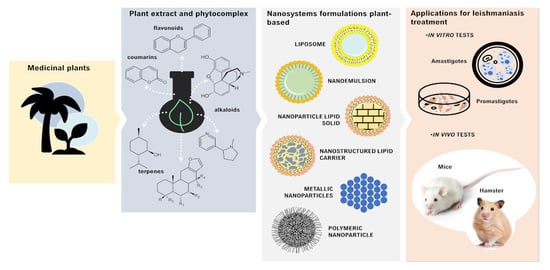
Current Applications of Plant-Based Drug Delivery Nano Systems for Leishmaniasis Treatment
Abstract
:1. Introduction
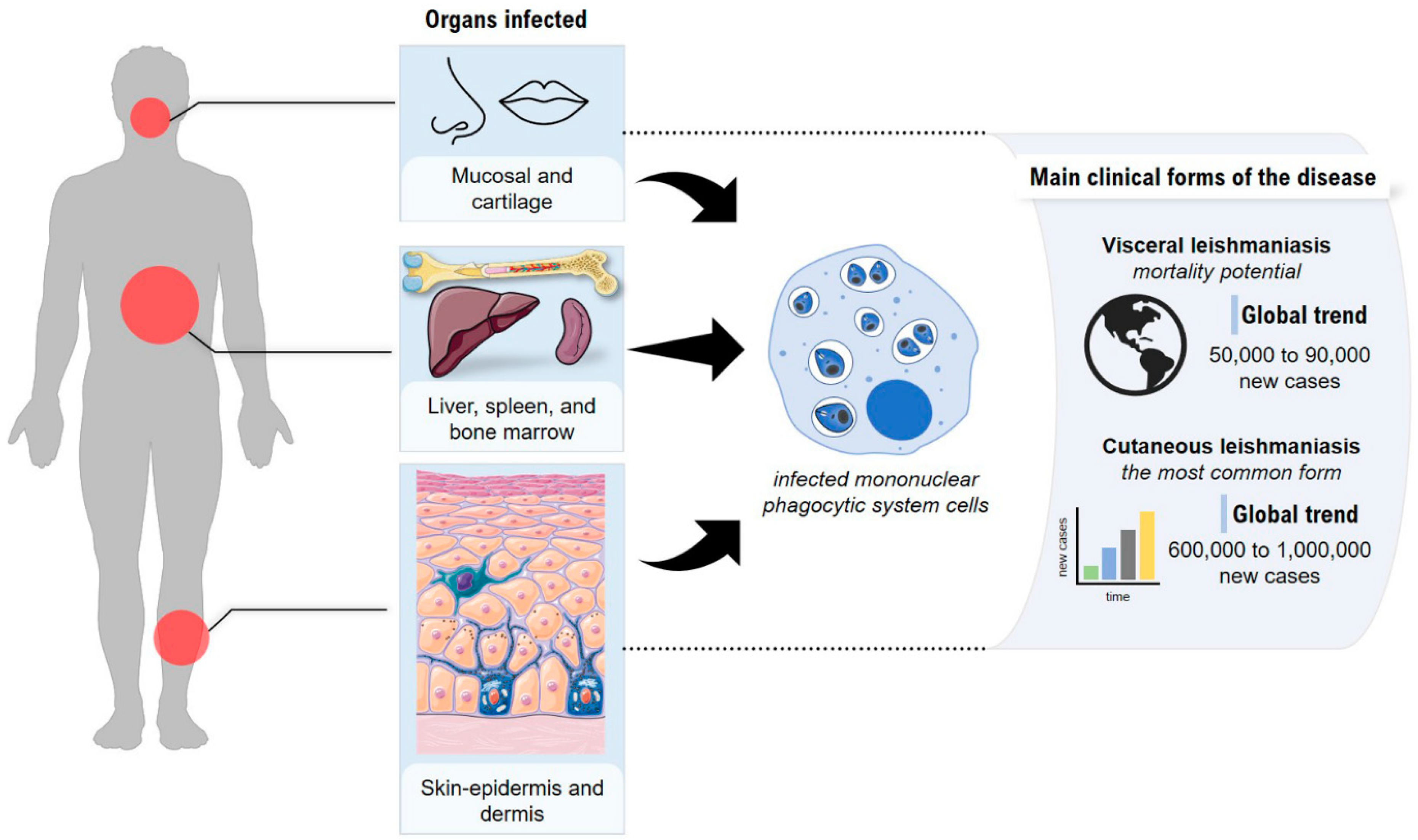
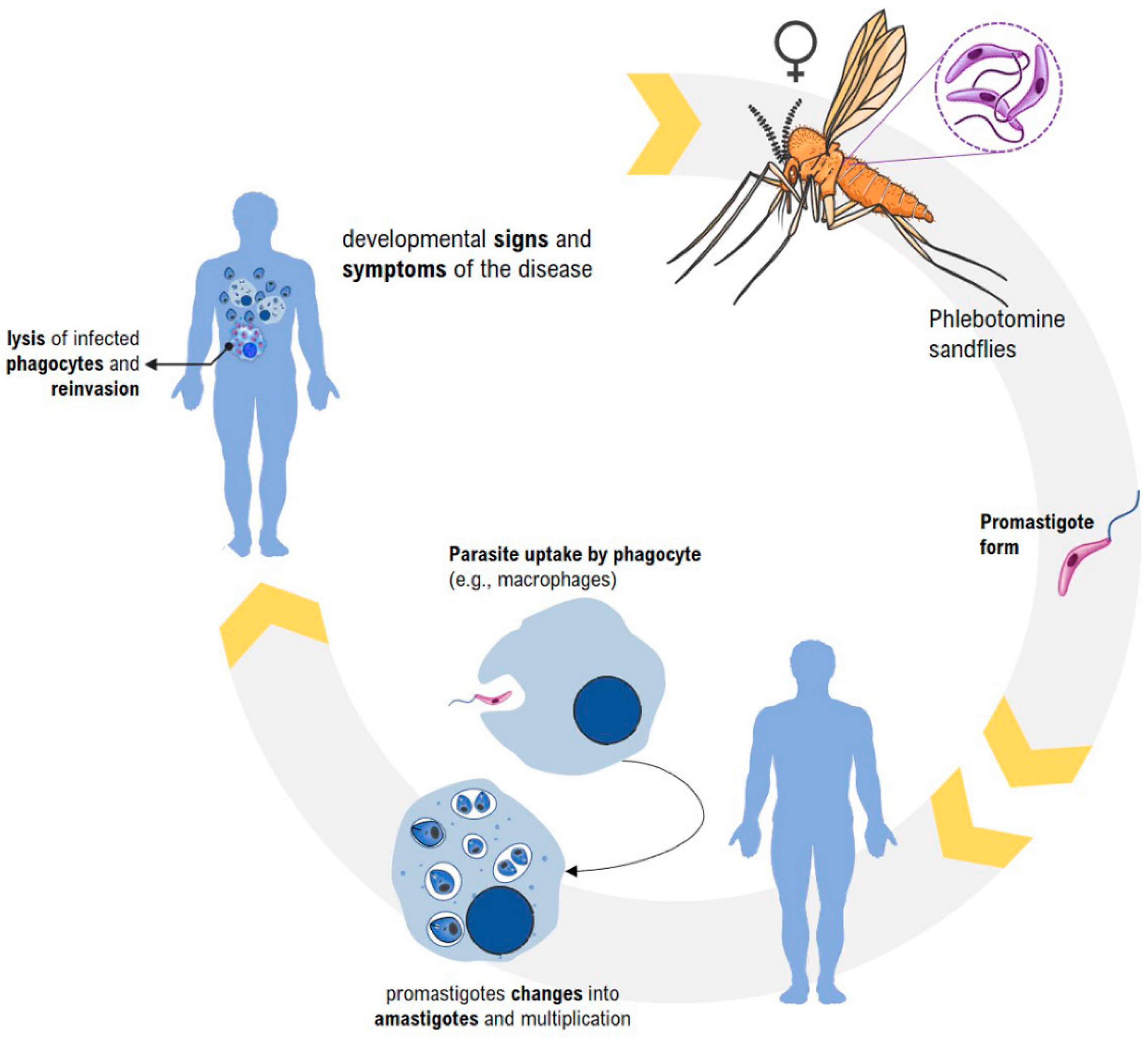
2. Leishmaniasis—General Aspects
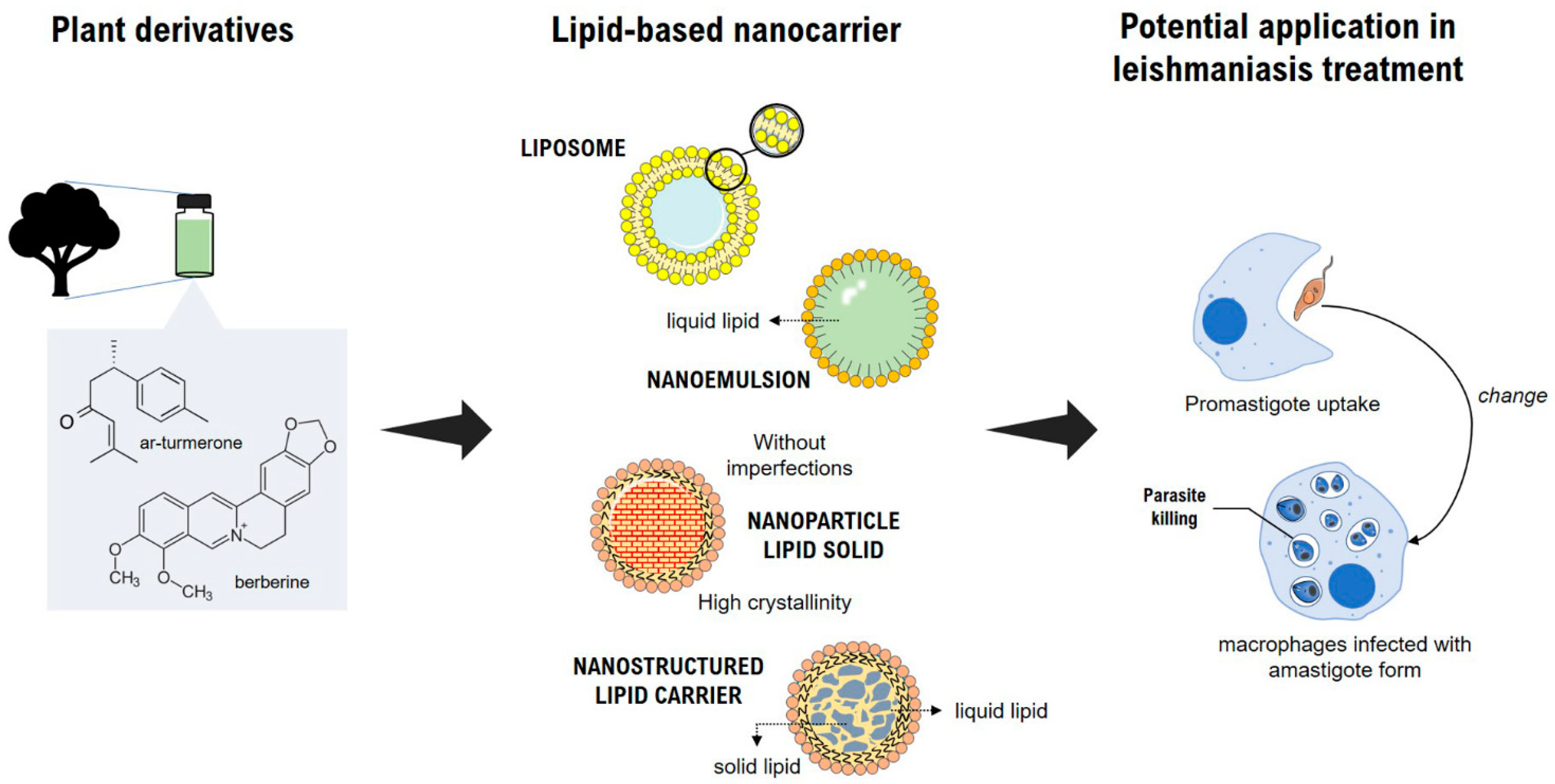
3. Plant-Based Nanocarriers and Their Potential Application in Leishmaniasis Treatment
3.1. Nanoemulsions
3.2. Liposomes
3.3. Lipid Nanoparticles
3.3.1. Solid Lipid Nanoparticles
3.3.2. Nanostructured Lipid Carriers
| Species | Vegetable Derivative | Physicochemical Characteristics | Leishmania spp. | Life Stage | IC50 (µg·mL−1) | In Vivo Effect | Toxicity | Reference |
|---|---|---|---|---|---|---|---|---|
| Ocotea duckei Vattimo | Yangambin lignan | Mean size: 218.8 nm, PDI 0.44, ZP: −30 mV, EE 94.2% | L. amazonensis | Promastigote | 20 | n.d. | Yangambin-loaded SLN reduces the toxic effects of free yangambin on macrophages (BMDM) | [83] |
| L. chagasi | ||||||||
| Not showed | Ursolic acid | Mean size: 267 nm, PDI 0.18, ZP: −29.26 mV and EE 59% | L. infantum | Amastigote | n.d. | NLC showed the lowest parasitic load and superior activity to ursolic acid alone. No damage to kidneys and liver. | No histological or biochemical alterations were observed in healthy hamsters treated with the NLC | [84] |
| Origanum vulgare | Carvacrol | Mean size: 153 nm, PDI 0.2; ZP: between −20 and −30 mV | L. amazonensis | Promastigote | 28.2 | n.d. | IC50 for healthy THP1 cells was almost threefold higher than the IC50 for L. amazonensis promastigotes; the formulation was considered safe | [88] |
| Amastigote | 19.65 | |||||||
| Cedar threes | Cedrol | Mean size: 54.73 nm, PDI 0.254, ZP: −23.7 and EE 96.4 % | L. donovani | Promastigote | CRD-NLC improved cedrol anti-leishmanial activity—0.85- to 1.5-fold higher IC50 than free cedrol; CRD-NLC was twofold more selective to intracellular amastigotes | CRD-NLC reduced more than 99% of the parasitic load in the liver and spleen (two oral doses every 7 days). CRD-NLC had superior anti-leishmanial efficacy compared to free miltefosine at the same doses. | CRD-NLC were successfully internalized by macrophages without causing any damage | [89] |
| Amastigote | ||||||||
| Bixa orellana L. | Seeds oil fraction | Size: 200 nm PDI 0.27 ZP: −30 mV spherical structures | L. major | Amastigote | Uptake of drug NLC, resulting in parasite elimination into the cell | n.d. | NLC loaded with 4% (w/w) seed oil showed IC50 of 153.6 and 123.4 mg/mL for 3T3 and HaCaT, respectively. Efficacy against L. major (concentration 2 and 5 µg/mL) was considerably lower than the concentrations that caused toxicity to 3T3 and HaCaT. | [90] |
3.4. Polymeric Nanoparticles
3.5. Metallic Nanoparticles
| Species | Vegetable Drivative | Physicochemical Characteristics | Leishmania spp. | Life Stage | IC50 (µg·mL −1) | In Vivo Effect | Toxicity | Reference |
|---|---|---|---|---|---|---|---|---|
| Nigella sativa | Essential oil | TiAgNps—n.d. | L. tropica | Amastigote | n.d. | n.d. | no cytotoxic for J774A.1 macrophages | [102] |
| Promastigote | ||||||||
| Rhazya stricta decne | Aqueous extract | AuNPs—size: 40 nm ZP: −46 mV | L. tropica | Amastigote | 43 | n.d. | biocompatible with THP-1 macrophages | [115] |
| Olax nana Wall. ex Benth | Leaves -aqueous extract | AgNPs— size: 31 nm ZP: +28 mV PDI 0.31 spherically shaped | L. tropica | Amastigote | 17.44 | n.d. | shown to be non-toxic to macrophages and compatible with the biological environment | [116] |
| Promastigote | 12.56 | |||||||
| AuNPs Size: 65 nm ZP: +32 mV PDI 0.51 spherically shaped | Amastigote | 42.20 | ||||||
| Promastigote | 21.52 | |||||||
| Trigonella foenum-graecum, Coriandrum sativum, and soybean | Leaf extracts | Au-Ag BNPs—size: 10–12 nm, monodispersed, spherically shaped | L. donovani | Amastigote | 46% reduction, 31% reduction, and 45% reduction in BNPs of macrophages treated with soybean, fenugreek, and coriander leaf extracts, respectively | n.d. | biocompatible with THP-1 macrophages | [117] |
| Promastigote | 0.04 | |||||||
| Rhamnus virgata | Leaf extract | CoONPs—Size: 123.9 nm ZP: +26.9 mV PDI 0.215 | L. tropica | Amastigote | 58.63 | n.d. | compatibility with the biological environment | [118] |
| Promastigote | 32.64 | |||||||
| Rhamnus virgata | Leaf extract | Cr2O3NPs—size: 274.1 and 1.91 nmZP: +45.5 mV PDI 0.505 | L. tropica | Amastigote | 44.31 | n.d. | nontoxic macrophages and biocompatible | [119] |
| Promastigote | 33.24 | |||||||
| Promastigote | 24.9 | |||||||
| Verbena officinalis | Leaf extracts | ZnONPs—size: 14–31 nm rod and flower shapes | L. tropica | Promastigote | 243 | n.d. | n.d. | [120] |
| Verbena tenuisecta | Size: 65–75 nm rod and flower shapes | 414 | ||||||
| Silybum marianum | Aqueous extract | ZnONPs—size: 25 nm ZP: −2.28 mV spherically shaped | L. tropica | Promastigote | 246 | n.d. | n.d. | [121] |
| Mentha longifolia (L.) L. | Leaves—aqueous extract | AgNPs—size: <100 nm PDI: 0.263 spherically shaped | L. tropica | Promastigote | 8.73 | n.d. | n.d. | [122] |
| Euphorbia prostrata | Leaves—aqueous extract | AgNPs—size: 12.82 nm polydisperse spherical and irregular shaped | L. donovani | Amastigote | 14.94 | n.d. | no cytotoxic for J774A.1 macrophages | [123] |
| Promastigote | 3.89 | |||||||
| Zingiber officinale | Rizhome—aqueous extract | AgNPs— size 10 nm approximately spherical | L. major | Amastigote | 2.35 (ppm) | n.d. | high concentrations have more toxic impacts on macrophage RAW.264.7 (≥ 2.5 ppm) | [124] |
| Promastigote | n.d. | |||||||
| Moringa oleifera | Leaves—aqueous extract | AgNPs—size: 116.2 nm PDI: 0.2 spherical shaped | L. major | Promastigote | n.d. | lesion size and complete healing after 14 days | minimized ROS-mediated toxic effect | [125] |
| Anethum graveolens | Leaves—aqueous extract | AgNPs—size: 35 nm spherical shaped | L. donovani | Amastigote | n.d. | n.d. | biocompatible with RAW 264.7 macrophages | [126] |
| Promastigote | ||||||||
| Maytenus royleanus | Stem—aqueous extract | AuNPs—mean size of 30 nm, ZP of −42.9 mV, and hexagonal shape | L. tropica | Promastigote | AuNPs inhibited the parasite growth by 75% after 72 h of incubation | n.d. | n.d. | [127] |
| Promastigote | ||||||||
| Elaeagnus angustifolia | Leaf extracts | ZnONPs—size: 205.9 nm, a ZP: 13.8 mV and a PDI 0.132 | L. tropica | Amastigote | 32.83 | n.d. | n.d. | [128] |
| Promastigote | 24.9 | |||||||
| Callistemon viminalis | Floral extracts | NiONPs—size: 20 nm–22 nm (300 °C); 16 nm–17 nm (400 °C), and 14–16 nm (500 °C) | L. tropica | Promastigote | 37 | n.d. | n.d. | [129] |
4. Possible Mechanisms Involved with Increased Antileishmanial Activity by Nanosystems
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Engels, D.; Zhou, X.N. Neglected Tropical Diseases: An Effective Global Response to Local Poverty-Related Disease Priorities. Infect. Dis. Poverty 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oerther, S.; Jöst, H.; Heitmann, A.; Lühken, R.; Krüger, A.; Steinhausen, I.; Brinker, C.; Lorentz, S.; Marx, M.; Schmidt-Chanasit, J.; et al. Phlebotomine Sand Flies in Southwest Germany: An Update with Records in New Locations. Parasites Vectors 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Urbanization: An Increasing Risk Factor for Leishmaniasis Urbanisation: Facteur de Risque Croissant Pour La Leishmaniose. Wkly. Epidemiol. Rec. 2002, 77, 365–370. [Google Scholar]
- Desjeux, P. Leishmaniasis: Current Situation and New Perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the Host-Pathogen Interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent Advances and New Strategies on Leishmaniasis Treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Van Griensven, J.; Boelaert, M. Combination Therapy for Visceral Leishmaniasis. Lancet 2011, 377, 443–444. [Google Scholar] [CrossRef]
- Bruni, N.; Stella, B.; Giraudo, L.; Della Pepa, C.; Gastaldi, D.; Dosio, F. Nanostructured Delivery Systems with Improved Leishmanicidal Activity: A Critical Review. Int. J. Nanomed. 2017, 12, 5289–5311. [Google Scholar] [CrossRef] [Green Version]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Al Thaher, Y.; Satoof, A.; Kamal, A.; Almani, D.; Shaban, D.; Kassab, G.; Surchi, H.; Abu-Qtaish, H.; Fatouh, J.; Ajaleh, S.A. Instrumental Analytical Techniques for Physicochemical Characterization of Bio-Nanomaterials. Handb. Nanobiomaterials Ther. Diagn. Appl. 2021, 7, 133–150. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Oryan, A.; Akbari, M. Worldwide Risk Factors in Leishmaniasis. Asian Pac. J. Trop. Med. 2016, 9, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Postigo, J.A.; Jain, S.; Mikhailov, A.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Mona Osman, Z.L.; Beshah, A.; Yajima, A.; Gasimovg, E. Global Leishmaniasis a Baseline for the 2030 Roadmap Surveillance Mondiale de La Leishmaniose: 2019–2020, Une Période de Référence Pour La Feuille de Route à l ’ Horizon 2030. Revel. Épidemiologique Hebd. 2021, 35, 401–419. [Google Scholar]
- WHO. World Health Organization: Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 22 October 2022).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; de Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Wamai, R.G.; Kahn, J.; McGloin, J.; Ziaggi, G. Visceral Leishmaniasis: A Global Overview. J. Glob. Health Sci. 2020, 2, e3. [Google Scholar] [CrossRef]
- Séguin, O.; Descoteaux, A. Leishmania, the Phagosome, and Host Responses: The Journey of a Parasite. Cell. Immunol. 2016, 309, 1–6. [Google Scholar] [CrossRef]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69, S10–S18. [Google Scholar] [CrossRef]
- Forestier, C.L.; MacHu, C.; Loussert, C.; Pescher, P.; Späth, G.F. Imaging Host Cell-Leishmania Interaction Dynamics Implicates Parasite Motility, Lysosome Recruitment, and Host Cell Wounding in the Infection Process. Cell Host Microbe 2011, 9, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Bates, P.A. Leishmania Sand Fly Interaction: Progress and Challenges. Curr. Opin. Microbiol. 2008, 11, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Nagill, R.; Kaur, S. Vaccine Candidates for Leishmaniasis: A Review. Int. Immunopharmacol. 2011, 11, 1464–1488. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Jain, N.K. Vaccines for Visceral Leishmaniasis: A Review. J. Immunol. Methods 2015, 422, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Le Rutte, E.A.; Coffeng, L.E.; Malvolti, S.; Kaye, P.M.; de Vlas, S.J. The Potential Impact of Human Visceral Leishmaniasis Vaccines on Population Incidence. PLoS Negl. Trop. Dis. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gillespiea, P.M.; Beaumier, C.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of Vaccine Research and Development of Vaccines for Leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef]
- Jain, K.; Jain, N.K. Novel Therapeutic Strategies for Treatment of Visceral Leishmaniasis. Drug Discov. Today 2013, 18, 1272–1281. [Google Scholar] [CrossRef]
- De Menezes, J.P.B.; Guedes, C.E.S.; De Oliveira Almeida Petersen, A.L.; Fraga, D.B.M.; Veras, P.S.T. Advances in Development of New Treatment for Leishmaniasis. BioMed Res. Int. 2015, 2015, 815023. [Google Scholar] [CrossRef]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural Products as Antiparasitic Drugs. Parasitol. Res. 2003, 90, 55–62. [Google Scholar] [CrossRef]
- Calla-Magariños, J.; Quispe, T.; Giménez, A.; Freysdottir, J.; Troye-Blomberg, M.; Fernández, C. Quinolinic Alkaloids from Galipea Longiflora Krause Suppress Production of Proinflammatory Cytokines in Vitro and Control Inflammation in Vivo upon Leishmania Infection in Mice. Scand. J. Immunol. 2013, 77, 30–38. [Google Scholar] [CrossRef]
- Bekele, B.; Adane, L.; Tariku, Y.; Hailu, A. Evaluation of Antileishmanial Activities of Triglycerides Isolated from Roots of Moringa Stenopetala. Med. Chem. Res. 2013, 22, 4592–4599. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Biswas, M.; Haldar, P. The Triterpenoid Fraction from Trichosanthes Dioica Root Exhibits in Vitro Antileishmanial Effect against Leishmania Donovani Promastigotes. Pharmacogn. Res. 2013, 5, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Mandlik, V.; Patil, S.; Bopanna, R.; Basu, S.; Singh, S. Biological Activity of Coumarin Derivatives as Anti-Leishmanial Agents. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef]
- Lage, P.S.; Chávez-Fumagalli, M.A.; Mesquita, J.T.; Mata, L.M.; Fernandes, S.O.A.; Cardoso, V.N.; Soto, M.; Tavares, C.A.P.; Leite, J.P.V.; Tempone, A.G.; et al. Antileishmanial Activity and Evaluation of the Mechanism of Action of Strychnobiflavone Flavonoid Isolated from Strychnos Pseudoquina against Leishmania Infantum. Parasitol. Res. 2015, 114, 4625–4635. [Google Scholar] [CrossRef] [PubMed]
- de Mello, M.V.P.; Abrahim-Vieira, B.D.A.; Domingos, T.F.S.; de Jesus, J.B.; de Sousa, A.C.C.; Rodrigues, C.R.; de Souza, A.M.T. A Comprehensive Review of Chalcone Derivatives as Antileishmanial Agents. Eur. J. Med. Chem. 2018, 150, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The Role of Natural Products in Drug Discovery and Development against Neglected Tropical Diseases. Molecules 2017, 22, 58. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A. Plant-Derived Compounds in Treatment of Leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar] [PubMed]
- Ribeiro, T.G.; Chávez-Fumagalli, M.A.; Valadares, D.G.; Franca, J.R.; Lage, P.S.; Duarte, M.C.; Andrade, P.H.R.; Martins, V.T.; Costa, L.E.; Arruda, A.L.A.; et al. Antileishmanial Activity and Cytotoxicity of Brazilian Plants. Exp. Parasitol. 2014, 143, 60–68. [Google Scholar] [CrossRef]
- Sen, R.; Chatterjee, M. Plant Derived Therapeutics for the Treatment of Leishmaniasis. Phytomedicine 2011, 18, 1056–1069. [Google Scholar] [CrossRef]
- de Sousa, V.P.; Crean, J.; Borges, V.R.D.A.; Tajber, L.; Boylan, F.; Rodrigues, C.R.; Cabral, L.M. Nanostructured Systems Containing Babassu (Orbignya Speciosa) Oil as a Potential Alternative Therapy for Benign Prostatic Hyperplasia. Int. J. Nanomed. 2013, 8, 3129–3139. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Edible Nanoemulsions: Fabrication, Properties, and Functional Performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as Pharmaceutical Carrier for Dermal and Transdermal Drug Delivery: Formulation Development, Stability Issues, Basic Considerations and Applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Carneiro, G.; Aguiar, M.G.; Fernandes, A.P.; Ferreira, L.A.M. Drug Delivery Systems for the Topical Treatment of Cutaneous Leishmaniasis. Expert Opin. Drug Deliv. 2012, 9, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.C.M.; De Souza, M.L.S.; Teixeira, E.M.; Alves, L.L.; Vilela, J.M.C.; Andrade, M.; Carvalho, M.D.G.; Fernandes, A.P.; Ferreira, L.; Aguiar, M.M.G. A New Nanoemulsion Formulation Improves Antileishmanial Activity and Reduces Toxicity of Amphotericin B. J. Drug Target. 2018, 26, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-Mediated Biosynthesis of Metallic Nanoparticles: A Review of Literature, Factors Affecting Synthesis, Characterization Techniques and Applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Bajerski, L.; Michels, L.R.; Colomé, L.M.; Bender, E.A.; Freddo, R.J.; Bruxel, F.; Haas, S.E. The Use of Brazilian Vegetable Oils in Nanoemulsions: An Update on Preparation and Biological Applications. Braz. J. Pharm. Sci. 2016, 52, 347–363. [Google Scholar] [CrossRef] [Green Version]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green Synthesis of Silver-Nanoparticles from Annona Reticulata Leaves Aqueous Extract and Its Mosquito Larvicidal and Anti-Microbial Activity on Human Pathogens. Biotechnol. Rep. 2019, 21, e00297. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous Emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- De Campos, V.E.B.; Ricci-Júnior, E.; Mansur, C.R.E. Nanoemulsions as Delivery Systems for Lipophilic Drugs. J. Nanosci. Nanotechnol. 2012, 12, 2881–2890. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K.; et al. Nanotherapeutics: An Insight into Healthcare and Multi-Dimensional Applications in Medical Sector of the Modern World. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, L.R.; Fernandes, F.R.; Costa, D.F.; Frézard, F.; Afonso, L.C.C.; Ferreira, L.A.M. Nanoemulsions Loaded with Amphotericin B: A New Approach for the Treatment of Leishmaniasis. Eur. J. Pharm. Sci. 2015, 70, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, M.Y.M.; Zamora, L.O.; Araújo, R.S.; Fernandes, C.P.; Ricotta, T.Q.N.; de Oliveira, L.G.; Queiroz-Junior, C.M.; Fernandes, A.P.; da Conceição, E.C.; Ferreira, L.A.M.; et al. Efficacy of Nanoemulsion with Pterodon Emarginatus Vogel Oleoresin for Topical Treatment of Cutaneous Leishmaniasis. Biomed. Pharmacother. 2021, 134, 1–12. [Google Scholar] [CrossRef]
- Santos, É.d.S.; Garcia, F.P.; Outuki, P.M.; Hoscheid, J.; Nunes de Goes, P.R.; Cardozo-Filho, L.; Nakamura, C.V.; Carvalho Cardoso, M.L. Optimization of Extraction Method and Evaluation of Antileishmanial Activity of Oil and Nanoemulsions of Pterodon Pubescens Benth. Fruit Extracts. Exp. Parasitol. 2016, 170, 252–260. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Ramos, A.D.S.; Falcão, D.Q.; Ferreira, J.L.P.; Basso, S.L.; Silva, J.R.D.A.; Amaral, A.C.F. Development of Nanoemulsions to Enhance the Antileishmanial Activity of Copaifera paupera Oleoresins. BioMed Res. Int. 2018, 2018, 9781724. [Google Scholar] [CrossRef] [Green Version]
- de Moraes, A.R.D.P.; Tavares, G.D.; Rocha, F.J.S.; de Paula, E.; Giorgio, S. Effects of Nanoemulsions Prepared with Essential Oils of Copaiba- and Andiroba against Leishmania Infantum and Leishmania Amazonensis Infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef]
- Ghanbariasad, A.; Amoozegar, F.; Rahmani, M.; Zarenezhad, E.; Osanloo, M. Impregnated Nanofibrous Mat with Nanogel of Citrus Sinensis Essential Oil as a New Type of Dressing in Cutaneous Leishmaniasis. Biointerface Res. Appl. Chem. 2021, 11, 11066–11076. [Google Scholar] [CrossRef]
- Ghanbariasad, A.; Valizadeh, A.; Ghadimi, S.N.; Fereidouni, Z.; Osanloo, M. Nanoformulating Cinnamomum zeylanicum essential oil with an extreme effect on Leishmania tropica and Leishmania major. J. Drug Deliv. Sci. Technol. 2021, 63, 1–6. [Google Scholar] [CrossRef]
- Shokri, A.; Saeedi, M.; Fakhar, M.; Morteza-Semnani, K.; Keighobadi, M.; Hosseini, T.S.; Kelidari, H.; Sadjadi, S. Antileishmanial Activity of Lavandula angustifolia and Rosmarinus Officinalis Essential Oils and Nano-emulsions on Leishmania major (MRHO/IR/75/ER). Iran. J. Parasitol. 2017, 12, 622–631. [Google Scholar]
- Carvalho, J.C.T.; Cascon, V.; Possebon, L.S.; Morimoto, M.S.S.; Cardoso, L.G.V.; Kaplan, M.A.C.; Gilbert, B. Topical Antiinflammatory and Analgesic Activities of Copaifera Duckei Dwyer. Phyther. Res. 2005, 19, 946–950. [Google Scholar] [CrossRef]
- Valentim, D.S.S.; Duarte, J.L.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Carvalho, J.C.T.; Solans, C.; Fernandes, C.P.; Tavares-Dias, M. Effects of a Nanoemulsion with Copaifera Officinalis Oleoresin against Monogenean Parasites of Colossoma Macropomum: A Neotropical Serrasalmidae. J. Fish Dis. 2018, 41, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, O.D.; Adriana; Izumi, E.; Ueda-nakamura, T.; Dias-filho, B.P.; Veiga-júnior, V.F.; Nakamura, C.V. Antileishmanial Activity of Diterpene Acids in Copaiba Oil. Memórias Do Inst. Oswaldo Cruz 2013, 108, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.D.S.; Siani, A.C.; Saraiva, E.M. Trans-β-Caryophyllene: An Effective Antileishmanial Compound Found in Commercial Copaiba Oil (Copaifera spp.). Evid.-Based Complement. Altern. Med. 2013, 2013, 761323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.K.; Jaiswal, A.K.; Asthana, S.; Teja B, V.; Shukla, P.; Shukla, M.; Sagar, N.; Dube, A.; Rath, S.K.; Mishra, P.R. Synergistic Enhancement of Parasiticidal Activity of Amphotericin B Using Copaiba Oil in Nanoemulsified Carrier for Oral Delivery: An Approach for Non-Toxic Chemotherapy. Br. J. Pharmacol. 2015, 172, 3596–3610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoscheid, J.; Outuki, P.M.; Kleinubing, S.A.; De Goes, P.R.N.; Lima, M.M.S.; Cuman, R.K.N.; Cardoso, M.L.C. Pterodon Pubescens Oil Nanoemulsions: Physiochemical and Microbiological Characterization and in Vivo Anti-Inflammatory Efficacy Studies. Braz. J. Pharmacogn. 2017, 27, 375–383. [Google Scholar] [CrossRef]
- Lemos, J.d.A.; Oliveira, A.E.M.; Araujo, R.S.; Townsend, D.M.; Ferreira, L.A.M.; de Barros, A.L.B. Recent Progress in Micro and Nano-Encapsulation of Bioactive Derivatives of the Brazilian Genus Pterodon. Biomed. Pharmacother. 2021, 143, 112137. [Google Scholar] [CrossRef]
- da Silva Cardoso, V.; Vermelho, A.B.; Ricci Junior, E.; Almeida Rodrigues, I.; Mazotto, A.M.; Supuran, C.T. Antileishmanial Activity of Sulphonamide Nanoemulsions Targeting the β-Carbonic Anhydrase from Leishmania Species. J. Enzym. Inhib. Med. Chem. 2018, 33, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine Review: Clinical Developments in Liposomal Applications; Springer: Vienna, Austria, 2019; Volume 10, ISBN 1264501900. [Google Scholar]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.A.; Eloy, J.O.; Luiz, M.T.; Junior, S.L.R.; Borges, J.C.; de la Fuente, L.R.; Luis, C.O.-D.S.; Marchetti, J.M.; Santos-Martinez, M.J.; Chorilli, M. Transferrin-Functionalized Liposomes for Docetaxel Delivery to Prostate Cancer Cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125806. [Google Scholar] [CrossRef]
- Kim, E.; Jeong, H. Liposomes: Biomedical Applications. Chonnam Med. J. 2021, 57, 27–35. [Google Scholar] [CrossRef]
- Ortega, V.; Giorgio, S.; de Paula, E. Liposomal Formulations in the Pharmacological Treatment of Leishmaniasis: A Review. J. Liposome Res. 2017, 27, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania Infantum-Infected Balb/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.C.F.; Gomes, L.A.; Silva, J.R.D.A.; Ferreira, J.L.P.; Ramos, A.D.S.; Rosa, M.D.S.S.; Vermelho, A.B.; Rodrigues, I.A. Liposomal Formulation of Turmerone-Rich Hexane Fractions from Curcuma Longa Enhances Their Antileishmanial Activity. BioMed Res. Int. 2014, 2014, 694934. [Google Scholar] [CrossRef] [Green Version]
- Bafghi, A.F.; Haghirosadat, B.F.; Yazdian, F.; Mirzaei, F.; Pourmadadi, M.; Pournasir, F.; Hemati, M.; Pournasir, S. A Novel Delivery of Curcumin by the Efficient Nanoliposomal Approach against Leishmania Major. Prep. Biochem. Biotechnol. 2021, 51, 990–997. [Google Scholar] [CrossRef]
- Haddad, M.; Sauvain, M.; Deharo, E. Curcuma as a Parasiticidal Agent: A Review. Planta Med. 2011, 77, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Frézard, F.; Demicheli, C.; Ribeiro, R. Pentavalent Antimonials: New Perspectives for Old Drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.C.; Silva, R.A.; Novais, M.V.; Coelho, L.D.; Ferreira, L.A.; Souza, P.E.; Tedesco, A.; Azevedo, R.B.; Aguiar, M.G.; Oliveira, M.C. Topical Photodynamic Therapy with Chloroaluminum Phthalocyanine Liposomes Is as Effective as Systemic Pentavalent Antimony in the Treatment of Experimental Cutaneous Leishmaniasis. Photodiagnosis Photodyn. Ther. 2019, 28, 210–215. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid Lipid Nanoparticles: A Review on Recent Perspectives and Patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Soltani, S.; Mojiri-Forushani, H.; Soltani, S.; Kahvaz, M.S.; Foroutan, M. Evaluation of Antileishmanial Activity Employing Conventional and Solid Lipid Nanoparticles of Amphotericin B on Leishmania Major In Vitro and In Vivo. Infect. Disord.-Drug Targets 2021, 20, 822–827. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; Torres, E.C.; Barud, H.D.S.; Zoccal, K.F.; Faccioli, L.H.; Hori, J.I.; Berretta, A.A. Physicochemical Characterization by AFM, FT-IR and DSC and Biological Assays of a Promising Antileishmania Delivery System Loaded with a Natural Brazilian Product. J. Pharm. Biomed. Anal. 2016, 123, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Jesus, J.A.; Sousa, I.M.O.; da Silva, T.N.F.; Ferreira, A.F.; Laurenti, M.D.; Antonangelo, L.; Faria, C.S.; da Costa, P.C.; de Carvalho Ferreira, D.; Passero, L.F.D. Preclinical Assessment of Ursolic Acid Loaded into Nanostructured Lipid Carriers in Experimental Visceral Leishmaniasis. Pharmaceutics 2021, 13, 908. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.L.M.; Filho, J.M.B.; Sousa, L.M.A.; Filho, P.F.A.; Dias, C.S.; Oliveira, M.R. Crude Ethanolic Extract, Lignoid Fraction and Yangambin from Ocotea Duckei (Lauraceae) Show Antileishmanial Activity. Z. Für Naturforsch. C 2007, 62, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Fülöp, Z.; Ritthidej, G.C. Amphotericin B Loaded Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carrier (NLCs): Physicochemical and Solid-Solution State Characterizations. Drug Dev. Ind. Pharm. 2019, 45, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Galvão, J.G.; Santos, R.L.; Silva, A.R.S.T.; Santos, J.S.; Costa, A.M.B.; Chandasana, H.; Nunes, R.S. Carvacrol loaded nanostructured lipid carriers as a promising parenteral formulation for leishmaniasis treatment. Eur. J. Pharm. Sci. 2020, 105335, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kar, N.; Chakraborty, S.; De, A.K.; Ghosh, S.; Bera, T. Development and Evaluation of a Cedrol-Loaded Nanostructured Lipid Carrier System for in Vitro and in Vivo Susceptibilities of Wild and Drug Resistant Leishmania Donovani Amastigotes. Eur. J. Pharm. Sci. 2017, 104, 196–211. [Google Scholar] [CrossRef]
- Ferreira, M.A.; de Almeida Júnior, R.F.; Onofre, T.S.; Casadei, B.R.; Farias, K.J.S.; Severino, P.; de Oliveira Franco, C.F.; Raffin, F.N.; de Lima e Moura, T.F.A.; de Melo Barbosa, R. Annatto Oil Loaded Nanostructured Lipid Carriers: A Potential New Treatment for Cutaneous Leishmaniasis. Pharmaceutics 2021, 13, 1912. [Google Scholar] [CrossRef]
- Singh, O.P.; Gedda, M.R.; Mudavath, S.L.; Srivastava, O.N.; Sundar, S. Envisioning the Innovations in Nanomedicine to Combat Visceral Leishmaniasis: For Future Theranostic Application. Nanomedicine 2019, 14, 1911–1927. [Google Scholar] [CrossRef]
- Jamshaid, H.; Din, F.U.; Khan, G.M. Nanotechnology Based Solutions for Anti-Leishmanial Impediments: A Detailed Insight. J. Nanobiotechnology 2021, 19, 1–51. [Google Scholar] [CrossRef]
- Pires, V.C.; Magalhães, C.P.; Ferrante, M.; Rebouças, J.D.S.; Nguewa, P.; Severino, P.; Barral, A.; Veras, P.S.T.; Formiga, F.R. Solid Lipid Nanoparticles as a Novel Formulation Approach for Tanespimycin (17-AAG) against Leishmania Infections: Preparation, Characterization and Macrophage Uptake. Acta Trop. 2020, 211, 105595. [Google Scholar] [CrossRef]
- de Souza, A.; Marins, D.S.S.; Mathias, S.L.; Monteiro, L.M.; Yukuyama, M.N.; Scarim, C.B.; Löbenberg, R.; Bou-Chacra, N.A. Promising Nanotherapy in Treating Leishmaniasis. Int. J. Pharm. 2018, 547, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koester, L.; Mattos, C.; Argenta, D.; Melchiades, G.; Cordeiro, M.; Tonini, M.; Moraes, M.; Weber, T.; Roman, S.; Nunes, R.; et al. Nanoemulsions Containing a Synthetic Chalcone as an Alternative for Treating Cutaneous Leshmaniasis: Optimization Using a Full Factorial Design. Int. J. Nanomed. 2015, 10, 5529–5542. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-S.; Cho, C.-W. Surface Modification of Solid Lipid Nanoparticles for Oral Delivery of Curcumin: Improvement of Bioavailability through Enhanced Cellular Uptake, and Lymphatic Uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Madkour, L.H. Nanoparticle and Polymeric Nanoparticle-Based Targeted Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128197776. [Google Scholar]
- Castro, K.C.; de Costa, J.M.; Campos, M.G.N. Drug-Loaded Polymeric Nanoparticles: A Review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V. Recent Advances in Leishmaniasis Treatment. Int. J. Infect. Dis. 2011, 15, e525–e532. [Google Scholar] [CrossRef] [Green Version]
- Saleem, K.; Khursheed, Z.; Hano, C.; Anjum, I.; Anjum, S. Applications of Nanomaterials in Leishmaniasis: A Focus on Recent Advances and Challenges. Nanomaterials 2019, 9, 1749. [Google Scholar] [CrossRef] [Green Version]
- Abamor, E.S.; Allahverdiyev, A.M. A Nanotechnology Based New Approach for Chemotherapy of Cutaneous Leishmaniasis: TIO2@AG Nanoparticles-Nigella Sativa Oil Combinations. Exp. Parasitol. 2016, 166, 150–163. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.X.; Zheng, C.L. Intracellular Disposition of Chitosan Nanoparticles in Macrophages: Intracellular Uptake, Exocytosis, and Intercellular Transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, T.G.; da Silva, P.F.; Azevedo, L.F.; da Rocha, L.G.; de Moraes Porto, I.C.C.; Lima e Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; Fonseca, E.J.d.S.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- da Silva, E.R.; Maquiaveli, C.D.C.; Magalhães, P.P. The Leishmanicidal Flavonols Quercetin and Quercitrin Target Leishmania (Leishmania) Amazonensis Arginase. Exp. Parasitol. 2012, 130, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Van De Ven, H.; Vermeersch, M.; Matheeussen, A.; Vandervoort, J.; Weyenberg, W.; Apers, S.; Cos, P.; Maes, L.; Ludwig, A. PLGA Nanoparticles Loaded with the Antileishmanial Saponin β-Aescin: Factor Influence Study and in Vitro Efficacy Evaluation. Int. J. Pharm. 2011, 420, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Mehrizi, T.Z.; Shafiee Ardestani, M.; Haji Molla Hoseini, M.; Khamesipour, A.; Mosaffa, N.; Ramezani, A. Novel Nanosized Chitosan-Betulinic Acid Against Resistant Leishmania Major and First Clinical Observation of Such Parasite in Kidney. Sci. Rep. 2018, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Karam, T.K.; Ortega, S.; Ueda Nakamura, T.; Auzély-Velty, R.; Nakamura, C.V. Development of Chitosan Nanocapsules Containing Essential Oil of Matricaria Chamomilla L. for the Treatment of Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Abamor, E.S.; Tosyali, O.A.; Bagirova, M.; Allahverdiyev, A. Nigella Sativa Oil Entrapped Polycaprolactone Nanoparticles for Leishmaniasis Treatment. IET Nanobiotechnology 2018, 12, 1018–1026. [Google Scholar] [CrossRef]
- Azevedo, L.F.; Silva, P.D.F.; Brandão, M.P.; da Rocha, L.G.; Aragão, C.F.S.; da Silva, S.A.S.; Porto, I.C.C.M.; Basílio-Júnior, I.D.; Fonseca, E.J.D.S.; de Moura, M.A.B.F.; et al. Polymeric Nanoparticle Systems Loaded with Red Propolis Extract: A Comparative Study of the Encapsulating Systems, PCL-Pluronic versus Eudragit® E100-Pluronic. J. Apic. Res. 2018, 57, 255–270. [Google Scholar] [CrossRef]
- Carter, N.S.; Stamper, B.D.; Elbarbry, F.; Nguyen, V.; Lopez, S.; Kawasaki, Y.; Poormohamadian, R.; Roberts, S.C. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms 2021, 9, 267. [Google Scholar] [CrossRef]
- Ma, L.; Shen, C.A.; Gao, L.; Li, D.W.; Shang, Y.R.; Yin, K.; Zhao, D.X.; Cheng, W.F.; Quan, D.Q. Anti-Inflammatory Activity of Chitosan Nanoparticles Carrying NF-ΚB/P65 Antisense Oligonucleotide in RAW264.7 Macropghage Stimulated by Lipopolysaccharide. Colloids Surf. B Biointerfaces 2016, 142, 297–306. [Google Scholar] [CrossRef]
- Venkatesh, N. Metallic Nanoparticle: A Review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar] [CrossRef] [Green Version]
- Ajitha, B.; Reddy, Y.A.K.; Jeon, H.J.; Ahn, C.W. Synthesis of Silver Nanoparticles in an Eco-Friendly Way Using Phyllanthus Amarus Leaf Extract: Antimicrobial and Catalytic Activity. Adv. Powder Technol. 2018, 29, 86–93. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Ullah, S.; Shah, S.I.; Nasir, F.; Shah, A.; Iqbal, Z.; Tahir, K.; Khan, U.A.; Yuan, Q. Synthesis of Phytochemicals-Stabilized Gold Nanoparticles and Their Biological Activities against Bacteria and Leishmania. Microb. Pathog. 2017, 110, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Raza, A.; Islam, N.U.; Ayaz, M.; Saravanan, M.; Ali, M.; Ahmad, I.; Shahid, M.; Shinwari, Z.K. Multifunctional Theranostic Applications of Biocompatible Green-Synthesized Colloidal Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Alti, D.; Veeramohan Rao, M.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold-Silver Bimetallic Nanoparticles Reduced with Herbal Leaf Extracts Induce ROS-Mediated Death in Both Promastigote and Amastigote Stages of Leishmania Donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.A.; Iqbal, J.; Khan, Z.; Ahmad, R.; Uddin, S.; Shahbaz, A.; Zahra, S.A.; Shaukat, M.; Kiran, F.; Kanwal, S.; et al. Phytofabrication of Cobalt Oxide Nanoparticles from Rhamnus Virgata Leaves Extract and Investigation of Different Bioactivities. Microsc. Res. Tech. 2020, 84, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Munir, A.; Uddin, S.; Kanwal, S.; Mahmood, T. Facile Green Synthesis Approach for the Production of Chromium Oxide Nanoparticles and Their Different in Vitro Biological Activities. Microsc. Res. Tech. 2020, 83, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Sumaira; Afridi, M.S.; Hashmi, S.S.; Ali, G.S.; Zia, M.; Abbasi, B.H. Comparative Antileishmanial Efficacy of the Biosynthesised ZnO NPs from Genus Verbena. IET Nanobiotechnology 2018, 12, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Khalil, A.T.; Ali, M.; Numan, M.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Greener Synthesis of ZnO and Ag-ZnO Nanoparticles Using Silybum Marianum for Diverse Biomedical Applications. Nanomedicine 2019, 14, 655–673. [Google Scholar] [CrossRef]
- Javed, B.; Mashwani, Z.-U.; Sarwer, A.; Raja, N.I.; Nadhman, A. Synergistic Response of Physicochemical Reaction Parameters on Biogenesis of Silver Nanoparticles and Their Action against Colon Cancer and Leishmanial Cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1340–1353. [Google Scholar] [CrossRef]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green Synthesis of Silver and Titanium Dioxide Nanoparticles Using Euphorbia Prostrata Extract Shows Shift from Apoptosis to G0/G1 Arrest Followed by Necrotic Cell Death in Leishmania Donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Zaki, L.; Saryazdi, A.K.P.; Tavakoli, P.; Tavajjohi, A.; Poursalehi, R.; Delavari, H.; Ghaffarifar, F. Efficacy of Green Synthesized Silver Nanoparticles via Ginger Rhizome Extract against Leishmania Major in Vitro. PLoS ONE 2021, 16, 1–12. [Google Scholar] [CrossRef]
- El-Khadragy, M.; Alolayan, E.M.; Metwally, D.M.; El-Din, M.F.S.; Alobud, S.S.; Alsultan, N.I.; Alsaif, S.S.; Awad, M.A.; Moneim, A.E.A. Clinical Efficacy Associated with Enhanced Antioxidant Enzyme Activities of Silver Nanoparticles Biosynthesized Using Moringa Oleifera Leaf Extract, against Cutaneous Leishmaniasis in a Murine Model of Leishmania Major. Int. J. Environ. Res. Public Health 2018, 15, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalangi, S.K.; Dayakar, A.; Gangappa, D.; Sathyavathi, R.; Maurya, R.S.; Narayana Rao, D. Biocompatible Silver Nanoparticles Reduced from Anethum Graveolens Leaf Extract Augments the Antileishmanial Efficacy of Miltefosine. Exp. Parasitol. 2016, 170, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Syed, F.; Imran, M.; Khan, A.U.; Tahir, K.; Khan, Z.U.H.; Yuan, Q. Phytosynthesis and Antileishmanial Activity of Gold Nanoparticles by Maytenus Royleanus. J. Food Biochem. 2015, 40, 420–427. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Yaseen, T.; El-Serehy, H.A.; Ahmad, P. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Hassan, D.; Khalil, A.T.; Mughal, A.; El-Mallul, A.; Ayaz, M.; Maaza, M. Floral extracts-mediated green synthesis of NiO nanoparticles and their diverse pharmacological evaluations. J. Biomol. Struct. Dyn. 2020, 39, 4133–4147. [Google Scholar] [CrossRef]
- Jain, V.; Gupta, A.; Pawar, V.K.; Asthana, S.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Chitosan-Assisted Immunotherapy for Intervention of Experimental Leishmaniasis via Amphotericin B-Loaded Solid Lipid Nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 1309–1330. [Google Scholar] [CrossRef]
- Lopes, R.M.; Pereira, J.; Esteves, M.A.; Gaspar, M.M.; Carvalheiro, M.; Eleutério, C.V.; Gonçalves Lídia Jiménez-Ruiz, A.; Almeida, A.J.; Cruz, M.E.M. Lipid-Based Nanoformulations of Trifluralin Analogs in the Management of Leishmania Infantum Infections. Nanomedicine 2016, 11, 153–170. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and Surface Characteristics of Gold Nanoparticles Influence Their Biodistribution and Uptake by Macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, P.; Kumar, P.; Kumar, S.; Rajana, V.K.; Kant, V.; Prasad, S.R.; Mohan, U.; Ravichandiran, V.; Mandal, D. Current Status of Nanoscale Drug Delivery and the Future of Nano-Vaccine Development for Leishmaniasis—A Review. Biomed. Pharmacother. 2021, 141, 111920. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruenraroengsak, P.; Cook, J.M.; Florence, A.T. Nanosystem Drug Targeting: Facing up to Complex Realities. J. Control. Release 2010, 141, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.C.; Ferreira, C.S.; Branquinha, M.H.; Santos, A.L.S.; Chaud, M.V.; Jain, S.; Cardoso, J.C.; Kovačević, A.B.; Souto, E.B.; Severino, P. Overcoming Multi-Resistant Leishmania Treatment by Nanoencapsulation of Potent Antimicrobials. J. Chem. Technol. Biotechnol. 2021, 96, 2123–2140. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous Leishmaniasis: Immune Responses in Protection and Pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Assolini, J.P.; da Silva, T.P.; da Silva Bortoleti, B.T.; Gonçalves, M.D.; Tomiotto-Pellissier, F.; Sahd, C.S.; Carloto, A.C.M.; Feuser, P.E.; Cordeiro, A.P.; Sayer, C.; et al. 4-Nitrochalcone Exerts Leishmanicidal Effect on L. Amazonensis Promastigotes and Intracellular Amastigotes, and the 4-Nitrochalcone Encapsulation in Beeswax Copaiba Oil Nanoparticles Reduces Macrophages Cytotoxicity. Eur. J. Pharmacol. 2020, 884, 173392. [Google Scholar] [CrossRef]
- Moreno, E.; Schwartz, J.; Larrea, E.; Conde, I.; Font, M.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Assessment of β-Lapachone Loaded in Lecithin-Chitosan Nanoparticles for the Topical Treatment of Cutaneous Leishmaniasis in L. Major Infected BALB/c Mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2003–2012. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.K.; Bedi, P.M.S. Nanoemulsion Based Gel for Transdermal Delivery of Meloxicam: Physico-Chemical, Mechanistic Investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Júnior, V.F.; Nakamura, C.V. Natural Products and Chagas’ Disease: A Review of Plant Compounds Studied for Activity against Trypanosoma Cruzi. Nat. Prod. Rep. 2011, 28, 809–823. [Google Scholar] [CrossRef]
- Varela, M.T.; Lima, M.L.; Galuppo, M.K.; Tempone, A.G.; de Oliveira, A.; Lago, J.H.G.; Fernandes, J.P.S. New Alkenyl Derivative from Piper Malacophyllum and Analogues: Antiparasitic Activity against Trypanosoma Cruzi and Leishmania Infantum. Chem. Biol. Drug Des. 2017, 90, 1007–1011. [Google Scholar] [CrossRef]
- Da Silva, B.J.M.; Hage, A.A.P.; Silva, E.O.; Rodrigues, A.P.D. Medicinal Plants from the Brazilian Amazonian Region and Their Antileishmanial Activity: A Review. J. Integr. Med. 2018, 16, 211–222. [Google Scholar] [CrossRef]
- Callahan, H.L.; Portal, A.C.; Devereaux, R.; Grogl, M. An Axenic Amastigote System for Drug Screening. Antimicrob. Agents Chemother. 1997, 41, 818–822. [Google Scholar] [CrossRef] [PubMed]
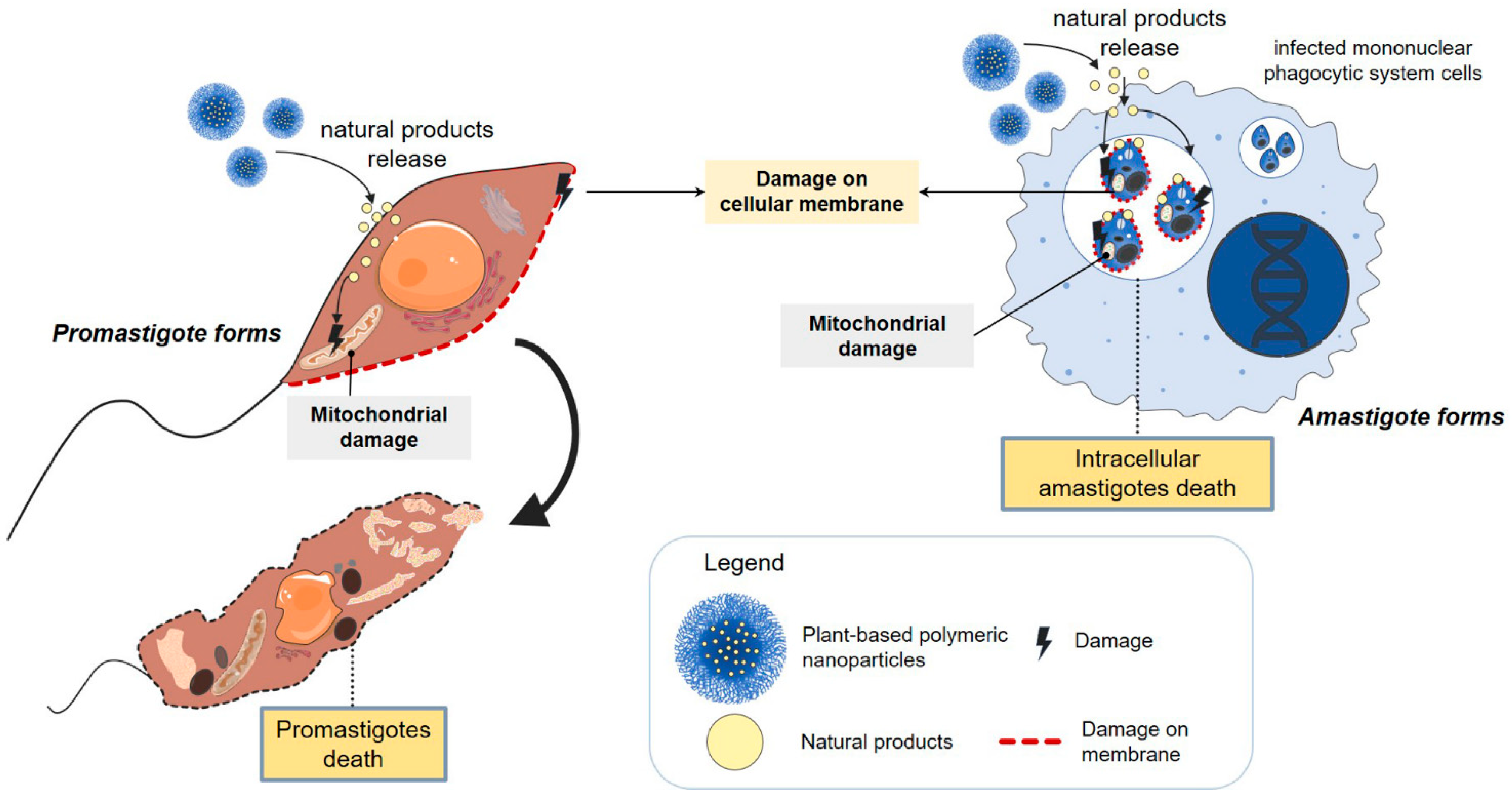
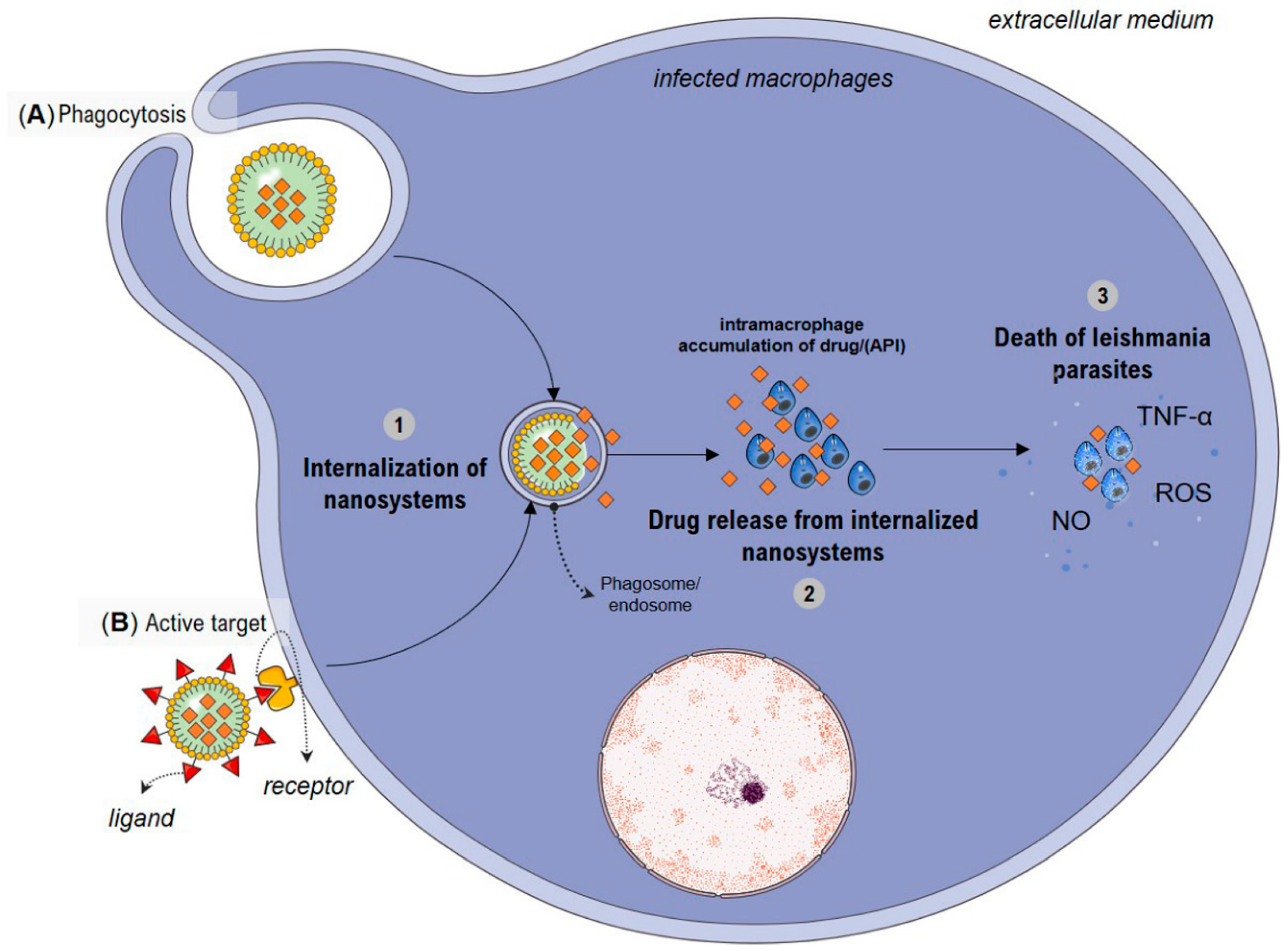
| Species | Vegetable Derivative | Physicochemical Characteristics | Leishmania spp. | Life Stage | IC50 (µg·mL−1) | In Vivo Effect | Toxicity | Reference |
|---|---|---|---|---|---|---|---|---|
| Pterodon emarginatus vogel | Fruits—oleoresin | Mean size: 158.10 nm, PDI 0.136 | L. amazonensis | Amastigote | n.d. | Decrease in lesion diameter; improvement in histopathological parameters, reduction in parasite load, and reduction in cytokine levels. | No cytotoxic effect in vitro | [55] |
| Pterodon pubescens benth | Fruits—extracts | Mean size: 185 nm, PDI 0.170, and consistency index of 1405.00 | L. amazonensis | Amastigote | 1.9 ± 0.30 | n.d. | Moderate cytotoxicity against J774 | [56] |
| Copaifera paupera | Trunks—oleoresin | Mean size: 114.9 ± 1.2 nm, PDI 0.18, ZP: −24.46 mV | L. amazonensis | Promastigote | 62.5 ± 8.3 | n.d. | n.d. | [57] |
| L. infantum | 65.9 ± 8.8 | |||||||
| Copaifera sp. Linnaeu | Essential oil | Mean size: 76.10 nm, PDI: 0.14, ZP: −2.5 mV | L. infantum | Promastigote | 16 ± 0.9 (24 h) | Decreased development of lesions, reduction of parasite load in liver and spleen. | Low in vitro toxicity against macrophages | [58] |
| L. amazonensis | 18 ± 0.16 (24 h) | |||||||
| L. infantum | Amastigote | Diminution of IF (≈90%) | ||||||
| L. amazonensis | Diminution of IF (≈96%) | |||||||
| Carapa guianensis Aublet | Essential oil | Mean size: 88.17 nm, PDI 0.16, ZP: −3.9 mV | L. infantum | Promastigote | 366 ± 21 (24 h) | Decreased development of lesions, reduced parasite load in liver and spleen, and improved histopathological features. | Low in vitro toxicity against macrophage | [58] |
| L. amazonensis | 590 ± 23 (24 h) | |||||||
| L. infantum | Amastigote | Diminution of IF (~50%) | ||||||
| L. amazonensis | Diminution of IF (~90%) | |||||||
| Citrus sinensis | Essential oil | Mean size of 225 nm (nanoemulsion-based nanogel) | L. tropica | Promastigote | 151.13 | n.d. | n.d. | [59] |
| L. major | 108.31 | |||||||
| Cinnamomum zeylanicum | Essential oil | Mean size: 52 nm | L. tropica | Promastigote | n.d. | n.d. | n.d. | [60] |
| L. major | ||||||||
| Lavandula angustifolia | Not shown | Mean size: 104.2 nm, PDI 0.312, negative ZP: −15.8 mV | L. major | Promastigote | 0.11 | n.d. | Low in vitro toxicity against J774 | [61] |
| Amastigote | 0.06 | |||||||
| Rosmarinus officinalis | Not shown | Mean size: 98.7 nm, PDI 0.298, ZP: −17.3 mV | L. major | Promastigote | 0.08 | n.d. | Low in vitro toxicity against J774 | [61] |
| Amastigote | 0.06 |
| Species | Vegetable Derivative | Physicochemical Characteristics | Leishmania spp. | Life Stage | IC50 (µg·mL−1) | In Vivo Effect | Toxicity | Reference |
|---|---|---|---|---|---|---|---|---|
| Berberis vulgaris and Berberis aristata | Berberine | Mean size: 120 nm, ZP: −38 mV, and loaded 6 nmol/µmol lipid. | L. infantum | Promastigote | 6.8 | LP enhanced accumulation in liver and spleen; reduced parasitic load (up to 99%) | Sevenfold decrease in toxicity for macrophages | [75] |
| Amastigote | 1.4 | |||||||
| Curcuma longa | Cortex—crude methanol extract | Not shown | L. amazonensis | Promastigote | 5.5 | n.d. | n.d. | [76] |
| Curcuma longa | Curcumin | Mean size: 176.5 nm, PDI: 0.187, mean ZP: +34.99 mV, EE of 92% | L. major | Promastigote | 2.33 | n.d. | HFF cell line treated with nanoliposomes demonstrated biocompatibility | [77] |
| Amburana cearensis | Coumarin derivative (3-(1,3-benzodioxol-5-yl)-2-oxo-2H-chromen-6yl acetate) | Mean size: 173.6 nm, EE 93.2% | L. major | Promastigote | Ranged from 10 to 15 | Reduced footpad thickness and lower lymph-node parasite load compared to untreated mice | Nanoliposome formulation showed less toxicity to the host cell J774 | [33] |
| Species | Vegetable Derivative | Physicochemical Characteristics | Leishmania spp. | Life Stage | IC50 (µg·mL −1) | In Vivo Effect | Toxicity | Reference |
|---|---|---|---|---|---|---|---|---|
| Apis mellifera | Red propolis extract | Mean size: 280.2 nm, PDI 0.089, ZP: −26.8 mV and, EE 81.2% flavonoids | L. braziliensis | Promastigote | 31.2 | n.d. | n.d. | [104] |
| Aesculus hippocastanum | β-aescin (preparation of NPs the commercially available was used) | Mean size: 261.4 nm, PDI 0.12, ZP: −24.7 mV and, EE of 32% | L. infantum | Amastigote | 1.04 | n.d. | Lytic effects of β-aescin on the investigated MRC-5 cells were blocked by encapsulating in PLGA NPs (17.87 µg∙mL1) | [106] |
| - | Betulinic acid | Mean size: 112 nm, PDI 0.3, ZP: 8 mV, and EE 93% | L.major | Amastigote and promastigote | n.d. | Wound healing. Decrease in parasite burden on lesion, liver, and spleen | The highest dose of BA NPs 20 mg/kg: no changes in serum concentrations of BUN, AST, ALT, and ALP; no morphological changes in liver, kidney, and spleen | [107] |
| Matricaria chamomilla L. | Floral chapters—essential oil | Mean size: 801 nm; PDI 0.3, spherical shaped, and EE 89.9% | L. amazonensis | Amastigote | 14.3 | n.d. | Essential oil encapsulation reduces toxic effects on cell lines HaCat, macrophages, and Vero (increase CC50) | [108] |
| Promastigote | 7.2 | |||||||
| Nigella sativa | Fixed oil | Mean size: 202–389 nm, PDI 0.08–0.16, ZP of −4.92 (−8.29) mV, and EE ≈ 87% | L. infantum | Amastigote | 102 | n.d. | No cytotoxic effect in vitro | [109] |
| Promastigote | 159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, D.B.; Lemos, J.A.; Miranda, S.E.M.; Di Filippo, L.D.; Duarte, J.L.; Ferreira, L.A.M.; Barros, A.L.B.; Oliveira, A.E.M.F.M. Current Applications of Plant-Based Drug Delivery Nano Systems for Leishmaniasis Treatment. Pharmaceutics 2022, 14, 2339. https://doi.org/10.3390/pharmaceutics14112339
dos Santos DB, Lemos JA, Miranda SEM, Di Filippo LD, Duarte JL, Ferreira LAM, Barros ALB, Oliveira AEMFM. Current Applications of Plant-Based Drug Delivery Nano Systems for Leishmaniasis Treatment. Pharmaceutics. 2022; 14(11):2339. https://doi.org/10.3390/pharmaceutics14112339
Chicago/Turabian Styledos Santos, Darline B., Janaina A. Lemos, Sued E. M. Miranda, Leonardo D. Di Filippo, Jonatas L. Duarte, Lucas A. M. Ferreira, Andre L. B. Barros, and Anna E. M. F. M. Oliveira. 2022. "Current Applications of Plant-Based Drug Delivery Nano Systems for Leishmaniasis Treatment" Pharmaceutics 14, no. 11: 2339. https://doi.org/10.3390/pharmaceutics14112339