Favorable Effect of Pemafibrate on Insulin Resistance and β-Cell Function in Subjects with Type 2 Diabetes and Hypertriglyceridemia: A Subanalysis of the PARM-T2D Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2018, 42, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes Mellitus, Fasting Glucose, and Risk of Cause-Specific Death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H. Reinventing Type 2 Diabetes. JAMA 2008, 299, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R. Insulin Action, Diabetogenes, and the Cause of Type II Diabetes. Diabetes 1994, 43, 1066–1085. [Google Scholar] [CrossRef]
- Kendall, D.M.; Cuddihy, R.M.; Bergenstal, R.M. Clinical Application of Incretin-Based Therapy: Therapeutic Potential, Patient Selection and Clinical Use. Am. J. Med. 2009, 122, S37–S50. [Google Scholar] [CrossRef]
- U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes 1995, 44, 1249–1258. [Google Scholar] [CrossRef]
- Blüher, M.; Malhotra, A.; Bader, G. Beta-cell function in treatment-naïve patients with type 2 diabetes mellitus: Analyses of baseline data from 15 clinical trials. Diabetes Obes. Metab. 2023, 25, 1403–1407. [Google Scholar] [CrossRef]
- Bellia, A.; Andreadi, A.; Giudice, L.; De Taddeo, S.; Maiorino, A.; D’ippolito, I.; Giorgino, F.M.; Ruotolo, V.; Romano, M.; Magrini, A.; et al. Atherogenic Dyslipidemia on Admission Is Associated with Poorer Outcome in People With and Without Diabetes Hospitalized for COVID-19. Diabetes Care 2021, 44, 2149–2157. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin resistance and compensatory hyperinsulinemia: Role in hypertension, dyslipidemia, and coronary heart disease. Am. Heart J. 1991, 121, 1283–1288. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E. Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.-C. Mechanism of Action of Fibrates on Lipid and Lipoprotein Metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From Orphan Receptors to Drug Discovery. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Arai, H.; Yokote, K.; Araki, E.; Suganami, H.; Yamashita, S. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: Results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J. Clin. Lipidol. 2018, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Effects of Pemafibrate, a Novel Selective PPARα Modulator, on Lipid and Glucose Metabolism in Patients with Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2018, 41, 538–546. [Google Scholar] [CrossRef]
- Andreadi, A.; Muscoli, S.; Tajmir, R.; Meloni, M.; Muscoli, C.; Ilari, S.; Mollace, V.; Della Morte, D.; Bellia, A.; Di Daniele, N.; et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1646. [Google Scholar] [CrossRef]
- Kito, K.; Nomoto, H.; Sakuma, I.; Nakamura, A.; Cho, K.Y.; Kameda, H.; Miya, A.; Omori, K.; Yanagiya, S.; Handa, T.; et al. Effects of pemafibrate on lipid metabolism in patients with type 2 diabetes and hypertriglyceridemia: A multi-center prospective observational study, the PARM-T2D study. Diabetes Res. Clin. Pactr. 2022, 192, 110091. [Google Scholar] [CrossRef]
- Caumo, A.; Perseghin, G.; Brunani, A.; Luzi, L. New Insights on the Simultaneous Assessment of Insulin Sensitivity and β-Cell Function with the HOMA2 Method. Diabetes Care 2006, 29, 2733–2734. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Yokote, K.; Yamashita, S.; Arai, H.; Araki, E.; Matsushita, M.; Nojima, T.; Suganami, H.; Ishibashi, S. Effects of pemafibrate on glucose metabolism markers and liver function tests in patients with hypertriglyceridemia: A pooled analysis of six phase 2 and phase 3 randomized double-blind placebo-controlled clinical trials. Cardiovasc. Diabetol. 2021, 20, 96. [Google Scholar] [CrossRef]
- Damci, T.; Tatliagac, S.; Osar, Z.; Ilkova, H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur. J. Intern. Med. 2003, 14, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Anderlová, K.; Doležalová, R.; Housová, J.; Bošanská, L.; Haluzíková, D.; Křemen, J.; Škrha, J.; Haluzík, M. Influence of PPAR-alpha agonist fenofibrate on insulin sensitivity and selected adipose tissue-derived hormones in obese women with type 2 diabetes. Physiol. Res. 2007, 56, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen-Markkola, H.; Taskinen, M.-R. Lowering of triglycerides by gemfibrozil affects neither the glucoregulatory nor antilipolytic effect of insulin in Type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993, 36, 161–169. [Google Scholar] [CrossRef]
- Avogaro, A.; Miola, M.; Favaro, A.; Gottardo, L.; Pacini, G.; Manzato, E.; Zambon, S.; Sacerdoti, D.; De Kreutzenberg, S.; Piliego, T.; et al. Gemfibrozil improves insulin sensitivity and flow-mediated vasodilatation in type 2 diabetic patients. Eur. J. Clin. Investig. 2001, 31, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Banach, M.; Atkin, S.L.; Gotto, A.M.; Sahebkar, A. Effect of fibrates on glycemic parameters: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol. Res. 2018, 132, 232–241. [Google Scholar] [CrossRef]
- Kostadinova, R.; Wahli, W.; Michalik, L. PPARs in Diseases: Control Mechanisms of Inflammation. Curr. Med. Chem. 2005, 12, 2995–3009. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kagaya, Y.; Saito, H.; Kanazawa, M.; Sato, K.; Miura, M.; Kondo, M.; Endo, H. Efficacy and Safety of Pemafibrate Versus Bezafibrate to Treat Patients with Hypertriglyceridemia: A Randomized Crossover Study. J. Atheroscler. Thromb. 2023, 30, 443–454. [Google Scholar] [CrossRef]
- Hennuyer, N.; Duplan, I.; Paquet, C.; Vanhoutte, J.; Woitrain, E.; Touche, V.; Colin, S.; Vallez, E.; Lestavel, S.; Lefebvre, P.; et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis 2016, 249, 200–208. [Google Scholar] [CrossRef]
- Keinicke, H.; Sun, G.; Junker Mentzel, C.M.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin. Exp. Hepatol. 2020, 6, 270–274. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate improves hepatic inflammation, function and fibrosis in patients with non-alcoholic fatty liver disease: A one-year observational study. Clin. Exp. Hepatol. 2021, 7, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Changing the Concept of Type 2 Diabetes: Beta Cell Workload Hypothesis Revisited. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Eguchi, Y.; Yoneda, M.; Imajo, K.; Tamaki, N.; Suganami, H.; Nojima, T.; Tanigawa, R.; Iizuka, M.; Iida, Y.; et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2021, 54, 1263–1277. [Google Scholar] [CrossRef]
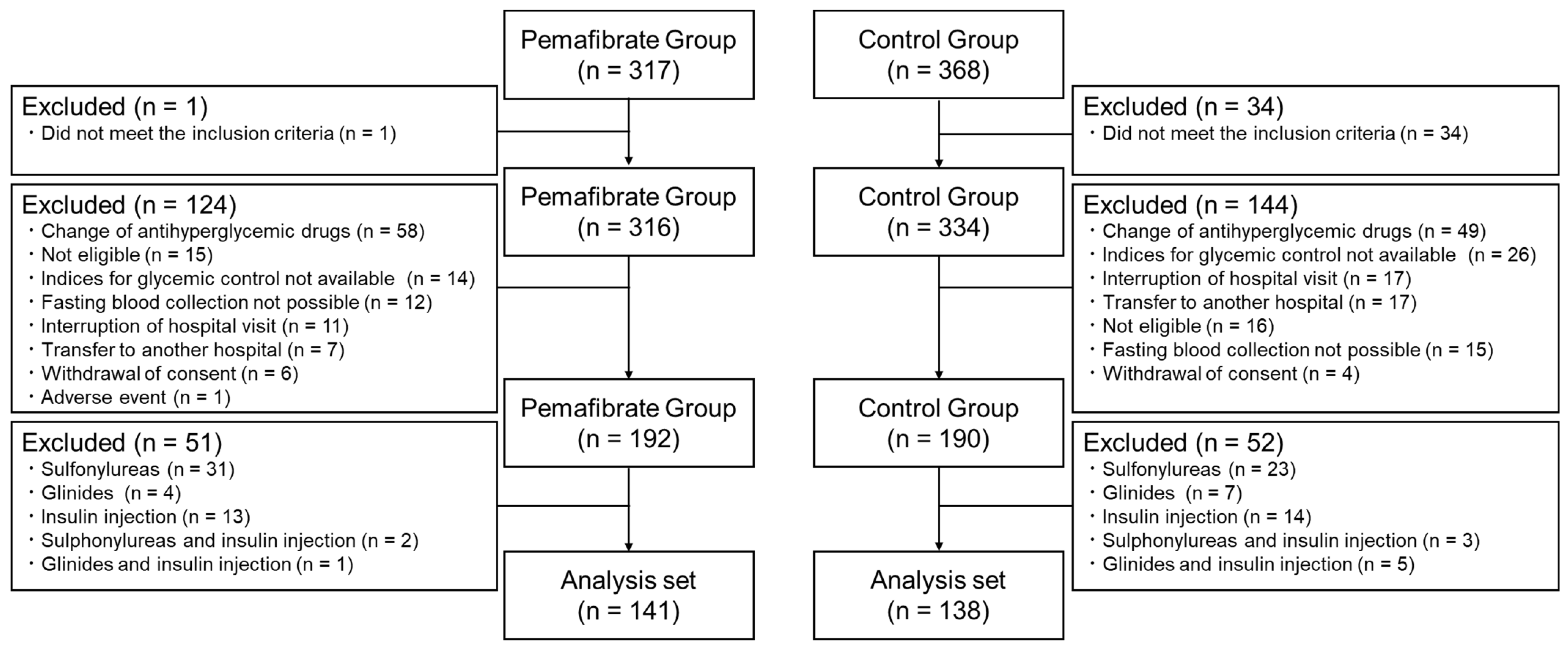
| Variables | Pemafibrate (n = 141) | Control (n = 138) | p-Value |
|---|---|---|---|
| Age (years) | 60.1 ± 12.3 | 60.9 ± 11.6 | 0.573 |
| Female sex (n, %) | 45 (31.9) | 54 (39.1) | 0.214 |
| Body mass index (kg/m2) | 27.3 ± 4.1 | 27.4 ± 4.8 | 0.932 |
| HbA1c (%) | 6.92 ± 0.78 | 6.83 ± 0.59 | 0.254 |
| FPG (mg/dL) | 135.2 ± 30.3 | 132.5 ± 26.3 | 0.423 |
| C-peptide (mg/dL) | 2.87 ± 1.32 | 2.84 ± 1.53 | 0.840 |
| T-Cho (mg/dL) | 185.0 ± 31.7 | 186.1 ± 32.8 | 0.732 |
| Triglyceride (mg/dL) | 171 (133, 235) | 168 (126, 226) | 0.708 |
| HDL-C (mg/dL) | 52.4 ± 11.9 | 52.0 ± 13.7 | 0.780 |
| AST (IU/L) | 31.5 ± 15.7 | 28.2 ± 14.5 | 0.069 |
| ALT (IU/L) | 30 (20, 45) | 28 (17, 39) | 0.102 |
| γ-GTP (IU/L) | 39 (24, 72) | 41 (23, 56) | 0.349 |
| eGFR (mL/min/1.73 m2) | 69.6 ± 18.3 | 69.7 ± 22.1 | 0.878 |
| Fibrates (n, %) | 52 (36.9) | 43 (31.2) | 0.377 |
| α-glucosidase inhibitors (n, %) | 2 (1.4) | 2 (1.5) | 1.000 |
| Biguanides (n, %) | 90 (63.8) | 87 (63.0) | 0.902 |
| DPP-4 inhibitors (n, %) | 66 (46.8) | 65 (47.1) | 1.000 |
| GLP-1 receptor agonists (n, %) | 7 (5.0) | 10 (7.2) | 0.463 |
| SGLT2 inhibitors (n, %) | 66 (46.8) | 49 (35.5) | 0.068 |
| Thiazolidines (n, %) | 7 (5.0) | 3 (2.2) | 0.335 |
| Sulfonylureas (n, %) | 0 | 0 | NA |
| Glinides (n, %) | 0 | 0 | NA |
| Insulin injections (n, %) | 0 | 0 | NA |
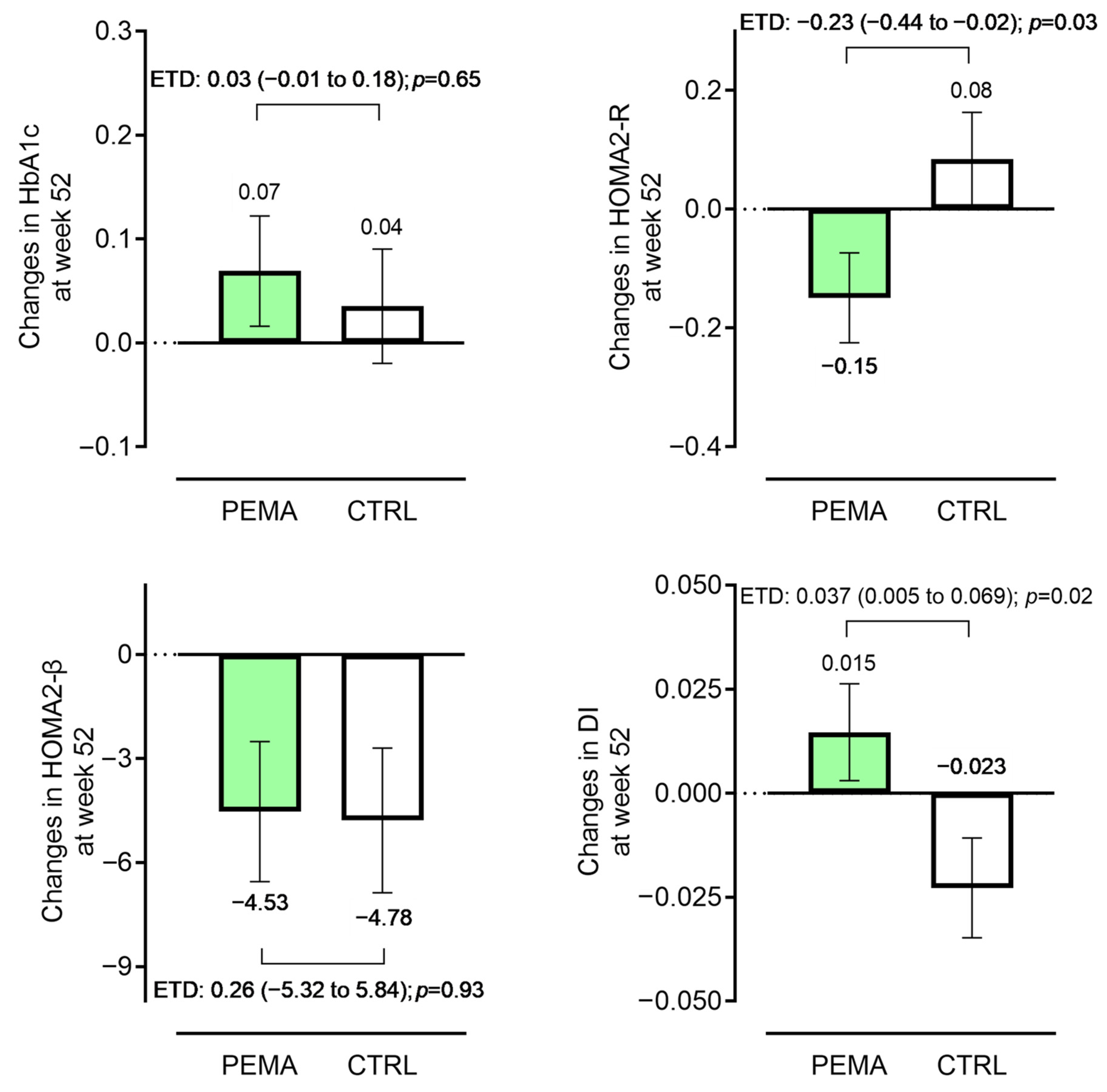
| Week 0 | Week 24 | Week 52 | Mean Change at Week 52 | p-Value between Groups at Week 52 | ||
|---|---|---|---|---|---|---|
| HbA1c (%) | PEMA (n = 141) | 6.92 ± 0.78 | 7.03 ± 0.93 | 6.99 ± 0.81 | 0.06(−0.04 to 0.16) | 0.940 |
| CTRL (n = 138) | 6.83 ± 0.59 | 6.94 ± 0.76 | 6.89 ± 0.80 | 0.06(−0.05 to 0.18) | ||
| HOMA2-R | PEMA (n = 141) | 2.11 (1.53, 2.95) | a 2.01 (1.43, 2.77) | 1.92 (1.45, 2.66) * | −0.20(−0.33 to −0.20) | 0.017 |
| CTRL (n = 138) | 1.99 (1.47, 2.82) | b 1.98 (1.51, 2.74) | 1.94 (1.47, 2.87) | 0.03(−0.08 to 0.11) | ||
| HOMA2-β | PEMA (n = 141) | 75.0 (57.2, 95.3) | a 69.0 (52.0, 84.8) | 69.4 (55.5, 91.7) | −3.9(−5.6 to −1.0) | 0.451 |
| CTRL (n = 138) | 75.9 (59.1, 94.0) | b 78.0 (55.9, 100.1) | 71.3 (54.4, 93.0) | −1.1(−3.0 to 1.5) | ||
| Disposition index | PEMA (n = 141) | 0.36 (0.28, 0.46) | a 0.35 (0.26, 0.47) | 0.36 (0.27, 0.53) | 0.02(−0.01 to 0.04) | 0.030 |
| CTRL (n = 138) | 0.37 (0.27, 0.48) | b 0.36 (0.30, 0.47) | 0.36 (0.27, 0.47) | −0.01(−0.04 to 0.01) |
| ΔHOMA-2R | ΔDI | |||
|---|---|---|---|---|
| Variables | ρ | p-Value | ρ | p-Value |
| ΔBMI (kg/m2) | 0.119 | 0.160 | −0.018 | 0.037 |
| ΔTriglyceride (mg/dL) | 0.265 | 0.002 | −0.258 | 0.002 |
| ΔHDL-C (ng/mL) | −0.190 | 0.002 | 0.130 | 0.124 |
| ΔAST (IU/L) | 0.017 | 0.838 | 0.032 | 0.709 |
| ΔALT (IU/L) | 0.148 | 0.079 | −0.142 | 0.093 |
| Δγ-GTP (IU/L) | 0.329 | <0.001 | −0.335 | <0.001 |
| ΔeGFR (mL/min/1.73 m2) | 0.017 | 0.838 | −0.161 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomoto, H.; Kito, K.; Iesaka, H.; Oe, Y.; Kawata, S.; Tsuchida, K.; Yanagiya, S.; Miya, A.; Kameda, H.; Cho, K.Y.; et al. Favorable Effect of Pemafibrate on Insulin Resistance and β-Cell Function in Subjects with Type 2 Diabetes and Hypertriglyceridemia: A Subanalysis of the PARM-T2D Study. Pharmaceutics 2023, 15, 1838. https://doi.org/10.3390/pharmaceutics15071838
Nomoto H, Kito K, Iesaka H, Oe Y, Kawata S, Tsuchida K, Yanagiya S, Miya A, Kameda H, Cho KY, et al. Favorable Effect of Pemafibrate on Insulin Resistance and β-Cell Function in Subjects with Type 2 Diabetes and Hypertriglyceridemia: A Subanalysis of the PARM-T2D Study. Pharmaceutics. 2023; 15(7):1838. https://doi.org/10.3390/pharmaceutics15071838
Chicago/Turabian StyleNomoto, Hiroshi, Kenichi Kito, Hiroshi Iesaka, Yuki Oe, Shinichiro Kawata, Kazuhisa Tsuchida, Shingo Yanagiya, Aika Miya, Hiraku Kameda, Kyu Yong Cho, and et al. 2023. "Favorable Effect of Pemafibrate on Insulin Resistance and β-Cell Function in Subjects with Type 2 Diabetes and Hypertriglyceridemia: A Subanalysis of the PARM-T2D Study" Pharmaceutics 15, no. 7: 1838. https://doi.org/10.3390/pharmaceutics15071838
APA StyleNomoto, H., Kito, K., Iesaka, H., Oe, Y., Kawata, S., Tsuchida, K., Yanagiya, S., Miya, A., Kameda, H., Cho, K. Y., Sakuma, I., Manda, N., Nakamura, A., & Atsumi, T. (2023). Favorable Effect of Pemafibrate on Insulin Resistance and β-Cell Function in Subjects with Type 2 Diabetes and Hypertriglyceridemia: A Subanalysis of the PARM-T2D Study. Pharmaceutics, 15(7), 1838. https://doi.org/10.3390/pharmaceutics15071838






