Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapy for Diabetes Mellitus and Diabetic Complications
Abstract
1. Introduction
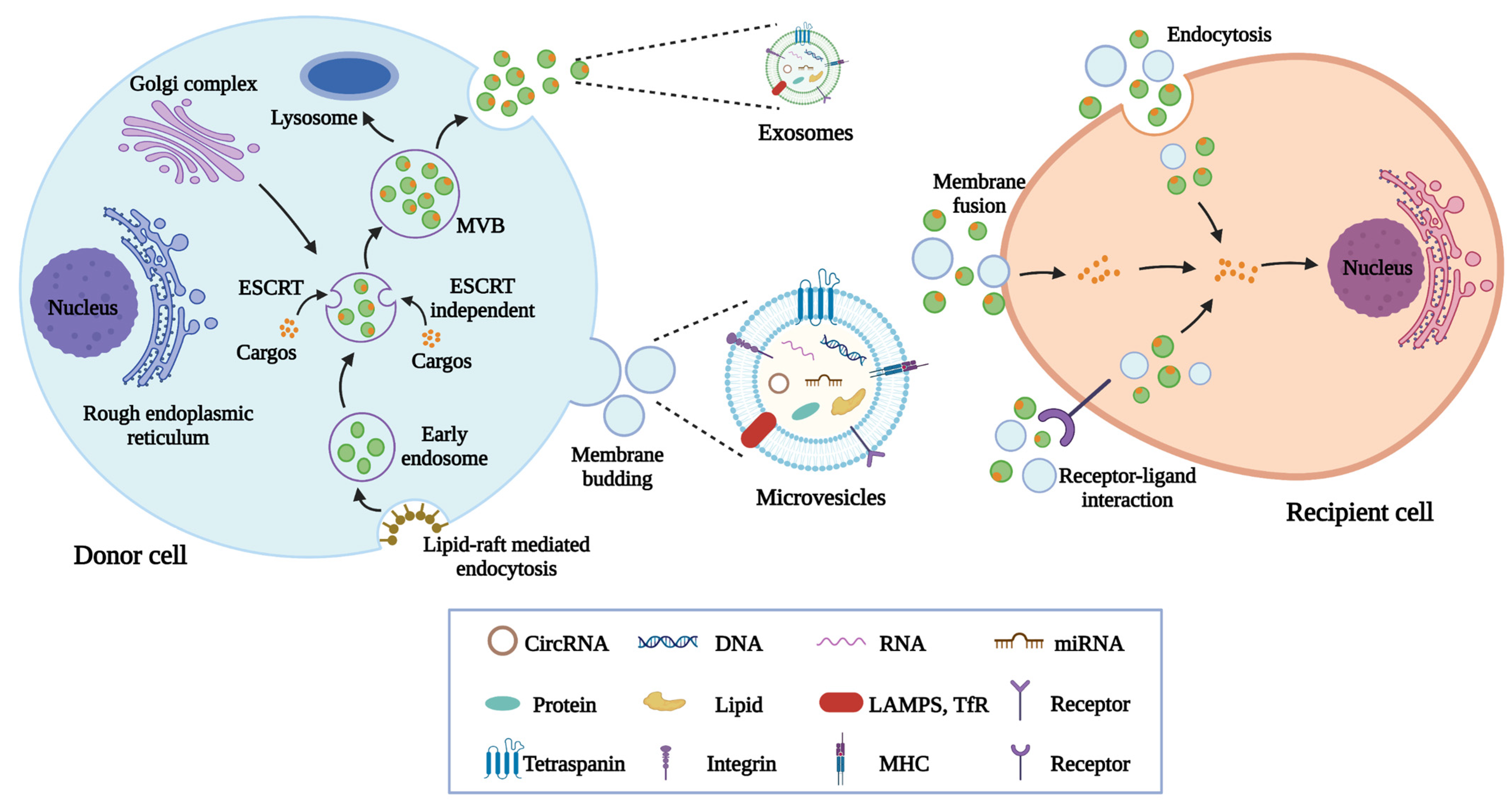
2. Biogenesis, Contents, and Characteristics of MSC-EVs
3. Natural MSC-EVs in the Treatment of DM and Diabetic Complications
3.1. Diabetes Mellitus
3.2. Diabetic Nephropathy
3.3. Diabetic Retinopathy
3.4. Diabetic Wound Healing
3.5. Diabetic Neuropathy
4. Engineered MSC-EVs in the Treatment of DM and Diabetic Complications
4.1. Modification of MSC
4.2. Direct Modification of Isolated MSC-EVs
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Jeyagaran, A.; Lu, C.E.; Zbinden, A.; Birkenfeld, A.L.; Brucker, S.Y.; Layland, S.L. Type 1 Diabetes and Engineering Enhanced Islet Transplantation. Adv. Drug Deliv. Rev. 2022, 189, 114481. [Google Scholar] [CrossRef]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 2022, 33, 333–344. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, Z.; Han, J.; Xia, P.; Shen, Y.; Ma, J.; Liu, X.; Zhang, J.; Yu, P. Advances in the study of RNA-binding proteins in diabetic complications. Mol. Metab. 2022, 62, 101515. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Ortega, A.; Forner, M.J.; Cortes, R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Non-Coding RNA Therapeutic Vehicles in Autoimmune Diseases. Pharmaceutics 2022, 14, 733. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- An, T.; Chen, Y.; Tu, Y.; Lin, P. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev. Rep. 2021, 17, 369–378. [Google Scholar] [CrossRef]
- Zhuang, X.; Hu, X.; Zhang, S.; Li, X.; Yuan, X.; Wu, Y. Mesenchymal Stem Cell-Based Therapy as a New Approach for the Treatment of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2022, 15, 3. [Google Scholar] [CrossRef]
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhao, Z.; Gao, H. Effect of hTIMP-1 overexpression in human umbilical cord mesenchymal stem cells on the repair of pancreatic islets in type-1 diabetic mice. Cell Biol. Int. 2021, 45, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Kuljanin, M.; Bell, G.I.; Sherman, S.E.; Lajoie, G.A.; Hess, D.A. Proteomic characterisation reveals active Wnt-signalling by human multipotent stromal cells as a key regulator of beta cell survival and proliferation. Diabetologia 2017, 60, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Galiè, M.; Konstantinidou, G.; Peroni, D.; Scambi, I.; Marchini, C.; Lisi, V.; Krampera, M.; Magnani, P.; Merigo, F.; Montani, M.; et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene 2008, 27, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, B.; Chen, Z.; Li, G.; Zhang, Z. MSC-induced lncRNA HCP5 drove fatty acid oxidation through miR-3619-5p/AMPK/PGC1α/CEBPB axis to promote stemness and chemo-resistance of gastric cancer. Cell Death Dis. 2020, 11, 233. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, X.; Qiu, R.; Gong, Z.; Huang, F.; Yu, W.; Shen, B.; Sha, X.; Dong, H.; Huang, J.; et al. Lymph node metastasis-derived gastric cancer cells educate bone marrow-derived mesenchymal stem cells via YAP signaling activation by exosomal Wnt5a. Oncogene 2021, 40, 2296–2308. [Google Scholar] [CrossRef]
- Lei, F.; Li, M.; Lin, T.; Zhou, H.; Wang, F.; Su, X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 2022, 141, 333–343. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, S.; Um, W.; Shin, S.; Kim, C.H.; Park, J.H.; Kim, B.S. Functional Extracellular Vesicles for Regenerative Medicine. Small 2022, 18, e2106569. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, L.; Zhang, M.; Chen, Z. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles: A Novel Approach for Kidney Disease Treatment. Int. J. Nanomed. 2022, 17, 3603–3618. [Google Scholar] [CrossRef] [PubMed]
- Psaraki, A.; Ntari, L.; Karakostas, C.; Korrou-Karava, D.; Roubelakis, M.G. Extracellular vesicles derived from mesenchymal stem/stromal cells: The regenerative impact in liver diseases. Hepatology 2022, 75, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, e2005709. [Google Scholar] [CrossRef]
- Lu, B.; Ku, J.; Flojo, R.; Olson, C.; Bengford, D.; Marriott, G. Exosome- and extracellular vesicle-based approaches for the treatment of lysosomal storage disorders. Adv. Drug Deliv. Rev. 2022, 188, 114465. [Google Scholar] [CrossRef]
- Estes, S.; Konstantinov, K.; Young, J.D. Manufactured extracellular vesicles as human therapeutics: Challenges, advances, and opportunities. Curr. Opin. Biotechnol. 2022, 77, 102776. [Google Scholar] [CrossRef]
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- L Ramos, T.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef]
- Burrello, J.; Biemmi, V.; Dei Cas, M.; Amongero, M.; Bolis, S.; Lazzarini, E.; Bollini, S.; Vassalli, G.; Paroni, R.; Barile, L. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci. Rep. 2020, 10, 16182. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Rustam, Y.H.; Masters, C.L.; Makalic, E.; McLean, C.A.; Hill, A.F.; Barnham, K.J.; Reid, G.E.; Vella, L.J. Characterization of brain-derived extracellular vesicle lipids in Alzheimer’s disease. J. Extracell. Vesicles 2021, 10, e12089. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Vietri, M.; Radulovic, M.; Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Tallon, C.; Hollinger, K.R.; Pal, A.; Bell, B.J.; Rais, R.; Tsukamoto, T.; Witwer, K.W.; Haughey, N.J.; Slusher, B.S. Nipping disease in the bud: nSMase2 inhibitors as therapeutics in extracellular vesicle-mediated diseases. Drug Discov. Today 2021, 26, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, H.; Nomi, A.; Zhang, L.; Zhang, B.; Qian, H. Mesenchymal stem cell-derived extracellular vesicles: A new impetus of promoting angiogenesis in tissue regeneration. Cytotherapy 2019, 21, 497–508. [Google Scholar] [CrossRef]
- Roura, S.; Monguió-Tortajada, M.; Munizaga-Larroudé, M.; Clos-Sansalvador, M.; Franquesa, M.; Rosell, A.; Borràs, F.E. Potential of Extracellular Vesicle-Associated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 6761. [Google Scholar] [CrossRef]
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733. [Google Scholar] [CrossRef]
- Tan, K.L.; Chia, W.C.; How, C.W.; Tor, Y.S.; Show, P.L.; Looi, Q.H.D.; Foo, J.B. Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells. Mol. Biotechnol. 2021, 63, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wu, P.; Zhou, X.; Qian, H.; Xu, W. Extracellular Vesicles: Novel Roles in Neurological Disorders. Stem Cells Int. 2021, 2021, 6640836. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Huang, W.; Liu, J.; Tian, J.; Wang, S.; Rui, K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front. Immunol. 2021, 12, 749192. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-de la Torre, R.; Quiñones-Vico, M.I.; Fernández-González, A.; Sánchez-Díaz, M.; Montero-Vílchez, T.; Sierra-Sánchez, Á.; Arias-Santiago, S. Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. J. Clin. Med. 2021, 10, 2991. [Google Scholar] [CrossRef]
- Hu, C.; Wu, Z.; Li, L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 2020, 16, 893–903. [Google Scholar] [CrossRef]
- Zhao, A.G.; Shah, K.; Cromer, B.; Sumer, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells Int. 2020, 2020, 8825771. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Zhang, L.; Bao, Q.; Li, L. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020, 11, 377. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef]
- Sohrabi, B.; Dayeri, B.; Zahedi, E.; Khoshbakht, S.; Nezamabadi Pour, N.; Ranjbar, H.; Davari Nejad, A.; Noureddini, M.; Alani, B. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022, 29, 1105–1116. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lai, L.C. The potential roles of stem cell-derived extracellular vesicles as a therapeutic tool. Ann. Transl. Med. 2019, 7, 693. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Sarvestani, F.S.; Ghahremani, M.H.; Aghdaei, M.H.; Al-Abdullah, I.H.; Azarpira, N. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J. 2020, 19, 1064–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.K.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell. Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.T.; Sherman, S.E.; Bell, G.I.; Dayarathna, T.; McRae, D.M.; Ma, J.; Lagugné-Labarthet, F.; Pasternak, S.H.; Lajoie, G.A.; Hess, D.A. Ultrafiltration and Injection of Islet Regenerative Stimuli Secreted by Pancreatic Mesenchymal Stromal Cells. Stem Cells Dev. 2021, 30, 247–264. [Google Scholar] [CrossRef]
- Mahdipour, E.; Salmasi, Z.; Sabeti, N. Potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell. Physiol. 2019, 234, 20310–20321. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, M.; Mishra, S.; Chaudhary, D.K.; Kumar, A.; Avni, B.; Tiwari, S. Exosomes Secreted by Umbilical Cord Blood-Derived Mesenchymal Stem Cell Attenuate Diabetes in Mice. J. Diabetes Res. 2021, 2021, 9534574. [Google Scholar] [CrossRef]
- He, Q.; Song, J.; Cui, C.; Wang, J.; Hu, H.; Guo, X.; Yang, M.; Wang, L.; Yan, F.; Liang, K.; et al. Mesenchymal stem cell-derived exosomal miR-146a reverses diabetic β-cell dedifferentiation. Stem Cell Res. Ther. 2021, 12, 449. [Google Scholar] [CrossRef]
- Yap, S.K.; Tan, K.L.; Abd Rahaman, N.Y.; Saulol Hamid, N.F.; Ooi, J.; Tor, Y.S.; Daniel Looi, Q.H.; Stella Tan, L.K.; How, C.W.; Foo, J.B. Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Ameliorated Insulin Resistance in Type 2 Diabetes Mellitus Rats. Pharmaceutics 2022, 14, 649. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, L.; Zhao, R.; Yan, F.; Sha, S.; Cui, C.; Song, J.; Hu, H.; Guo, X.; Yang, M.; et al. Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res. Ther. 2020, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Q.; Liu, D.; Liu, Z. Exosomes: Advances, development and potential therapeutic strategies in diabetic nephropathy. Metabolism 2021, 122, 154834. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, D.; Feng, Q.; Liu, Z. Diabetic Nephropathy: Perspective on Extracellular Vesicles. Front. Immunol. 2020, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, S.K.; Guo, B.; Li, F.; Zheng, M.H.; Lei, L.M.; Xu, Q.S.; Ullah, M.H.E.; Xu, F.; Lin, X.; et al. The Multi-Therapeutic Role of MSCs in Diabetic Nephropathy. Front. Endocrinol. 2021, 12, 671566. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Saijo, Y.; Tsuchida, H.; Ishioka, S.; Nishikawa, A.; Saito, T.; et al. Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci. Rep. 2017, 7, 8484. [Google Scholar] [CrossRef]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, Y.; Zhang, J.; Dong, Q.; Yang, M.; Wang, W. Mouse Umbilical Cord Mesenchymal Stem Cell Paracrine Alleviates Renal Fibrosis in Diabetic Nephropathy by Reducing Myofibroblast Transdifferentiation and Cell Proliferation and Upregulating MMPs in Mesangial Cells. J. Diabetes Res. 2020, 2020, 3847171. [Google Scholar] [CrossRef]
- Wang, S.; Bao, L.; Fu, W.; Deng, L.; Ran, J. Protective effect of exosomes derived from bone marrow mesenchymal stem cells on rats with diabetic nephropathy and its possible mechanism. Am. J. Transl. Res. 2021, 13, 6423–6430. [Google Scholar]
- Hao, Y.; Miao, J.; Liu, W.; Cai, K.; Huang, X.; Peng, L. Mesenchymal Stem Cell-Derived Exosomes Carry MicroRNA-125a to Protect Against Diabetic Nephropathy by Targeting Histone Deacetylase 1 and Downregulating Endothelin-1. Diabetes Metab. Syndr. Obes. 2021, 14, 1405–1418. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef]
- Cui, C.; Zang, N.; Song, J.; Guo, X.; He, Q.; Hu, H.; Yang, M.; Wang, Y.; Yang, J.; Zou, Y.; et al. Exosomes derived from mesenchymal stem cells attenuate diabetic kidney disease by inhibiting cell apoptosis and epithelial-to-mesenchymal transition via miR-424-5p. FASEB J. 2022, 36, e22517. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef]
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Stefańska, K.; Zieliński, M.; Sakowska, J.; Jankowiak, M.; Trzonkowski, P.; Marek-Trzonkowska, N.; Kwiatkowski, S. Podocytes-The Most Vulnerable Renal Cells in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 5051. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, Y.; Zhao, L.; Zou, W.; Tan, M.; He, Q. Exosomal miRNA-215-5p Derived from Adipose-Derived Stem Cells Attenuates Epithelial-Mesenchymal Transition of Podocytes by Inhibiting ZEB2. Biomed. Res. Int. 2020, 2020, 2685305. [Google Scholar] [CrossRef]
- Zhao, T.; Jin, Q.; Kong, L.; Zhang, D.; Teng, Y.; Lin, L.; Yao, X.; Jin, Y.; Li, M. microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis. J. Bioenerg. Biomembr. 2022, 54, 17–30. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, Q.; Wang, Y.; Ma, Y.; Chen, F.; Zhu, X.; Shi, J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020, 295, 12868–12884. [Google Scholar] [CrossRef]
- Cai, X.; Zou, F.; Xuan, R.; Lai, X.Y. Exosomes from mesenchymal stem cells expressing microribonucleic acid-125b inhibit the progression of diabetic nephropathy via the tumour necrosis factor receptor-associated factor 6/Akt axis. Endocr. J. 2021, 68, 817–828. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef]
- Hu, J.; Dziumbla, S.; Lin, J.; Bibli, S.I.; Zukunft, S.; de Mos, J.; Awwad, K.; Frömel, T.; Jungmann, A.; Devraj, K.; et al. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 2017, 552, 248–252. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xue, L.D.; Di, Y.; Li, T.; Tian, Y.J.; Song, Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021, 272, 119232. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gao, X.; He, G.H.; Chen, S.; Gu, Z.H.; Zhang, Y.L.; Li, L.Y. Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging. Int. J. Ophthalmol. 2021, 14, 1828–1833. [Google Scholar] [CrossRef]
- Ebrahim, N.; El-Halim, H.E.A.; Helal, O.K.; El-Azab, N.E.; Badr, O.A.M.; Hassouna, A.; Saihati, H.A.A.; Aborayah, N.H.; Emam, H.T.; El-Wakeel, H.S.; et al. Effect of bone marrow mesenchymal stem cells-derived exosomes on diabetes-induced retinal injury: Implication of Wnt/ b-catenin signaling pathway. Biomed. Pharmacother. 2022, 154, 113554. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sun, Y.; Zhu, J.; Wang, X.; Ji, C.; Zhang, J.; Chen, S.; Yu, Y.; Xu, W.; Qian, H. Mesenchymal stem cells-derived small extracellular vesicles alleviate diabetic retinopathy by delivering NEDD4. Stem Cell Res. Ther. 2022, 13, 293. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, N.; Li, S.; Li, K.; Guo, H.; Zhang, H.; Jin, H.; An, M.; Yu, X. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. Int. Immunopharmacol. 2021, 101, 108234. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, L.Y.; Cui, Y.B.; Xie, N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int. Immunopharmacol. 2021, 90, 107010. [Google Scholar] [CrossRef]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J. Endocrinol. Investig. 2021, 44, 1193–1207. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Zhao, S.; He, D.; Gao, Y. Mesenchymal Stem Cell Exosomal miR-146a Mediates the Regulation of the TLR4/MyD88/NF-κB Signaling Pathway in Inflammation due to Diabetic Retinopathy. Comput. Math. Methods Med. 2022, 2022, 3864863. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Gao, Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J. Cell. Physiol. 2021, 236, 5036–5051. [Google Scholar] [CrossRef]
- Liang, G.; Qin, Z.; Luo, Y.; Yin, J.; Shi, Z.; Wei, R.; Ma, W. Exosomal microRNA-133b-3p from bone marrow mesenchymal stem cells inhibits angiogenesis and oxidative stress via FBN1 repression in diabetic retinopathy. Gene Ther. 2022, 7, 35125496. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; He, G.H.; Zhang, X.T.; Chen, S. Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway. Int. J. Ophthalmol. 2021, 14, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Torres, L.A.; Gamboa Acha, L.; Tran, S.; Liu, A.; Patel, R.; Chennakesavalu, M.; Aneesh, A.; Huang, C.C.; Feinstein, D.L.; et al. Uptake and Distribution of Administered Bone Marrow Mesenchymal Stem Cell Extracellular Vesicles in Retina. Cells 2021, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Qiao, G.H.; Wang, M.; Yu, L.; Sun, Y.; Shi, H.; Ma, T.L. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front. Cell Dev. Biol. 2022, 10, 812262. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, Y.; Li, L.; Wu, P.; Dkw, O.; Shi, H. Calcium Channels: Noteworthy Regulators and Therapeutic Targets in Dermatological Diseases. Front. Pharmacol. 2021, 12, 702264. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Zhang, Q.; Zhang, Y. Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells. Int. J. Stem Cells 2022, 15, 164–172. [Google Scholar] [CrossRef]
- Yan, C.; Xv, Y.; Lin, Z.; Endo, Y.; Xue, H.; Hu, Y.; Hu, L.; Chen, L.; Cao, F.; Zhou, W.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 829868. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Zhang, Y.; Lu, Y.; Zhang, W.; Lu, S.; Fu, Y.; Zhou, Y.; Zhang, J.; Zhang, J. Human Exosomes Accelerate Cutaneous Wound Healing by Promoting Collagen Synthesis in a Diabetic Mouse Model. Stem Cells Dev. 2021, 30, 922–933. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.; Shen, K.; Luo, L.; Zhao, M.; Xu, C.; Jia, Y.; Xiao, D.; Li, Y.; Gao, X.; et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells 2022, 11, 2568. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Liu, D. Extracellular Vesicles from Adipose-Derived Stem Cells Promote Diabetic Wound Healing via the PI3K-AKT-mTOR-HIF-1α Signaling Pathway. Tissue Eng. Regen. Med. 2021, 18, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef]
- Huang, C.; Luo, W.; Wang, Q.; Ye, Y.; Fan, J.; Lin, L.; Shi, C.; Wei, W.; Chen, H.; Wu, Y.; et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021, 52, 102235. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Y.; Ma, K.; Li, Q.; Li, B.; Hu, W.; Fu, X.; Zhang, C. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Diabetic Wound Healing Through MiR-17-5p-mediated Enhancement of Angiogenesis. Stem Cell Rev. Rep. 2022, 18, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Gallagher, G.; Fridman, V.; Feldman, E.L. Diabetic neuropathy: What does the future hold? Diabetologia 2020, 63, 891–897. [Google Scholar] [CrossRef]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Venkat, P.; Zacharek, A.; Landschoot-Ward, J.; Wang, F.; Culmone, L.; Chen, Z.; Chopp, M.; Chen, J. Exosomes derived from bone marrow mesenchymal stem cells harvested from type two diabetes rats promotes neurorestorative effects after stroke in type two diabetes rats. Exp. Neurol. 2020, 334, 113456. [Google Scholar] [CrossRef]
- Ding, H.; Jia, Y.; Lv, H.; Chang, W.; Liu, F.; Wang, D. Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neuroinflammation after diabetic intracerebral hemorrhage via the miR-183-5p/PDCD4/NLRP3 pathway. J. Endocrinol. Investig. 2021, 44, 2685–2698. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizue, Y.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, H.; Yan, J.; Ma, X. An experimental study on the treatment of diabetes-induced cognitive disorder mice model with exosomes deriving from mesenchymal stem cells (MSCs). Pak. J. Pharm. Sci. 2019, 32, 1965–1970. [Google Scholar] [PubMed]
- Lang, H.L.; Zhao, Y.Z.; Xiao, R.J.; Sun, J.; Chen, Y.; Hu, G.W.; Xu, G.H. Small extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells improve postoperative cognitive dysfunction in mice with diabetes. Neural Regen. Res. 2022, 18, 609–617. [Google Scholar] [CrossRef]
- Yin, S.; Liu, W.; Ji, C.; Zhu, Y.; Shan, Y.; Zhou, Z.; Chen, W.; Zhang, L.; Sun, Z.; Zhou, W.; et al. hucMSC-sEVs-Derived 14-3-3ζ Serves as a Bridge between YAP and Autophagy in Diabetic Kidney Disease. Oxid. Med. Cell. Longev. 2022, 2022, 3281896. [Google Scholar] [CrossRef]
- Ge, L.; Yang, L.; Bron, R.; van Rijn, P. Topography-Mediated Enhancement of Nonviral Gene Delivery in Stem Cells. Pharmaceutics 2022, 14, 1096. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Control. Release 2016, 238, 166–175. [Google Scholar] [CrossRef]
- Han, Z.F.; Cao, J.H.; Liu, Z.Y.; Yang, Z.; Qi, R.X.; Xu, H.L. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res. Clin. Pract. 2022, 183, 109126. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z.; Li, T.; Zhu, L.; Li, Z.; Tian, N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am. J. Transl. Res. 2017, 9, 5012–5021. [Google Scholar]
- Zhang, L.; Yu, Z.; Qu, Q.; Li, X.; Lu, X.; Zhang, H. Exosomal lncRNA HOTAIR Promotes the Progression and Angiogenesis of Endometriosis via the miR-761/HDAC1 Axis and Activation of STAT3-Mediated Inflammation. Int. J. Nanomed. 2022, 17, 1155–1170. [Google Scholar] [CrossRef]
- Born, L.J.; Chang, K.H.; Shoureshi, P.; Lay, F.; Bengali, S.; Hsu, A.T.W.; Abadchi, S.N.; Harmon, J.W.; Jay, S.M. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv. Healthc. Mater. 2022, 11, e2002070. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp. Neurol. 2021, 341, 113694. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y.; et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, X.; Chi, J.; Che, K.; Ma, X.; Qiu, M.; Fu, Z.; Wang, Y.; Wang, Y.; Wang, W. Resveratrol Enhances Wound Healing in Type 1 Diabetes Mellitus by Promoting the Expression of Extracellular Vesicle-Carried MicroRNA-129 Derived from Mesenchymal Stem Cells. J. Proteome Res. 2022, 21, 313–324. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sun, J.; Yuan, Y.; Sun, Z. Effects of atorvastatin on autophagy in skeletal muscles of diabetic rats. J. Diabetes Investig. 2018, 9, 753–761. [Google Scholar] [CrossRef]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- David, B.T.; Curtin, J.J.; Brown, J.L.; Coutts, D.J.C.; Boles, N.C.; Hill, C.E. Treatment with hypoxia-mimetics protects cultured rat Schwann cells against oxidative stress-induced cell death. Glia 2021, 69, 2215–2234. [Google Scholar] [CrossRef]
- Ding, J.; Wang, X.; Chen, B.; Zhang, J.; Xu, J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed Res. Int. 2019, 2019, 9742765. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, W.Q.; Zhu, Y.; Han, X.Q.; Liu, N. Exosomes Derived From Mesenchymal Stromal Cells Pretreated With Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells. Front. Endocrinol. 2018, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, F.; Wang, X.; Song, Q.; Ye, Z.; Mohammadniaei, M.; Zhang, M.; Chu, X.; Xi, S.; Zhou, N.; et al. An Optimally Designed Engineering Exosome-Reductive COF Integrated Nanoagent for Synergistically Enhanced Diabetic Fester Wound Healing. Small 2022, 18, e2200895. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef]
- Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol. J. 2016, 11, 473–486. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012, 347, 701–711. [Google Scholar] [CrossRef]
- Lo, Y.P.; Liu, Y.S.; Rimando, M.G.; Ho, J.H.; Lin, K.H.; Lee, O.K. Three-dimensional spherical spatial boundary conditions differentially regulate osteogenic differentiation of mesenchymal stromal cells. Sci. Rep. 2016, 6, 21253. [Google Scholar] [CrossRef]
- Yu, W.; Li, S.; Guan, X.; Zhang, N.; Xie, X.; Zhang, K.; Bai, Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater. Adv. 2022, 133, 112646. [Google Scholar] [CrossRef]
- Su, N.; Gao, P.L.; Wang, K.; Wang, J.Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Robinson, M.; Petrie, T.; Spandler, V.; Boyd, W.D.; Sondergaard, C.S. Small intestinal submucosa-derived extracellular matrix bioscaffold significantly enhances angiogenic factor secretion from human mesenchymal stromal cells. Stem Cell Res. Ther. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, W.; Li, J.J.; Chai, S.; Xing, D.; Yu, H.; Zhang, Y.; Yan, W.; Xu, Z.; Zhao, B.; et al. A low dose cell therapy system for treating osteoarthritis: In vivo study and in vitro mechanistic investigations. Bioact. Mater. 2022, 7, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Wedzinska, A.; Kot, M.; Sarnowska, A. Effect of Long-Term 3D Spheroid Culture on WJ-MSC. Cells 2021, 10, 719. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111671. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Ma, S.; Hu, H.; Wu, J.; Li, X.; Ma, X.; Zhao, Z.; Liu, Z.; Wu, C.; Zhao, B.; Wang, Y.; et al. Functional extracellular matrix hydrogel modified with MSC-derived small extracellular vesicles for chronic wound healing. Cell Prolif. 2022, 55, e13196. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Tao, S.C.; Guo, S.C.; Li, M.; Ke, Q.F.; Guo, Y.P.; Zhang, C.Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl. Med. 2017, 6, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, M.; Jia, H.; Wu, P. Extracellular vesicles: Emerging anti-cancer drugs and advanced functionalization platforms for cancer therapy. Drug Deliv. 2022, 29, 2513–2538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Li, Y.; Su, L.; Duan, Y.; Zhang, H.; An, J.; Ni, T.; Li, X.; Zhang, X. Therapeutic Effect of Rapamycin-Loaded Small Extracellular Vesicles Derived from Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Front. Immunol. 2022, 13, 864956. [Google Scholar] [CrossRef]
- Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef]
- Ko, K.W.; Yoo, Y.I.; Kim, J.Y.; Choi, B.; Park, S.B.; Park, W.; Rhim, W.K.; Han, D.K. Attenuation of Tumor Necrosis Factor-α Induced Inflammation by Umbilical Cord-Mesenchymal Stem Cell Derived Exosome-Mimetic Nanovesicles in Endothelial Cells. Tissue Eng. Regen. Med. 2020, 17, 155–163. [Google Scholar] [CrossRef]
- Gondaliya, P.; Sayyed, A.A.; Bhat, P.; Mali, M.; Arya, N.; Khairnar, A.; Kalia, K. Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol. Pharm. 2022, 19, 1294–1308. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Long, Y.; Chen, Y. Emerging Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Chronic Respiratory Diseases: An Overview of Recent Progress. Front. Bioeng. Biotechnol. 2022, 10, 845042. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef]
- Upadhya, D.; Shetty, A.K. Promise of extracellular vesicles for diagnosis and treatment of epilepsy. Epilepsy Behav. 2021, 121, 106499. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Raz, I. Insulin Therapy: Future Perspectives. Am. J. Ther. 2020, 27, e121–e132. [Google Scholar] [CrossRef] [PubMed]
- Regazzi, R. MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin. Ther. Targets 2018, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]



| Method | Principle | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Ultracentrifugation | According to the size, density, and shape of MSC-EVs | Low cost, simple operation, suitable for large samples | Time-consuming, low yield, poor integrity | [47] |
| Density gradient centrifugation | Based on the density of MSC-EVs | High purity | Time consuming, complex operation | [48] |
| Size exclusion chromatography | Based on the size of MSC-EVs | Simple operation, high yield, high purity, good integrity | High cost, suitable for low sample volume | [49] |
| Immunoaffinity capture | Specific binding of antibody to the surface marker of MSC-EVs | High purity | High cost, low yield | [50] |
| Ultrafiltration | Based on the size of MSC-EVs | Efficiency, simple operation | Low purity | [51] |
| Polymer precipitation | Changing the solubility and dispersibility of MSC-EVs | High yield, simple operation | Low purity, high cost | [52] |
| Disease | Animal Model | Injection of MSC-EVs | Effect of MSC-EVs In Vivo | Cell Culture | Effect of MSC-EVs In Vitro | Reference |
|---|---|---|---|---|---|---|
| DM | N/A | N/A | N/A | Dendritic cells from diabetic patients | Induce immature IL-10- secreting dendritic cells to alleviate inflammation | [63] |
| DM | NOD mice | Intravenous | Inhibit the activation of antigen- presenting cells and the development of T helper cells | N/A | N/A | [64] |
| DM | STZ-induced diabetic mice | Intraperitoneal | Enhance the islet number and improve glycemic control by increasing regulatory T-cell population | N/A | N/A | [65] |
| DM | STZ-induced diabetic rats | Intravenous | Reduce blood glucose level, inhibit β-cell apoptosis, and alleviate peripheral insulin resistance | PA-treated LO2 cells and HG medium-treated L6 cells | Enhance glucose uptake and glycolysis in L6 cells, and reduce glycogenolysis in LO2 cells | [66] |
| DM | NOD mice injected with STZ | Intrapancreatic | Increase islet number and β-cell mass, elevate insulin level, and reduce blood glucose | HMVECs cultured in serum-starved conditions | Enhance the tube formation ability of HMVECs | [67] |
| DM | STZ-induced diabetic rats | Intravenous | Promote islet regeneration and insulin production through Pdx-1 mechanism | N/A | N/A | [68] |
| DM | STZ-induced diabetic mice | Intravenous | Alleviate hyperglycemia and facilitate pancreatic regeneration by regulating the Extl3-Reg-cyclinD1 pathway | N/A | N/A | [69] |
| DM | STZ-induced diabetic rats | Intravenous | Improve β-cell function by miR-146a-mediated inhibition on Numb expression | INS-1 cells cultured in HG medium | Alleviate cell dedifferentiation | [70] |
| DM | STZ-induced diabetic rats | Intravenous | Ameliorate insulin resistance and relieve the structural injury of pancreas, kidney, and liver | HSkMCs cultured in serum-starved conditions | Increase glucose uptake by HSkMCs | [71] |
| DM | STZ-induced diabetic rats | Intravenous | Promote hepatic glycolysis, glycogen storage and lipolysis, and reduce gluconeogenesis | PA-treated LO2 cells | Promote glycolysis and glycogen synthesis, and inhibit gluconeogenesis | [72] |
| Diabetic nephropathy | STZ-induced diabetic rats | Intravenous | Alleviate pathologic changes in the renal glomerulus and tubule | N/A | N/A | [78] |
| Diabetic nephropathy | STZ-induced diabetic rats | Intravenous | Suppress mesangial hyperplasia and kidney fibrosis by miR-125a-induced HDAC1 inhibition | Glomerular mesangial cells cultured in HG medium | Reduce IL-6, collagen I, and fibronectin expressions, and promote cell apoptosis | [79] |
| Diabetic nephropathy | STZ-induced diabetic mice | Intravenous | Suppress renal fibrosis | N/A | N/A | [80] |
| Diabetic nephropathy | Db/db mice | Intravenous | Alleviate renal apoptosis and EMT by miR-424-5p-induced YAP1 inhibition | HK2 cells cultured in HG medium | Promote cell proliferation and inhibit EMT process | [81] |
| Diabetic nephropathy | Db/db mice | Intravenous | Improve renal function and inhibit renal apoptosis by miR-486-induced Smad1 inhibition | MPC5 cells cultured in HG medium | Improve cell viability and inhibit cell apoptosis | [82] |
| Diabetic nephropathy | N/A | N/A | N/A | MPC5 cells cultured in HG medium | Alleviate EMT by miR-215-5p-induced ZEB2 inhibition | [85] |
| Diabetic nephropathy | N/A | N/A | N/A | MPC5 cells cultured in HG medium | Alleviate inflammation and apoptosis by miR-15b-5p-induced PDK4 inhibition | [86] |
| Diabetic nephropathy | Db/db mice | Intravenous | Improve renal function and inhibit renal apoptosis by miR-26a-5p- induced TLR4 inhibition | MPC5 cells cultured in HG medium | Promote cell proliferation | [87] |
| Diabetic nephropathy | N/A | N/A | N/A | HKCs cultured in HG medium | Promote cell proliferation by miR-125b-induced TRAF6 inhibition | [88] |
| Diabetic nephropathy | STZ-induced diabetic mice | Intravenous | Inhibit renal apoptosis, inflammation, and fibrosis. | N/A | N/A | [89] |
| Diabetic retinopathy | N/A | N/A | N/A | Human RMECs cultured in HG medium | Suppress EMT and tube formation by delivering lncRNA SNHG7 | [92] |
| Diabetic retinopathy | STZ-induced diabetic rats | Intravitreal | Alleviate retinal structure disruption and vascular damage | N/A | N/A | [93] |
| Diabetic retinopathy | STZ-induced diabetic rats | Intravitreal | Inhibit retinal oxidative stress, inflammation, and angiogenesis by suppressing Wnt/β-catenin signaling | N/A | N/A | [94] |
| Diabetic retinopathy | STZ-induced diabetic rats | Intravitreal | Alleviate retinal apoptosis and oxidative stress by NEDD4-mediated PTEN inhibition | RPE cells cultured in HG medium | Inhibit cell apoptosis and oxidative injury | [95] |
| Diabetic retinopathy | STZ-induced diabetic rats | Intravitreal | Ameliorate retinal vascular leakage and inflammation by miR-18b-induced MAP3K1 inhibition | Human RMECs cultured in HG medium | Inhibit cell inflammation and apoptosis | [96] |
| Diabetic retinopathy | STZ-induced diabetic mice | Intravitreal | Alleviate retinal inflammation and oxidative stress by miR-17-3p-induced STAT1 inhibition | N/A | N/A | [97] |
| Diabetic retinopathy | STZ-induced diabetic mice | Intravitreal | Alleviate retinal apoptosis, inflammation, and oxidative stress by miR-486-3p-induced TLR4 inhibition | Muller cells cultured in HG medium | Promote cell proliferation | [98] |
| Diabetic retinopathy | STZ-induced diabetic mice | Intravitreal | Suppress retinal inflammation by miR-146a-induced TLR4 inhibition | N/A | N/A | [99] |
| Diabetic retinopathy | STZ-induced diabetic rats | Intravitreal | Ameliorate retinal inflammation and angiogenesis by miR-192-induced ITGA1 inhibition | HG medium-cultured RPE cells, Muller cells and human RMECs | Inhibit RPE cell apoptosis, Muller cell activation and RMECs proliferation | [100] |
| Diabetic retinopathy | KK/Upj-Ay mice | Intravitreal | Ameliorate retinal oxidative stress and neovascularization by miR-133b-3p- induced fibrillin-1 inhibition | HG medium-cultured mouse RMECs | Alleviate cell oxidative stress and angiogenesis | [101] |
| Diabetic retinopathy | N/A | N/A | N/A | Rat retinal neurons cultured in HG medium | Alleviate neuronal apoptosis by activating BDNF-TrkB pathway | [102] |
| Diabetic wound healing | STZ-induced diabetic mice | At the wound site | Promote granulation tissue formation and angiogenesis | HUVECs cultured in HG medium | Promote cell proliferation and tube formation, and inhibit oxidative stress and inflammation | [108] |
| Diabetic wound healing | Db/db mice | At the wound site | Promote wound closure, re-epithelialization, and collagen synthesis | Human dermal fibroblasts cultured in HG medium | Enhance cell proliferation and migration | [109] |
| Diabetic wound healing | Db/db mice | Subcutaneous | Promote angiogenesis and wound closure by activating SIRT3/SOD2 signaling | HUVECs cultured in HG medium | Promote cell proliferation, migration, and tube formation | [110] |
| Diabetic wound healing | STZ-induced diabetic rats | Intradermal | Accelerate wound closure, collagen deposition, and angiogenesis | AGE-treated HUVECs | Promote cell proliferation, migration, and tube formation | [111] |
| Diabetic wound healing | STZ-induced diabetic mice | Injected into the skin around the wound | Promote angiogenesis and collagen deposition, and inhibit inflammation by delivering lncRNA H19 | Fibroblast cultured in HG medium | Promote cell proliferation and migration | [112] |
| Diabetic wound healing | STZ-induced diabetic rats | Intramuscular | Promote blood perfusion and angiogenesis by delivering miR-21-5p | HUVECs cultured in HG medium | Promote cell proliferation, migration, and tube formation | [113] |
| Diabetic wound healing | Db/db mice | Injected into the skin around the wound | Promote angiogenesis by miR-17-5p-induced PTEN inhibition | HUVECs cultured in HG medium | Promote cell proliferation, migration, and tube formation | [114] |
| Diabetic wound healing | STZ-induced diabetic mice | Injected into the skin around the wound | Accelerate wound closure and angiogenesis by miR-146a-induced Src inhibition | HUVECs cultured in HG medium | Promote cell proliferation, migration, and tube formation | [115] |
| Diabetic neuropathy | Db/db mice | Intravenous | Increase nerve conduction velocity, intraepidermal nerve fiber number, and myelin thickness | N/A | N/A | [119] |
| Diabetic neuropathy | STZ-induced diabetic rats | Intravenous | Improve blood brain barrier integrity, promote white matter remodeling, and inhibit inflammation | N/A | N/A | [120] |
| Diabetic neuropathy | Db/db mice | Intravenous | Alleviate neuroinflammation by miR-183-5p-induced inhibition on PDCD4/NLRP3 signaling | BV2 cells cultured in HG medium | Inhibit cell oxidative stress, inflammation, and apoptosis | [121] |
| Diabetic neuropathy | STZ-induced diabetic mice | ICV | Inhibit oxidative stress, increase synaptic density, and repair damaged neurons and astrocytes | N/A | N/A | [122] |
| Diabetic neuropathy | STZ-induced diabetic mice | Intracranial | Improve cognitive impairment and histological abnormalities | N/A | N/A | [123] |
| Diabetic neuropathy | STZ-induced diabetic mice | Intravenous | Recover proliferation and neuronal-differentiation capacity of hippocampal neural stem cells | N/A | N/A | [124] |
| EVs Source | Hydrogel Details | Hydrogel Loading | Diabetic Model | Outcomes | Reference |
|---|---|---|---|---|---|
| Umbilical cord-derived MSC | polyvinyl alcohol/alginate nanohydrogel | Mixing, stirring, and gelation | STZ-induced diabetic rats | Increased angiogenesis by promoting VEGF expression | [158] |
| Adipose-derived MSC | FHE hydrogel composed of Pluronic F127, oxidative hyaluronic acid, and poly-ε-lysine | Mixing, stirring, and gelation | STZ-induced diabetic mice | Enhanced wound closure rates, angiogenesis, re-epithelization, and collagen deposition | [159] |
| Umbilical cord-derived MSC | Porcine small intestinal submucosa-based hydrogel | Fusion peptide- mediated binding of MSC-EVs to hydrogel | STZ-induced diabetic rats | Elevated granulation tissue and collagen fiber formation and neovascularization | [160] |
| Umbilical cord-derived MSC | Pluronic F-127 hydrogel | Mixing and gelation | STZ-induced diabetic rats | Enhanced wound closure rate and granulation tissue regeneration by promoting CD31, Ki67 and VEGF expressions | [161] |
| Bone marrow MSC | Carboxyethyl chitosan-dialdehyde carboxymethyl cellulose hydrogel | Mixing, stirring, and gelation | STZ-induced diabetic rats | Increased angiogenesis and reduced inflammation | [162] |
| Gingival MSC | Chitosan/silk hydrogel sponge | Seeded on the hydrogel sponge | STZ-induced diabetic rats | Enhanced re-epithelialization, collagen deposition, angiogenesis, and neuronal ingrowth | [163] |
| Synovium MSC | Chitosan hydrogel | Mixing, stirring, and gelation | STZ-induced diabetic rats | Accelerated re-epithelialization, angiogenesis, and collagen maturity | [164] |
| Strategy | Method | Principle | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Modification of MSC | Transfection | Plasmids or virus-mediated cargos delivery | Simple; Maintain the integrity of MSC-EVs | Cytotoxicity; low specificity and efficiency | [126] |
| Direct modification of MSC-EVs | Incubation | Interaction between cargos and the membrane of MSC-EVs | Simple and feasible | Low loading efficiency | [165] |
| Direct modification of MSC-EVs | Electroporation | Transient voltage-induced the generation of pores on the membrane | Rapid; High loading efficiency | Aggregation of MSC-EVs; may impair the integrity of MSC-EVs | [166] |
| Direct modification of MSC-EVs | Sonication | Membrane deformation | High loading efficiency | Aggregation of MSC-EVs; may impair the integrity of MSC-EVs | [167] |
| Direct modification of MSC-EVs | Extrusion | Mechanical force-induced the temporary destruction of the membrane | Efficient packaging | May change the membrane property | [168] |
| Direct modification of MSC-EVs | Freeze–thaw cycles | Ice crystals-induced the temporary destruction of the membrane | High loading efficiency | Aggregation of MSC-EVs; may change the membrane structure | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Sun, Y.; Wu, F.; Xu, W.; Qian, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapy for Diabetes Mellitus and Diabetic Complications. Pharmaceutics 2022, 14, 2208. https://doi.org/10.3390/pharmaceutics14102208
Sun F, Sun Y, Wu F, Xu W, Qian H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapy for Diabetes Mellitus and Diabetic Complications. Pharmaceutics. 2022; 14(10):2208. https://doi.org/10.3390/pharmaceutics14102208
Chicago/Turabian StyleSun, Fengtian, Yuntong Sun, Feng Wu, Wenrong Xu, and Hui Qian. 2022. "Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapy for Diabetes Mellitus and Diabetic Complications" Pharmaceutics 14, no. 10: 2208. https://doi.org/10.3390/pharmaceutics14102208
APA StyleSun, F., Sun, Y., Wu, F., Xu, W., & Qian, H. (2022). Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapy for Diabetes Mellitus and Diabetic Complications. Pharmaceutics, 14(10), 2208. https://doi.org/10.3390/pharmaceutics14102208







