforms Signaling Specificity at the Membrane. Cancer Res. 2018, 78, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Porto, S.; Lusa, S.; Gazzano, E.; Salzano, G.; Giordano, A.; Desiderio, V.; Ghigo, D.; Caraglia, M.; De Rosa, G.; et al. Self-assembling nanoparticles encapsulating zoledronic acid revert multidrug resistance in cancer cells. Oncotarget 2015, 6, 31461–31478. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Riganti, C.; Vitale, C.; Griggio, V.; Campia, I.; Robino, M.; Foglietta, M.; Castella, B.; Sciancalepore, P.; Buondonno, I.; et al. Simvastatin and downstream inhibitors circumvent constitutive and stromal cell-induced resistance to doxorubicin in IGHV unmutated CLL cells. Oncotarget 2015, 6, 29833–29846. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Meng, X. Knockdown of PARP-1 Inhibits Proliferation and ERK Signals, Increasing Drug Sensitivity in Osteosarcoma U2OS Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 24, 279–286. [Google Scholar] [CrossRef]
- Pinzón-Daza, M.L.; Cuellar-Saenz, Y.; Nualart, F.; Ondo-Mendez, A.; Del Riesgo, L.; Castillo-Rivera, F.; Garzón, R. Oxidative Stress Promotes Doxorubicin-Induced Pgp and BCRP Expression in Colon Cancer Cells Under Hypoxic Conditions: O XIDATIVE S TRESS P ROMOTES D OXORUBICIN -I NDUCED Pgp AND BCRP. J. Cell Biochem. 2017, 118, 1868–1878. [Google Scholar] [CrossRef]
- Buravchenko, G.I.; Scherbakov, A.M.; Dezhenkova, L.G.; Bykov, E.E.; Solovieva, S.E.; Korlukov, A.A.; Sorokin, D.V.; Fidalgo, L.M.; Shchekotikhin, A.E. Discovery of derivatives of 6-amino-3-phenylquinoxaline-2-carbonitrile 1,4-dioxides: Novel, hypoxia-selective HIF-1α inhibitors with strong antiestrogenic potency. Bioorg. Chem. 2020, 104, 104324. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.Y.; Gao, X.H.; Bian, Q.; Jia, M.; Lai, X.L.; Wang, T.Y.; Bian, X.L.; Wang, H.Y. Low Molecular Weight Heparin (LMWH) Improves Peritoneal Function and Inhibits Peritoneal Fibrosis Possibly through Suppression of HIF-1α, VEGF and TGF-β1. PLoS ONE 2015, 10, e0118481. [Google Scholar] [CrossRef][Green Version]
- Li, L.F.; Yu, C.C.; Huang, H.Y.; Wu, H.P.; Chu, C.M.; Huang, C.Y.; Liu, P.C.; Liu, Y.Y. Suppression of Hypoxia-Inducible Factor 1α by Low-Molecular-Weight Heparin Mitigates Ventilation-Induced Diaphragm Dysfunction in a Murine Endotoxemia Model. Int. J. Mol. Sci. 2021, 22, 1702. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Shi, Y.; Yu, S.; Ma, X. Different signaling pathways involved in the anti-inflammatory effects of unfractionated heparin on lipopolysaccharide-stimulated human endothelial cells. J. Inflamm 2020, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xu, Z.; Yuan, Y.; Wang, T.; Xu, C.; Yin, C.; Xie, P.; Xu, P.; Ye, H.; Patel, N.; et al. Heparin impairs skeletal muscle glucose uptake by inhibiting insulin binding to insulin receptor. Endocrinol. Diab Metab 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lu, Y.; Wang, J.; Liu, X.; Keller, E.T.; Liu, Q.; Zhou, Q.; Zhang, J. Targeting the Notch signaling pathway in cancer therapeutics: Notch signaling pathway in cancer. Thorac. Cancer 2014, 5, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Herranz, D.; Ambesi-Impiombato, A.; Palomero, T.; Schnell, S.A.; Belver, L.; Wendorff, A.A.; Xu, L.; Castillo-Martin, M.; Llobet-Navás, D.; Cordon-Cardo, C.; et al. A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nat. Med. 2014, 20, 1130–1137. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ahmad, A.; Azmi, A.S.; Banerjee, S.; Kong, D.; Sarkar, F.H. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim. Et Biophys. Acta 2010, 1806, 258–267. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch Promotes Radioresistance of Glioma Stem Cells. Stem. Cells 2010, 28, 17–28. [Google Scholar] [CrossRef]
- Cho, S.; Lu, M.; He, X.; Ee, P.L.; Bhat, U.; Schneider, E.; Miele, L.; Beck, W.T. Notch1 regulates the expression of the multidrug resistance gene ABCC1 / MRP1 in cultured cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 20778–20783. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Li, J.; Zhang, K.; Chen, J.; Chen, D.; Feng, B.; Song, H.; Feng, J.; Wang, R.; et al. Notch-1 Confers Chemoresistance in Lung Adenocarcinoma to Taxanes through AP-1/microRNA-451 Mediated Regulation of MDR-1. Mol. Ther. -Nucleic Acids 2016, 5, e375. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Lu, S.; Chen, Y.; Shan, L.; Zhao, X.; Xu, Y. The role of Notch ligand Jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J. Orthop. Surg. Res. 2021, 16, 226. [Google Scholar] [CrossRef]
- Xiao, W.; Gao, Z.; Duan, Y.; Yuan, W.; Ke, Y. Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 41. [Google Scholar] [CrossRef]
- Niderla-Bielińska, J.; Bartkowiak, K.; Ciszek, B.; Jankowska-Steifer, E.; Krejner, A.; Ratajska, A. Sulodexide inhibits angiogenesis via decreasing Dll4 and Notch1 expression in mouse proepicardial explant cultures. Fundam. Clin. Pharmacol. 2019, 33, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, X.; Hu, Y.; Wu, J.; Ju, Y.; Sun, X.; Liu, Y.; Shan, B. Blocking the Notch signal transduction pathway promotes tumor growth in osteosarcoma by affecting polarization of TAM to M2 phenotype. Ann. Transl. Med. 2020, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Handschin, A.E.; Trentz, O.A.; Hoerstrup, S.P.; Kock, H.J.; Wanner, G.A.; Trentz, O. Effect of low molecular weight heparin (dalteparin) and fondaparinux (Arixtra®) on human osteoblasts in vitro. Br. J. Surg. 2005, 92, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Pollari, S.; Käkönen, R.S.; Mohammad, K.S.; Rissanen, J.P.; Halleen, J.M.; Wärri, A.; Nissinen, L.; Pihlavisto, M.; Marjamäki, A.; Perälä, M.; et al. Heparin-like Polysaccharides Reduce Osteolytic Bone Destruction and Tumor Growth in a Mouse Model of Breast Cancer Bone Metastasis. Mol. Cancer Res. 2012, 10, 597–604. [Google Scholar] [CrossRef]
- Sadaie, M.R. Can heparins stimulate bone cancer stem cells and interfere with tumorigenesis? Ther. Adv. Drug Saf. 2011, 2, 271–282. [Google Scholar] [CrossRef]
- Saito, M.; Ichikawa, J.; Ando, T.; Schoenecker, J.G.; Ohba, T.; Koyama, K.; Suzuki-Inoue, K.; Haro, H. Platelet-Derived TGF-β Induces Tissue Factor Expression via the Smad3 Pathway in Osteosarcoma Cells. J. Bone. Miner Res. 2018, 33, 2048–2058. [Google Scholar] [CrossRef]
- Ichikawa, J.; Cole, H.A.; Magnussen, R.A.; Mignemi, N.A.; Butler, M.; Holt, G.E.; O’Rear, L.; Yuasa, M.; Pabla, B.; Haro, H.; et al. Thrombin induces osteosarcoma growth, a function inhibited by low molecular weight heparin in vitro and in vivo: Procoagulant nature of osteosarcoma. Cancer 2012, 118, 2494–2506. [Google Scholar] [CrossRef]
- Nazari Soltan Ahmad, S.; Sanajou, D.; Kalantary-Charvadeh, A.; Hosseini, V.; Roshangar, L.; Khojastehfard, M.; Haiaty, S.; Mesgari-Abbasi, M. β- LAP achone ameliorates doxorubicin-induced cardiotoxicity via regulating autophagy and Nrf2 signalling pathways in mice. Basic Clin. Pharmacol. Toxicol 2020, 126, 364–373. [Google Scholar] [CrossRef]
- Ryoo, I.; Kim, G.; Choi, B.; Lee, S.; Kwak, M.K. Involvement of NRF2 Signaling in Doxorubicin Resistance of Cancer Stem. Cell-Enriched Colonospheres. Biomol. Ther. 2016, 24, 482–488. [Google Scholar] [CrossRef]
- Yalniz, M.; Demirel, U.; Orhan, C.; Bahcecioglu, I.H.; Ozercan, I.H.; Aygun, C.; Tuzcu, M.; Sahin, K. Nadroparin Sodium Activates Nrf2/HO-1 Pathway in Acetic Acid-Induced Colitis in Rats. Inflammation 2012, 35, 1213–1221. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, X.; Liu, Y.; Tang, S.; Miao, J.; Zhou, Q.; Chen, S. IL-1β-induced NF-κB activation down-regulates miR-506 expression to promotes osteosarcoma cell growth through JAG1. Biomed. Pharmacother. 2017, 95, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Cai, C.Y.; Assaraf, Y.G.; Guo, H.Q.; Cui, Q.; Wei, L.; Huang, J.J.; Ashby, C.R., Jr.; Chen, Z.S. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updates 2020, 48, 100663. [Google Scholar] [CrossRef] [PubMed]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator V3: A Reference Expression Database for the Meta-Analysis of Transcriptomes. Adv. Bioinform. 2008, 2008, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zheng, L.H.; Ma, B.A.; Zhou, Y.; Zhang, M.H.; Zhang, D.Z.; Fan, Q.Y. Clinical value of signal transducers and activators of transcription 3 (STAT3) gene expression in human osteosarcoma. Acta Histochem. 2011, 113, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Jiguet-Jiglaire, C.; Campion, L.; Bartoli, C.; Frassineti, F.; Deville, J.L.; Maues De Paula, A.; Forest, F.; Jézéquel, P.; Gentet, J.C.; et al. Correlation between ERK1 and STAT3 expression and chemoresistance in patients with conventional osteosarcoma. BMC Cancer 2014, 14, 606. [Google Scholar] [CrossRef][Green Version]
- Aggarwal, B.B.; Sethi, G.; Ahn, K.S.; Sandur, S.K.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Ichikawa, H. Targeting Signal-Transducer-and-Activator-of-Transcription-3 for Prevention and Therapy of Cancer. Ann. New York Acad. Sci. 2006, 1091, 151–169. [Google Scholar] [CrossRef]
- Tu, B.; Zhu, J.; Liu, S.; Wang, L.; Fan, Q.; Hao, Y.; Fan, C.; Tang, T.T. Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3. Oncotarget 2016, 7, 48296–48308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Zuo, D.; Zhang, T.; Yin, F.; Zhou, Z.; Wang, H.; Xu, J.; Mao, M.; Wang, G.; et al. Attenuation of STAT3 Phosphorylation Promotes Apoptosis and Chemosensitivity in Human Osteosarcoma Induced by Raddeanin A. Int. J. Biol. Sci. 2019, 15, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Jiang, D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS ONE 2018, 13, e0205918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol. Int. 2019, 43, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Liu, G.; Rus, I.A.; Yao, S.; Chen, Y.; Akiri, G.; Grumolato, L.; Aaronson, S.A. High-Frequency Canonical Wnt Activation in Multiple Sarcoma Subtypes Drives Proliferation through a TCF/β-Catenin Target Gene, CDC25A. Cancer Cell 2011, 19, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Pfankuchen, D.B.; Baltes, F.; Batool, T.; Li, J.P.; Schlesinger, M.; Bendas, G. Heparin antagonizes cisplatin resistance of A2780 ovarian cancer cells by affecting the Wnt signaling pathway. Oncotarget 2017, 8, 67553–67566. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Dong, Q.; Qin, L. CCN: Core regulatory proteins in the microenvironment that affect the metastasis of hepatocellular carcinoma? Oncotarget 2016, 7, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.E.; Howlett, M.; Cole, C.H.; Kees, U.R. Deregulated expression of connective tissue growth factor (CTGF/CCN2) is linked to poor outcome in human cancer: CTGF and Cancer. Int. J. Cancer 2015, 137, 504–511. [Google Scholar] [CrossRef]
- Li, L.; Kong, X.; Zang, M.; Hu, B.; Fang, X.; Gui, B.; Hu, Y. MicroRNA-584 Impairs Cellular Proliferation and Sensitizes Osteosarcoma Cells to Cisplatin and Taxanes by Targeting CCN2. Cancer Manag. Res. 2020, 12, 2577–2587. [Google Scholar] [CrossRef]
- Yeger, H.; Perbal, B. The CCN axis in cancer development and progression. J. Cell Commun. Signal 2021, 15, 491–517. [Google Scholar] [CrossRef]
- Hou, C.H.; Yang, R.; Tsao, Y.T. Connective tissue growth factor stimulates osteosarcoma cell migration and induces osteosarcoma metastasis by upregulating VCAM-1 expression. Biochem. Pharmacol. 2018, 155, 71–81. [Google Scholar] [CrossRef]
- Wang, L.H.; Tsai, H.C.; Cheng, Y.C.; Lin, C.Y.; Huang, Y.L.; Tsai, C.H.; Xu, G.H.; Wang, S.W.; Fong, Y.C.; Tang, C.H. CTGF promotes osteosarcoma angiogenesis by regulating miR-543/angiopoietin 2 signaling. Cancer Lett. 2017, 391, 28–37. [Google Scholar] [CrossRef]
- Jia, Q.; Bu, Y.; Wang, Z.; Chen, B.; Zhang, Q.; Yu, S.; Liu, Q. Maintenance of stemness is associated with the interation of LRP6 and heparin-binding protein CCN2 autocrined by hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 117. [Google Scholar] [CrossRef]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 2019, 8, 1584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, W.; Zhang, F. Poncirin downregulates ATP -binding cassette transporters to enhance cisplatin sensitivity in cisplatin-resistant osteosarcoma cells. Phytother. Res. 2021, 35, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, S.; Qu, L.; Zhu, J. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shueng, P.; Chou, C.; Lin, B.X.; Lin, M.H.; Kuo, D.Y.; Tsai, I.L.; Wu, S.M.; Lin, C.W. Elevation of CD109 promotes metastasis and drug resistance in lung cancer via activation of EGFR-AKT-mTOR signaling. Cancer Sci. 2020, 111, 1652–1662. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Yu, D.; Chen, X.; He, S. Modulation of binding to vascular endothelial growth factor and receptor by heparin derived oligosaccharide. Carbohydr. Polym. 2017, 174, 558–564. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef]
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, M.; Wang, W.; Zhang, P.; Wang, Y.; Wang, L. Global Characterization of Metabolic Genes Regulating Survival and Immune Infiltration in Osteosarcoma. Front. Genet. 2022, 12, 814843. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- Kodet, O.; Němejcova, K.; Strnadová, K.; Havlínová, A.; Dundr, P.; Krajsová, I.; Štork, J.; Smetana, K., Jr.; Lacina, L. The Abscopal Effect in the Era of CheckpoInt. Inhibitors. Int. J. Mol. Sci. 2021, 22, 7204. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; El-Baba, C.; Elnaggar, M.H.; Elkholy, Y.O.; Mottawea, M.; Johar, D.; Al Shehabi, T.S.; Kobeissy, F.; Moussalem, C.; Massaad, E.; et al. Novel therapeutic strategies for spinal osteosarcomas. Semin. Cancer Biol. 2020, 64, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, C.; Tian, S.; Wang, Y.; Shen, R.; Rao, H.; Li, J.; Yang, X.; Chen, B.; Ye, L. Comprehensive analysis of prognostic tumor microenvironment-related genes in osteosarcoma patients. BMC Cancer 2020, 20, 814. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhao, Q.; Zhao, M.; Zhu, S.; Liu, B.; Bukhari, I.; Zhang, K.; Wu, W.; Fu, Y.; Yu, Y.; et al. Immune infiltration profiling in gastric cancer and their clinical implications. Cancer Sci. 2021, 112, 3569–3584. [Google Scholar] [CrossRef]
- Zhang, J.C.; Xie, F.; Yu, X.H.; Deng, Z.Y.; Wang, Y.; Liang, P.; Sun, L.; Zhang, F.X. Expression levels of P-glycoprotein in peripheral blood CD8+ T lymphocytes from HIV-1-infected patients on antiretroviral therapy. Int. J. Mol. Med. 2014, 33, 431–440. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Gillam, F.B.; Hopkins, J.J.; Jayanthi, S.; Gundampati, R.K.; Su, G.; Bear, J.; Pilkington, G.R.; Jalah, R.; Felber, B.K.; et al. Molecular mechanisms of heparin-induced modulation of human interleukin 12 bioactivity. J. Biol. Chem. 2019, 294, 4412–4424. [Google Scholar] [CrossRef]
- Balestrieri, B.; Granata, F.; Loffredo, S.; Petraroli, A.; Scalia, G.; Morabito, P.; Cardamone, C.; Varricchi, G.; Triggiani, M. Phenotypic and Functional Heterogeneity of Low-Density and High-Density Human Lung Macrophages. Biomedicines 2021, 9, 505. [Google Scholar] [CrossRef]
- Swangphon, P.; Pientong, C.; Sunthamala, N.; Bumrungthai, S.; Azuma, M.; Kleebkaow, P.; Tangsiriwatthana, T.; Sangkomkamhang, U.; Kongyingyoes, B.; Ekalaksananan, T. Correlation of Circulating CD64+/CD163+ Monocyte Ratio and stroma/peri-tumoral CD163+ Monocyte Density with Human Papillomavirus Infected Cervical Lesion Severity. Cancer Microenviron. 2017, 10, 77–85. [Google Scholar] [CrossRef]
- Xiao, H.; Luo, G.; Son, H.; Zhou, Y.; Zheng, W. Upregulation of peripheral CD4+CXCR5+ T cells in osteosarcoma. Tumor Biol. 2014, 35, 5273–5279. [Google Scholar] [CrossRef]
- Koirala, P.; Roth, M.E.; Gill, J.; Piperdi, S.; Chinai, J.M.; Geller, D.S.; Hoang, B.H.; Park, A.; Fremed, M.A.; Zang, X.; et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016, 6, 30093. [Google Scholar] [CrossRef]
- McEachron, T.A.; Triche, T.J.; Sorenson, L.; Parham, D.M.; Carpten, J.D. Profiling targetable immune checkpoints in osteosarcoma. OncoImmunology 2018, 7, e1475873. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ruan, Z. Tim-3 and Tim-4 as the potential targets for antitumor therapy. Hum. Vaccines Immunother. 2015, 11, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Q.; Ren, J.P.; Zhao, J.; Wang, J.M.; Zhou, Y.; Li, G.Y.; Moorman, J.P.; Yao, Z.Q. MicroRNA-155 regulates interferon- γ production in natural killer cells via Tim-3 signalling in chronic hepatitis C virus infection. Immunology 2015, 145, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Li, J.; Fan, W.; Xu, D.; Sun, S. Tim-3 as a diagnostic and prognostic biomarker of osteosarcoma. Tumour Biol. 2017, 39, 101042831771564. [Google Scholar] [CrossRef]
- Gorman, J.V.; Colgan, J.D. Regulation of T cell responses by the receptor molecule Tim-3. Immunol. Res. 2014, 59, 56–65. [Google Scholar] [CrossRef]
- Song, Y.J.; Xu, Y.; Deng, C.; Zhu, X.; Fu, J.; Chen, H.; Lu, J.; Xu, H.; Song, G.; Tang, Q.; et al. Gene Expression Classifier Reveals Prognostic Osteosarcoma Microenvironment Molecular Subtypes. Front. Immunol. 2021, 12, 623762. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiao, Q.; Zhou, B.; Dai, Z.; Kang, Y. Prognostic Significance of ProgramMed. Death Ligand 1 Expression and Tumor-Infiltrating Lymphocytes in Axial Osteosarcoma. World Neurosurg. 2019, 129, e240–e254. [Google Scholar] [CrossRef]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef]
- Kashiwakura, Y.; Kojima, H.; Kanno, Y.; Hashiguchi, M.; Kobata, T. Heparin affects the induction of regulatory T cells independent of anti-coagulant activity and suppresses allogeneic immune responses. Clin. Exp. Immunol. 2020, 202, 119–135. [Google Scholar] [CrossRef]
- Verronèse, E.; Delgado, A.; Valladeau-Guilemond, J.; Garin, G.; Guillemaut, S.; Tredan, O.; Ray-Coquard, I.; Bachelot, T.; N’Kodia, A.; Bardin-Dit-Courageot, C.; et al. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. OncoImmunology 2016, 5, e1100791. [Google Scholar] [CrossRef]
- Becker-Hapak, M.K.; Shrestha, N.; McClain, E.; Dee, M.J.; Chaturvedi, P.; Leclerc, G.M.; Marsala, L.I.; Foster, M.; Schappe, T.; Tran, J.; et al. A Fusion Protein Complex that Combines IL-12, IL-15, and IL-18 Signaling to Induce Memory-Like NK Cells for Cancer Immunotherapy. Cancer Immunol. Res. 2021, 9, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Garnier, P.; Mummery, R.; Forster, M.J.; Mulloy, B.; Gibbs, R.V.; Rider, C.C. The localisation of the heparin binding sites of human and murine interleukin-12 within the carboxyterminal domain of the P40 subunit. Cytokine 2018, 110, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Koppolu, B.; Smith, S.G.; Jalah, R.; Bear, J.; Rosati, M.; Pavlakis, G.N.; Felber, B.K.; Zaharoff, D.A.; Kumar, T.K. Efficient production and purification of recombinant human interleukin-12 (IL-12) overexpressed in mammalian cells without affinity tag. Protein Expr. Purif. 2014, 102, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Koppolu, B.; Nguyen, K.G.; Smith, S.G.; Felber, B.K.; Kumar, T.K.; Zaharoff, D.A. Modulation of Interleukin-12 activity in the presence of heparin. Sci. Rep. 2017, 7, 5360. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.R.; Gonçalves, J.P.; McCulloch, T.; Delconte, R.B.; Hennessy, R.J.; Huntington, N.D.; Trindade, E.S.; Souza-Fonseca-Guimaraes, F. The Antitumor Effect of Heparin is not Mediated by Direct NK Cell Activation. J. Clin. Med. 2020, 9, 2666. [Google Scholar] [CrossRef]
- Xu, K.; Jin, L. The role of heparin/heparan sulphate in the IFN-γ-led Arena. Biochimie 2020, 170, 1–9. [Google Scholar] [CrossRef]
- Bruno, V.; Svensson-Arvelund, J.; Rubér, M.; Berg, G.; Piccione, E.; Jenmalm, M.C.; Ernerudh, J. Effects of low molecular weight heparin on the polarization and cytokine profile of macrophages and T helper cells in vitro. Sci. Rep. 2018, 8, 4166. [Google Scholar] [CrossRef]
- Wan, M.M.; Chen, H.; Da Wang, Z.; Liu, Z.Y.; Yu, Y.Q.; Li, L.; Miao, Z.Y.; Wang, X.W.; Wang, Q.; Mao, C. Nitric Oxide-Driven Nanomotor for Deep Tissue Penetration and Multidrug Resistance Reversal in Cancer Therapy. Adv. Sci. 2021, 8, 2002525. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Le Deley, M.C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.C.; Entz-Werlé, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Mohamed, S.; Coombe, D. Heparin Mimetics: Their Therapeutic Potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef]
- Lazo-Langner, A.; Goss, G.D.; Spaans, J.N.; Rodger, M.A. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J. Thromb. Haemost. 2007, 5, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Young, E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Boothello, R.S.; Patel, N.J.; Sharon, C.; Abdelfadiel, E.I.; Morla, S.; Brophy, D.F.; Lippman, H.R.; Desai, U.R.; Patel, B.B. A Unique Nonsaccharide Mimetic of Heparin Hexasaccharide Inhibits Colon Cancer Stem. Cells via p38 MAP Kinase Activation. Mol. Cancer Ther. 2019, 18, 51–61. [Google Scholar] [CrossRef]
- Casu, B.; Vlodavsky, I.; Sanderson, R.D. Non-Anticoagulant Heparins and Inhibition of Cancer. Pathophysiol. Haemost. Thromb. 2007, 36, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Banik, N.; Yang, S.B.; Kang, T.B.; Lim, J.H.; Park, J. Heparin and Its Derivatives: Challenges and Advances in Therapeutic Biomolecules. Int. J. Mol. Sci. 2021, 22, 10524. [Google Scholar] [CrossRef] [PubMed]
- Dredge, K.; Brennan, T.V.; Hammond, E.; Lickliter, J.D.; Lin, L.; Bampton, D.; Handley, P.; Lankesheer, F.; Morrish, G.; Yang, Y.; et al. A Phase I study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours. Br. J. Cancer. 2018, 118, 1035–1041. [Google Scholar] [CrossRef]
- Yang, X.; Du, H.; Liu, J.; Zhai, G. Advanced Nanocarriers Based on Heparin and Its Derivatives for Cancer Management. Biomacromolecules 2015, 16, 423–436. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. In Drug Delivery Systems; Jain, K.K., Ed.; Springer: New York, NY, USA, 2020; Volume 2059, pp. 1–54. [Google Scholar] [CrossRef]
- Sun, H.; Cao, D.; Wu, H.; Liu, H.; Ke, X.; Ci, T. Development of low molecular weight heparin based nanoparticles for metastatic breast cancer therapy. Int. J. Biol. Macromol. 2018, 112, 343–355. [Google Scholar] [CrossRef]
- Newland, B.; Varricchio, C.; Körner, Y.; Hoppe, F.; Taplan, C.; Newland, H.; Eigel, D.; Tornillo, G.; Pette, D.; Brancale, A.; et al. Focal drug administration via heparin-containing cryogel microcarriers reduces cancer growth and metastasis. Carbohydr. Polym. 2020, 245, 116504. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Hsu, H.K.; Hsu, K.H.; Cheng, Y.M.; Suen, H.Y.; Peng, S.F. Development and In Vitro Evaluation of Linear PEI-Shelled Heparin/Berberine Nanoparticles in Human Osteosarcoma U-2 OS Cells. Molecules 2018, 23, 3121. [Google Scholar] [CrossRef] [PubMed]
- Melim, C.; Jarak, I.; Veiga, F.; Figueiras, A. The potential of micelleplexes as a therapeutic strategy for osteosarcoma disease. 3 Biotech 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K.P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 2019, 363, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel-Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Prudowsky, Z.D.; Yustein, J.T. Recent Insights into Therapy Resistance in Osteosarcoma. Cancers 2020, 13, 83. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment—A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef]
- Morton, S.W.; Shah, N.J.; Quadir, M.A.; Deng, Z.J.; Poon, Z.; Hammond, P.T. Osteotropic Therapy via Targeted Layer-by-Layer Nanoparticles. Adv. Healthc. Mater. 2014, 3, 867–875. [Google Scholar] [CrossRef]
- Smorenburg, S.M.; Van Noorden, C.J. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol. Rev. 2001, 53, 93–105. [Google Scholar]
- O’Reilly, E.M.; Barone, D.; Mahalingam, D.; Bekaii-Saab, T.; Shao, S.H.; Wolf, J.; Rosano, M.; Krause, S.; Richards, D.A.; Yu, K.H.; et al. Randomised phase II trial of gemcitabine and nab-paclitaxel with necuparanib or placebo in untreated metastatic pancreas ductal adenocarcinoma. Eur. J. Cancer 2020, 132, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Kaur, S.; Tummala, H.; Sangamwar, A.T. Overcoming multiple drug resistance in cancer using polymeric micelles. Expert Opin. Drug Deliv. 2018, 15, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]

| Protumor Properties | References | Antitumor Properties | References |
|---|---|---|---|
| Macrophages M0 | [26,89,90] | Macrophages M0 | [91] |
| Macrophages M2 | [92] | Macrophages M1 | [89,90,93,94] |
| Tfh cells | [95] | CD8 cells | [26,90,96,97,98,99] |
| γδ T-cells | [100] | Th1 cells | [94,101] |
| Mast cells | [102] | Th1.17 cells | [103] |
| Dendritic cells | [26] | NK cells | [90,96,97] |
| Cancer stem cells (CSCs) | [104] | γδ T-cells | [105,106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todosenko, N.; Yurova, K.; Khaziakhmatova, O.; Malashchenko, V.; Khlusov, I.; Litvinova, L. Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells. Pharmaceutics 2022, 14, 2181. https://doi.org/10.3390/pharmaceutics14102181
Todosenko N, Yurova K, Khaziakhmatova O, Malashchenko V, Khlusov I, Litvinova L. Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells. Pharmaceutics. 2022; 14(10):2181. https://doi.org/10.3390/pharmaceutics14102181
Chicago/Turabian StyleTodosenko, Natalia, Kristina Yurova, Olga Khaziakhmatova, Vladimir Malashchenko, Igor Khlusov, and Larisa Litvinova. 2022. "Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells" Pharmaceutics 14, no. 10: 2181. https://doi.org/10.3390/pharmaceutics14102181
APA StyleTodosenko, N., Yurova, K., Khaziakhmatova, O., Malashchenko, V., Khlusov, I., & Litvinova, L. (2022). Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells. Pharmaceutics, 14(10), 2181. https://doi.org/10.3390/pharmaceutics14102181






