Magnetite Microspheres for the Controlled Release of Rosmarinic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
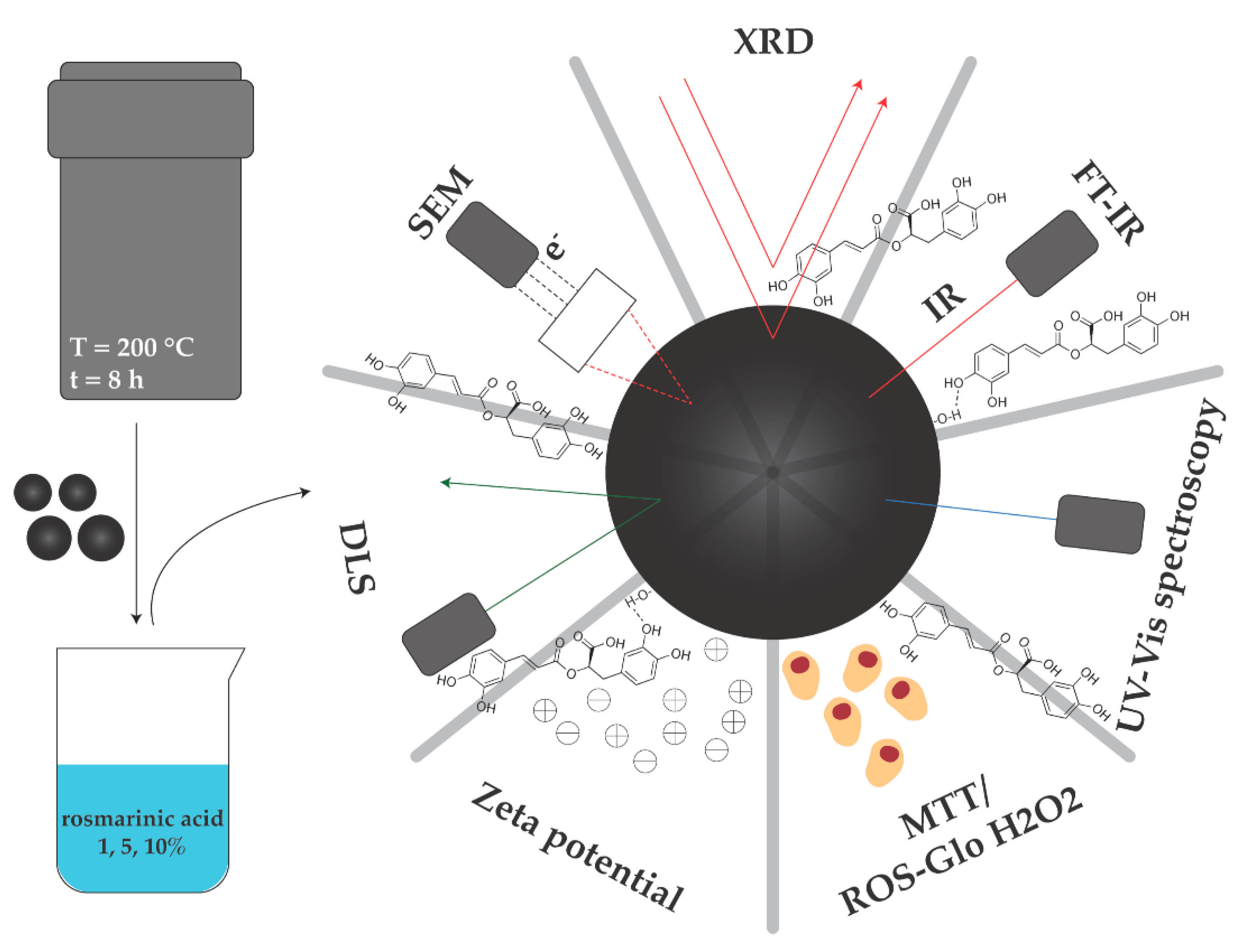
2.2. Synthesis of Magnetite Microspheres and RA Loading
2.3. Physicochemical Characterization
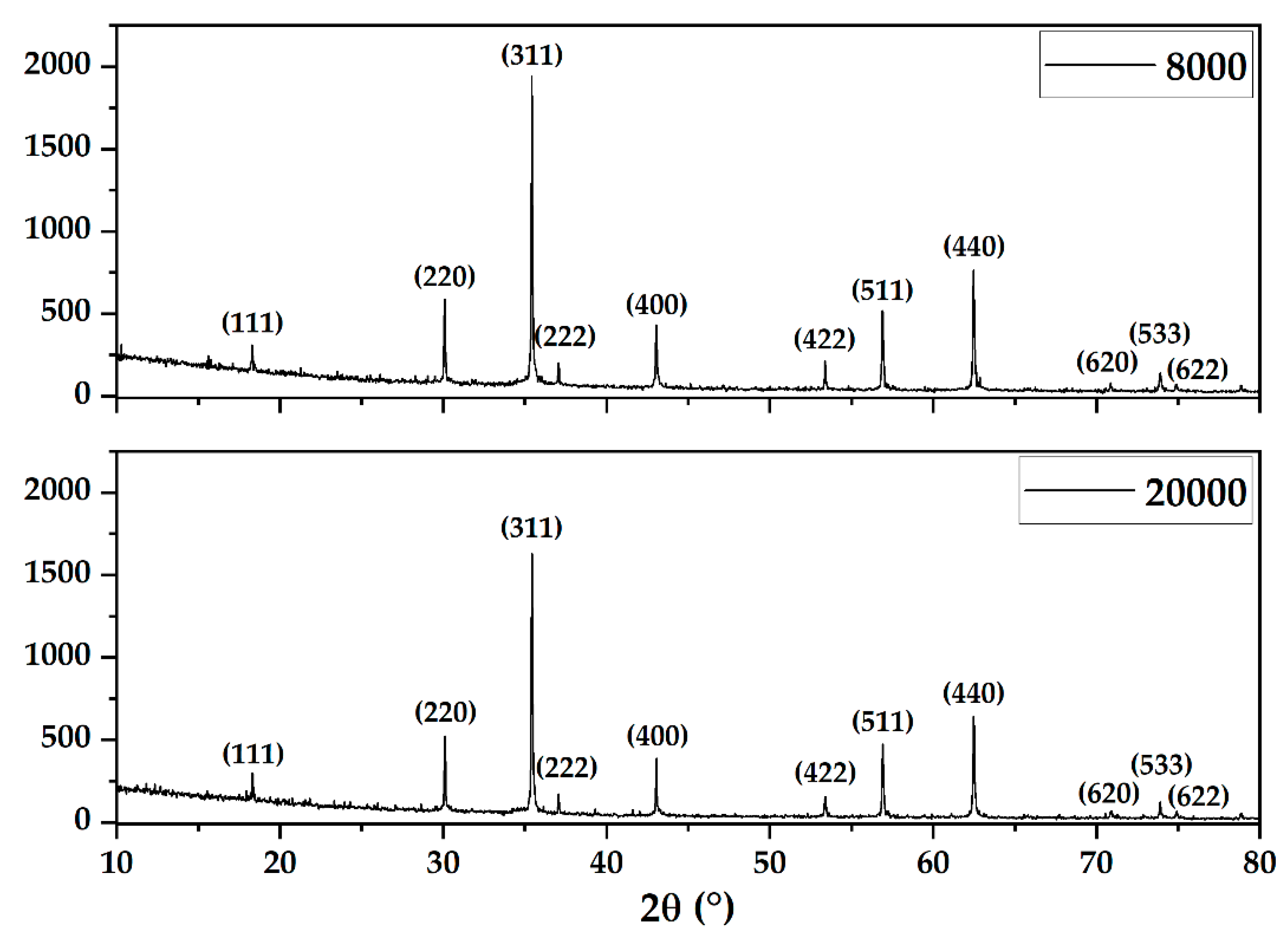
2.3.1. X-ray Diffraction (XRD) Coupled with Rietveld Refinement
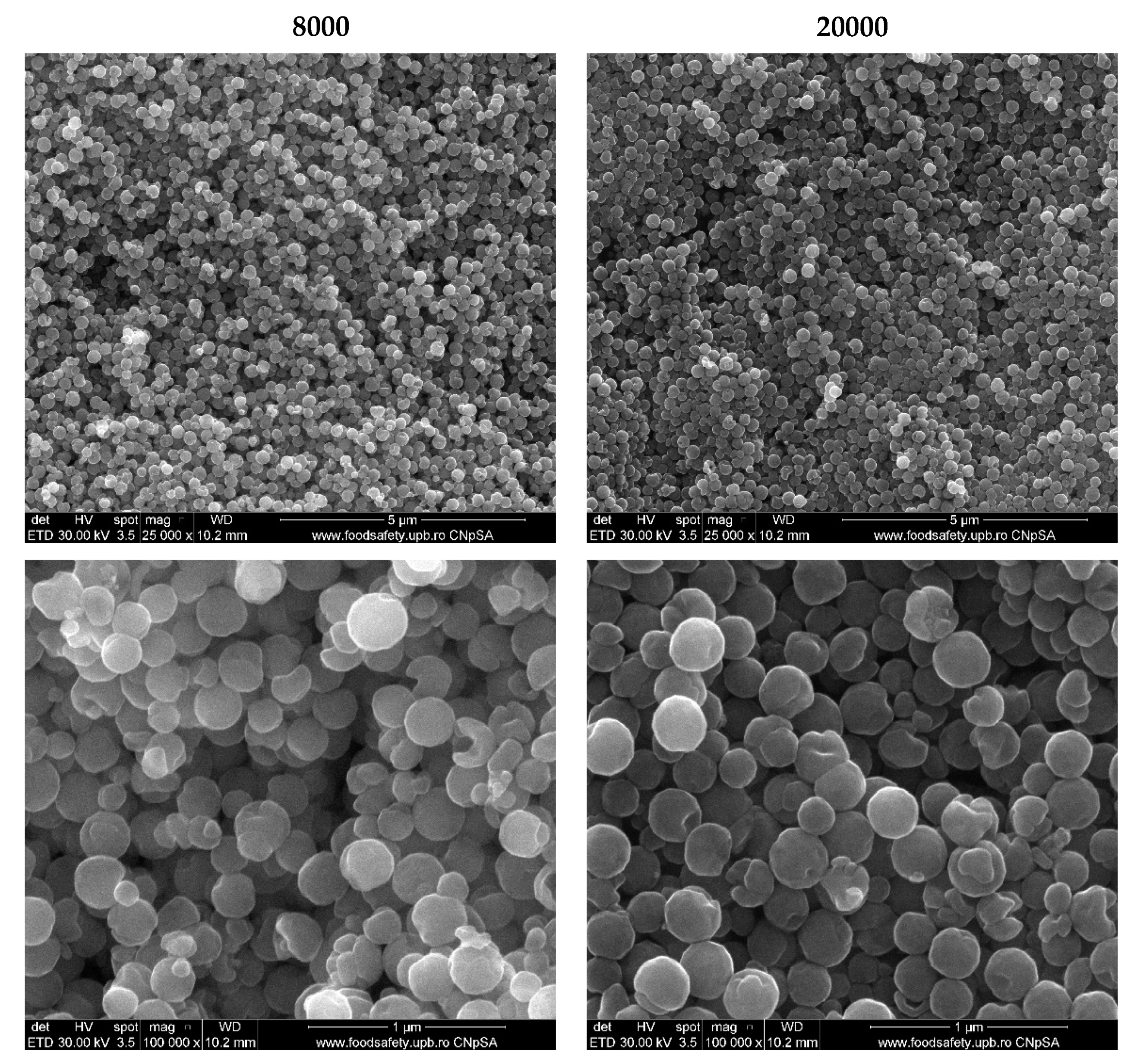
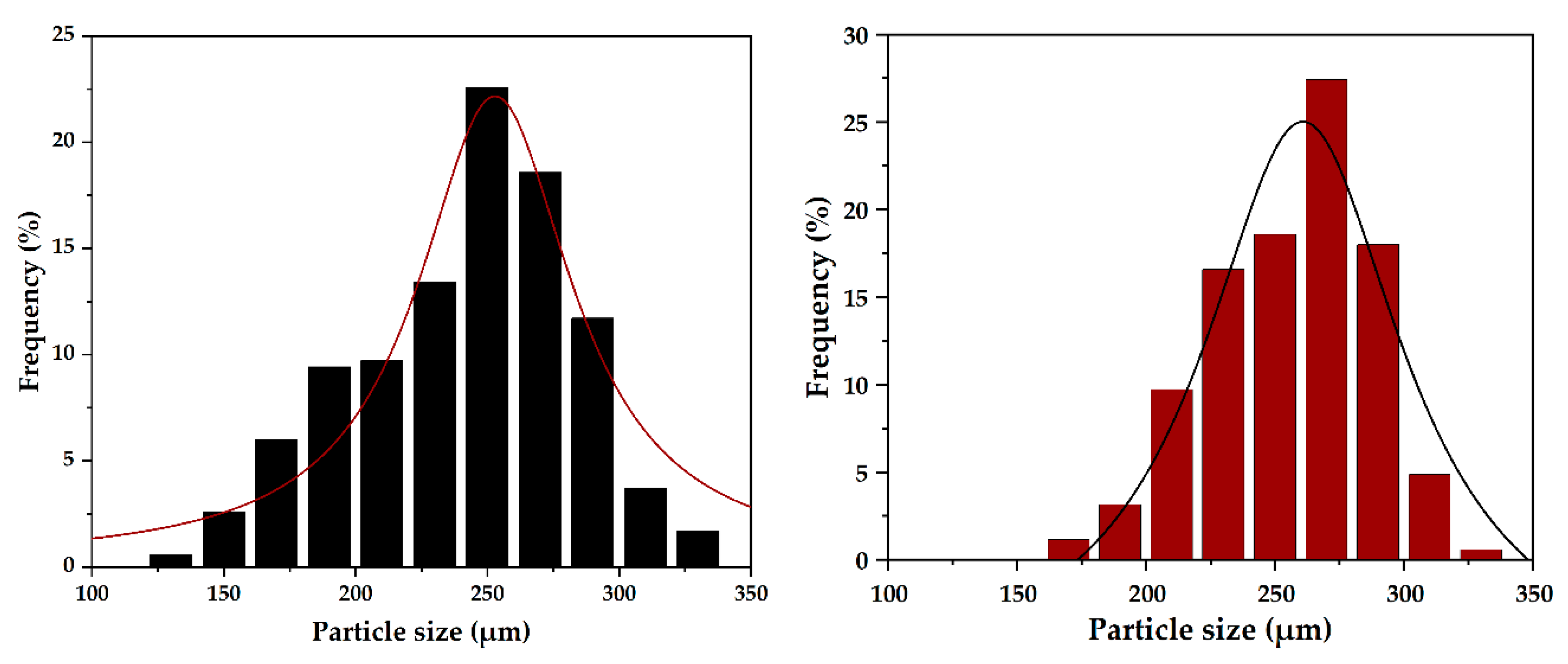
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.4. Dynamic Light Scattering (DLS) and Zeta Potential
2.3.5. UV–Vis Spectrophotometry
2.4. Biological Evaluations
2.4.1. ROS-Glo H2O2 Assay
2.4.2. MTT Assay
2.4.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Taufiq, A.; Nikmah, A.; Hidayat, A.; Sunaryono, S.; Mufti, N.; Hidayat, N.; Susanto, H. Synthesis of magnetite/silica nanocomposites from natural sand to create a drug delivery vehicle. Heliyon 2020, 6, e03784. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Biol. Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Tripodo, G. Micro and Nano-Drug Delivery Systems. 2020. Available online: https://pubs.rsc.org/en/content/chapterhtml/2020/bk9781788017725-00001?isbn=978-1-78801-772-5&sercode=bk (accessed on 23 August 2020).
- Mallick, S.P.; Panda, S.P.; Gayatri, A.; Kunaal, Y.; Naresh, C.; Suman, D.K.; Samineni, J.; Siddiqui, N.; Singh, B.N. Chitosan Oligosaccharide Based Hydrogel: An Insight into the Mechanical, Drug Delivery, and Antimicrobial Studies. Biointerface Res. Appl. Chem. 2020, 11, 10293–10300. [Google Scholar]
- Malviya, R. Non-invasive drug delivery system for the delivery of protein/peptide using neem gum and its derivatives. Biointerface Res. Appl. Chem. 2020, 10, 5460–5465. [Google Scholar]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Moodley, T.; Singh, M. Polymeric mesoporous silica nanoparticles for combination drug delivery in vitro. Biointerface Res. Appl. Chem. 2020, 11, 11905–11919. [Google Scholar]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Kanojia, N.; Singh, S.; Singh, J.; Sharma, N.; Grewal, A.; Rani, L.; Thapa, K.; Arora, S. Recent advancements and applications of inhalable microparticles based drug delivery systems in respiratory disorders. Biointerface Res. Appl. 2021, 11, 10099–10118. [Google Scholar]
- Abdelhamid, H.N.; Hussein, K.H. Graphene oxide as a carrier for drug delivery of methotrexate. Biointerface Res. Appl. Chem. 2021, 11, 14726–14735. [Google Scholar]
- Farcas, C.G.; Macasoi, I.; Pinzaru, I.; Chirita, M.; Chirita Mihaila, M.C.; Dehelean, C.; Avram, S.; Loghin, F.; Mocanu, L.; Rotaru, V.; et al. Controlled Synthesis and Characterization of Micrometric Single Crystalline Magnetite With Superparamagnetic Behavior and Cytocompatibility/Cytotoxicity Assessments. Front. Pharmacol. 2020, 11, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, G.; Hassan, N.; Shahat, A.; El-Didamony, A.; Ashraf, A. Synthesis and characterization of porous magnetite nanosphere iron oxide as a novel adsorbent of anionic dyes removal from aqueous solution. Biointerface Res. Appl. Chem. 2021, 11, 13377–13401. [Google Scholar]
- Zarei, S.; Sadighian, S.; Rostamizadeh, K.; Khalkhali, M. Theragnostic magnetic core-shell nanoparticle as versatile nanoplatform for magnetic resonance imaging and drug delivery. Biointerface Res. Appl. Chem. 2021, 11, 13276–13289. [Google Scholar]
- Ramazanov, M.; Karimova, A.; Shirinova, H. Magnetism for drug delivery, MRI and hyperthermia applications: A review. Biointerface Res. Appl. Chem. 2021, 11, 8654–8668. [Google Scholar]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Fortes Brollo, M.E.; Veintemillas-Verdaguer, S.; Morales, M.d.P. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef]
- Salami-Kalajahi, M.; Golshan, M. Chapter 14-Dendrimer hybrids with other nanoparticles as therapeutics. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 253–272. [Google Scholar] [CrossRef]
- Sakai, E.; Farhana, F.; Yamaguchi, Y.; Tsukuba, T. Chapter 1-Potentials of natural antioxidants from plants as antiosteoporotic agents. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 72, pp. 1–28. [Google Scholar]
- Ramos-Hryb, A.B.; Cunha, M.P.; Kaster, M.P.; Rodrigues, A.L.S. Chapter 6-Natural Polyphenols and Terpenoids for Depression Treatment: Current Status. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 55, pp. 181–221. [Google Scholar]
- Tupas, G.D.; Otero, M.C.B.; Ebhohimen, I.E.; Egbuna, C.; Aslam, M. Chapter 8—Antidiabetic lead compounds and targets for drug development. In Phytochemicals as Lead Compounds for New Drug Discovery; Egbuna, C., Kumar, S., Ifemeje, J.C., Ezzat, S.M., Kaliyaperumal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–141. [Google Scholar] [CrossRef]
- Silva, M.; Caro, V.; Guzmán, C.; Perry, G.; Areche, C.; Cornejo, A. Chapter 1—α-Synuclein and tau, two targets for dementia. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 1–25. [Google Scholar]
- Omar, S.H. Chapter 4—Biophenols: Impacts and Prospects in Anti-Alzheimer Drug Discovery. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–148. [Google Scholar] [CrossRef]
- Adeseko, C.J.; Fatoki, T.H. Isolation and Partial Purification of Polyphenol Oxidase from Seed of Melon (Cucumeropsis edulis). 2020. Available online: https://biointerfaceresearch.com/wp-content/uploads/2020/09/20695837112.90859096.pdf (accessed on 1 September 2020).
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Liu, L.; Cheng, X.L.; Cai, J.; Zhou, J.; Wang, T. Anticancer effects of Rosmarinic acid in OVCAR-3 ovarian cancer cells are mediated via induction of apoptosis, suppression of cell migration and modulation of lncRNA MALAT-1 expression. J. Buon 2018, 23, 763–768. [Google Scholar]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Hossan, M.S.; Rahman, S.; Bashar, A.B.M.; Jahan, R.; Nahian, A.; Rahmatullah, M. Rosmarinic acid: A review of its anticancer action. World J. Pharm. Pharm. Sci. 2014, 3, 57–70. [Google Scholar]
- Sheikh, I.; Sharma, V.; Tuli, H.S.; Aggarwal, D.; Sankhyan, A.; Vyas, P.; Sharma, A.K.; Bishayee, A. Cancer chemoprevention by flavonoids, dietary polyphenols and terpenoids. Biointerface Res. Appl. Chem. 2020, 11, 8502–8537. [Google Scholar]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse Magnetic Single-Crystal Ferrite Microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Jia, C.Y.; Theng, C.S.; Muniandy, T.; Muralidharan, S.; Dhanaraj, S.A. Invitro studies and evaluation of metformin marketed tablets-Malaysia. J. Appl. Pharm. Sci. 2011, 1, 214–217. [Google Scholar]
- Ganonyan, N.; He, J.; Temkin, A.; Felner, I.; Gvishi, R.; Avnir, D. Ultralight monolithic magnetite aerogel. Appl. Mater. Today 2021, 22, 100955. [Google Scholar] [CrossRef]
- Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.S.; Oprea, O.; Nicoară, A.I.; Yang, C.-H.; Huang, K.-S.; Andronescu, E. Synthesis of Magnetite Nanoparticles through a Lab-On-Chip Device. Materials 2021, 14, 5906. [Google Scholar] [CrossRef]
- Chircov, C.; Ștefan, R.-E.; Dolete, G.; Andrei, A.; Holban, A.M.; Oprea, O.-C.; Vasile, B.S.; Neacșu, I.A.; Tihăuan, B. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics 2022, 14, 1057. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Matei, M.-F.; Neacșu, I.A.; Vasile, B.S.; Oprea, O.-C.; Croitoru, A.-M.; Trușcă, R.-D.; Andronescu, E.; Sorescu, I.; Bărbuceanu, F. Iron Oxide–Silica Core–Shell Nanoparticles Functionalized with Essential Oils for Antimicrobial Therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Chieng, B.W.; Ibrahim, N.; Yunus, W.; Hussein, M. Effects of Graphene Nanopletelets on Poly(Lactic Acid)/Poly(Ethylene Glycol) Polymer Nanocomposites. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Mai, T.; Hilt, J.Z. Magnetic nanoparticles: Reactive oxygen species generation and potential therapeutic applications. J. Nanoparticle Res. 2017, 19, 253. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection-A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Haneda, M.; Qiao, S.; Naruse, M.; Yoshino, M. Prooxidant action of rosmarinic acid: Transition metal-dependent generation of reactive oxygen species. Toxicol. Vitr. 2007, 21, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite Nanoparticles: Synthesis and Applications in Optics and Nanophotonics. Materials 2022, 15, 2601. [Google Scholar] [CrossRef]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Chircov, C.; Vasile, B. New Approaches in Synthesis and Characterization Methods of Iron Oxide Nanoparticles; Intech Open: London, UK, 2022. [Google Scholar]
- Ren, L.; Xu, H. Effect of ethylene glycol as solvent on the composition and morphology of nickel phosphide. Micro Nano Lett. 2018, 13, 1646–1648. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Li, J.; Feng, Z.-W.; Xu, G.-Q.; Shi, X.; Ding, Q.-J.; Li, W.; Ma, C.-H.; Yu, B. Ethylene Glycol: A Green Solvent for Visible Light-Promoted Aerobic Transition Metal-Free Cascade Sulfonation/Cyclization Reaction. Adv. Synth. Catal. 2020, 362, 2609–2614. [Google Scholar] [CrossRef]
- Sun, X.; Sun, K.; Liang, Y. Hydrothermal synthesis of magnetite: Investigation of influence of aging time and mechanism. Micro Nano Lett. 2015, 10, 99–104. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, Y.-J.; Chang, J. Fe3O4 polyhedral nanoparticles with a high magnetization synthesized in mixed solvent ethylene glycol–water system. New J. Chem. 2008, 32, 1526–1530. [Google Scholar] [CrossRef]
- Wang, W.-W.; Zhu, Y.-J. Microwave-Assisted Synthesis of Magnetite Nanosheets in Mixed Solvents of Ethylene Glycol and Water. Curr. Nanosci. 2007, 3, 171–176. [Google Scholar] [CrossRef]
- Medinger, J.; Nedyalkova, M.; Lattuada, M. Solvothermal Synthesis Combined with Design of Experiments-Optimization Approach for Magnetite Nanocrystal Clusters. Nanomaterials 2021, 11, 360. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, M.; Ou, P.; Zhang, Y.; Han, G.R. Synthesis of monodispersed Fe3O4 magnetite nanoparticles by ethylene glycol solvothermal method. In Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2013; pp. 2276–2279. [Google Scholar]
- Iacovita, C.; Stiufiuc, R.; Radu, T.; Florea, A.; Stiufiuc, G.; Dutu, A.; Mican, S.; Tetean, R.; Lucaciu, C.M. Polyethylene Glycol-Mediated Synthesis of Cubic Iron Oxide Nanoparticles with High Heating Power. Nanoscale Res. Lett 2015, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Illés, E.; Tombácz, E.; Szekeres, M.; Toth, I.; Szabo, A.; Iván, B. Novel carboxylated PEG-coating on magnetite nanoparticles designed for biomedical applications. J. Magn. Magn. Mater. 2014, 2014, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Sabit, H.; Darwish, Y.; Abdelhakim, M.; Abd El Maksoud, A.; El-Zawahri, M. Antitumor Activity of Rosmarinic Acid Encapsulated in Chitosan Nanoparticles. Acad. J. Cancer Res. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Madureira, A.R.; Nunes, S.; Campos, D.A.; Fernandes, J.C.; Marques, C.; Zuzarte, M.; Gullon, B.; Rodriguez-Alcala, L.M.; Calhau, C.; Sarmento, B. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, 11, 3621. [Google Scholar]
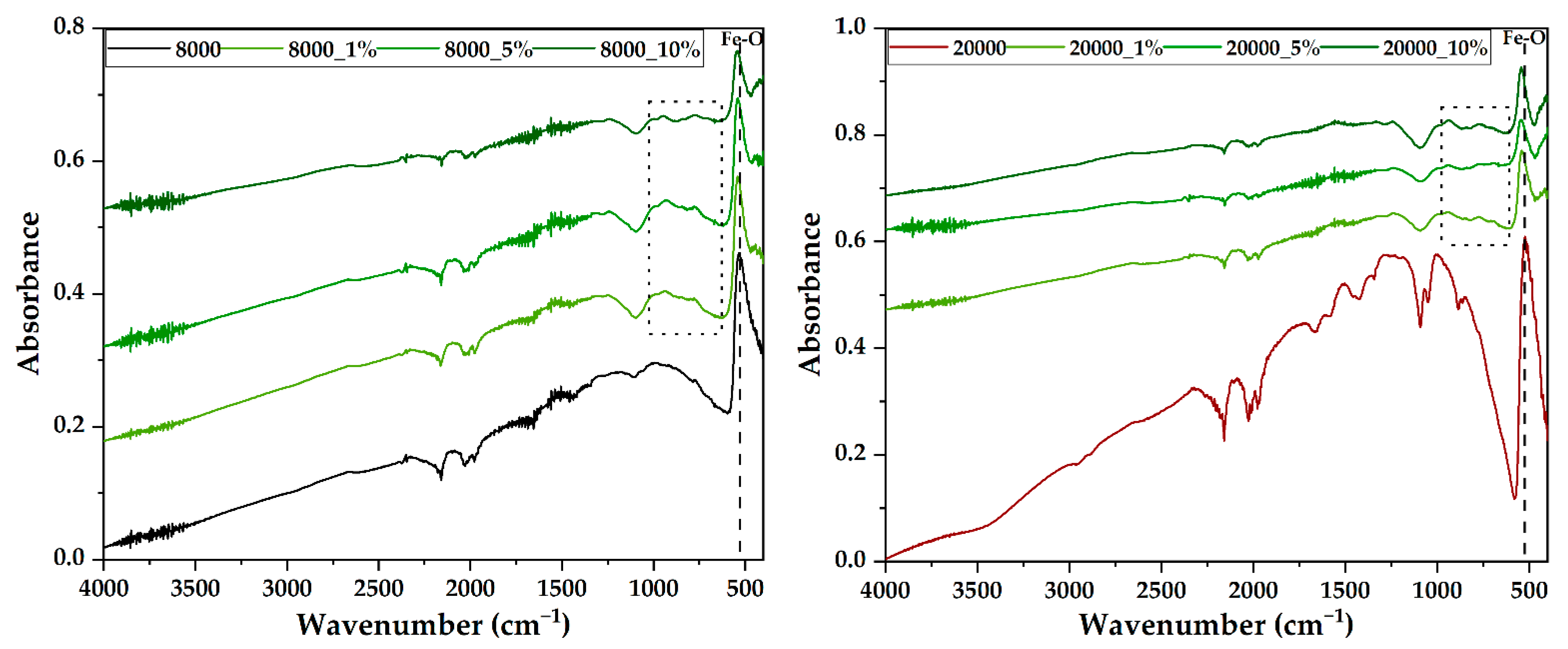
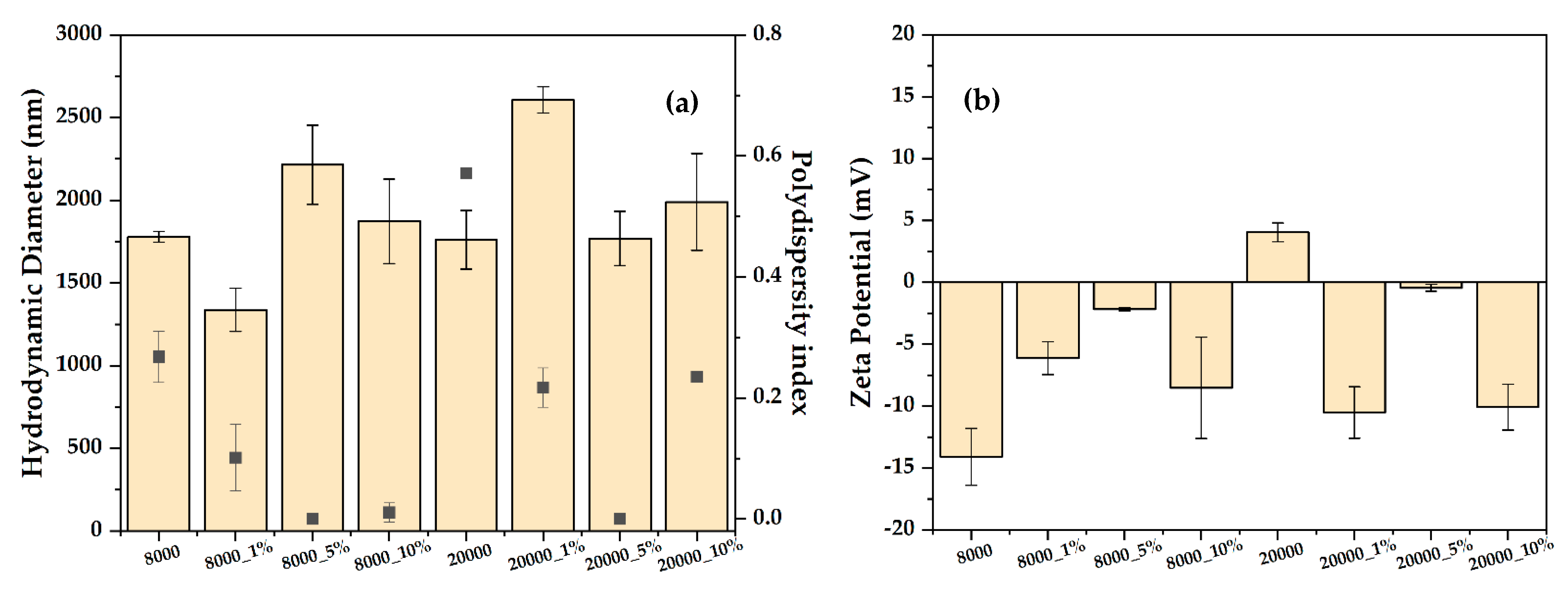


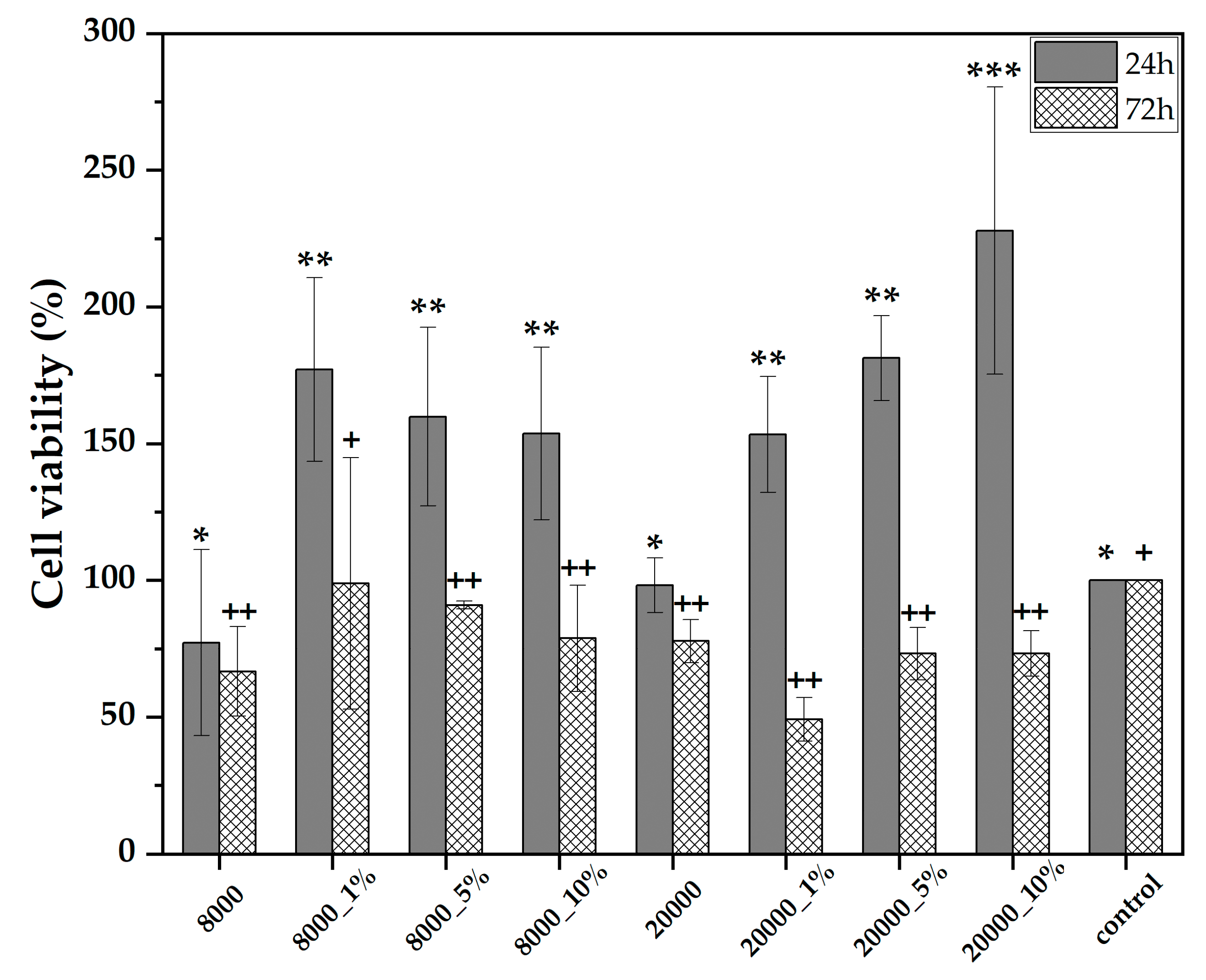
| Type of PEG (g/mol) | RA Concentration (%) | Sample Code |
|---|---|---|
| 8000 | 0 | 8000 |
| 1 | 8000_1% | |
| 5 | 8000_5% | |
| 10 | 8000_10% | |
| 20000 | 0 | 20000 |
| 1 | 20000_1% | |
| 5 | 20000_5% | |
| 10 | 20000_10% |
| Sample | Unit Cell Parameters | Average Crystallite Size [nm] | |||||
|---|---|---|---|---|---|---|---|
| a [Å] | b [Å] | c [Å] | α [°] | β [°] | γ [°] | ||
| 8000 | 8.40 | 8.40 | 8.40 | 90 | 90 | 90 | 218.44 |
| 20000 | 8.40 | 8.40 | 8.40 | 90 | 90 | 90 | 287.85 |
| Sample | Amount of RA Added (mg) | LC% | EE% | Amount of RA Released (mg) | ||
| 24 h | 72 h | 21 Days | ||||
| 8000_1% | 4 | 0.50 | 50.34 | 0.19 | 0.24 | 0.32 |
| 8000_5% | 20 | 0.93 | 18.50 | 0.26 | 0.30 | 0.41 |
| 8000_10% | 40 | 1.83 | 18.27 | 0.27 | 0.27 | 0.37 |
| 20000_1% | 4 | 0.31 | 31.36 | 0.13 | 0.19 | 0.30 |
| 20000_5% | 20 | 0.78 | 15.55 | 0.31 | 0.30 | 0.43 |
| 20000_10% | 40 | 1.16 | 11.59 | 0.48 | 0.49 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chircov, C.; Pîrvulescu, D.-C.; Bîrcă, A.C.; Andronescu, E.; Grumezescu, A.M. Magnetite Microspheres for the Controlled Release of Rosmarinic Acid. Pharmaceutics 2022, 14, 2292. https://doi.org/10.3390/pharmaceutics14112292
Chircov C, Pîrvulescu D-C, Bîrcă AC, Andronescu E, Grumezescu AM. Magnetite Microspheres for the Controlled Release of Rosmarinic Acid. Pharmaceutics. 2022; 14(11):2292. https://doi.org/10.3390/pharmaceutics14112292
Chicago/Turabian StyleChircov, Cristina, Diana-Cristina Pîrvulescu, Alexandra Cătălina Bîrcă, Ecaterina Andronescu, and Alexandru Mihai Grumezescu. 2022. "Magnetite Microspheres for the Controlled Release of Rosmarinic Acid" Pharmaceutics 14, no. 11: 2292. https://doi.org/10.3390/pharmaceutics14112292
APA StyleChircov, C., Pîrvulescu, D.-C., Bîrcă, A. C., Andronescu, E., & Grumezescu, A. M. (2022). Magnetite Microspheres for the Controlled Release of Rosmarinic Acid. Pharmaceutics, 14(11), 2292. https://doi.org/10.3390/pharmaceutics14112292









