Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Purification of MSLPs (MMP-Sensitive Lipopeptides)
2.2. MMP2 Activity Assay on MSLPs
2.3. Preparation and Characterization of Liposomes
2.3.1. Preparation and Characterization of MSLP-Liposomes Functionalized with mApoE Peptide
2.3.2. Preparation and Characterization of Liposomes Loaded with Glibenclamide
2.4. Preparation and Characterization of Calcein-Loaded MSLP-Liposomes
2.5. In Vitro BBB Permeability of MSLP-Liposomes
2.6. Isolation, Culture, and Treatment of Primary Neurons and Cell Lineages
2.7. Extraction of Total RNA
2.8. Cellular Assays Using MLSP-Liposomes
2.8.1. LDH-Glo Cytotoxicity Assay
2.8.2. Cell Counting Kit-8 Assay
2.8.3. ELISA Assay
2.9. Statistical Methods
3. Results
3.1. Synthesis of MSLPs
3.2. Characterization of MSLP-Liposomes
3.3. MMP-Responsiveness of MSLP Constructs and MSLP-Liposomes
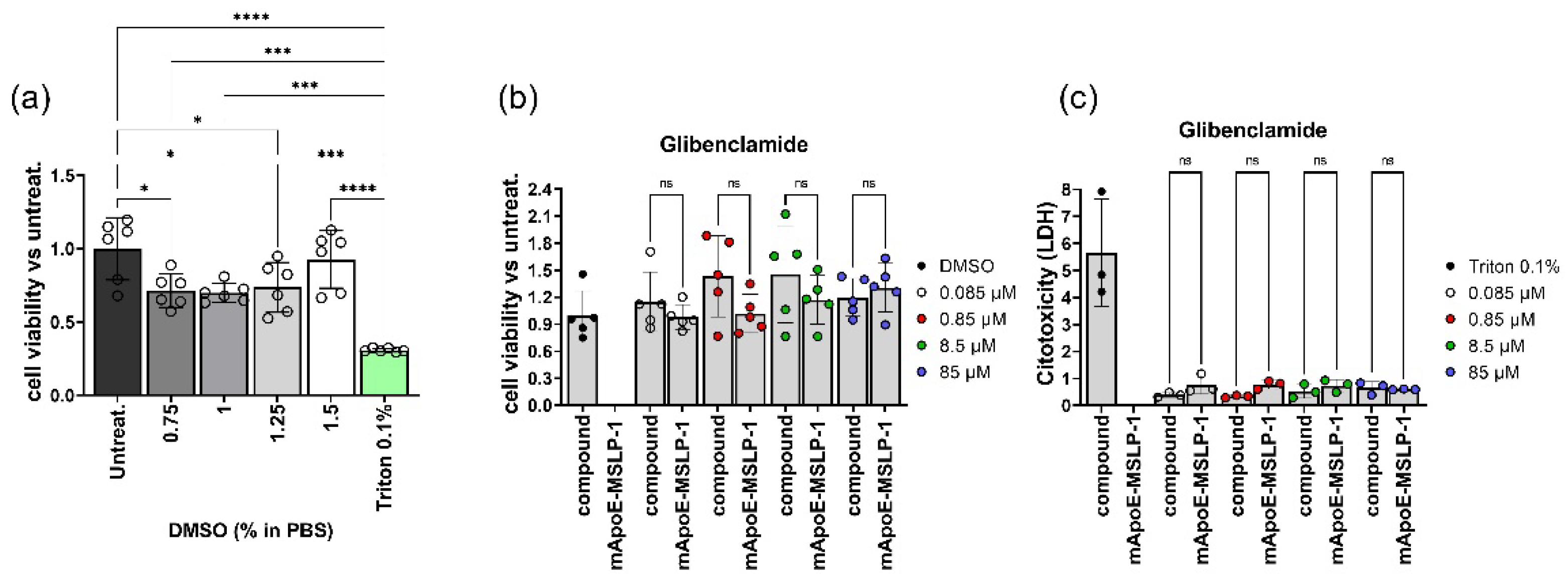
3.4. Biocompatibility of mApoE-MSLP-Liposomes
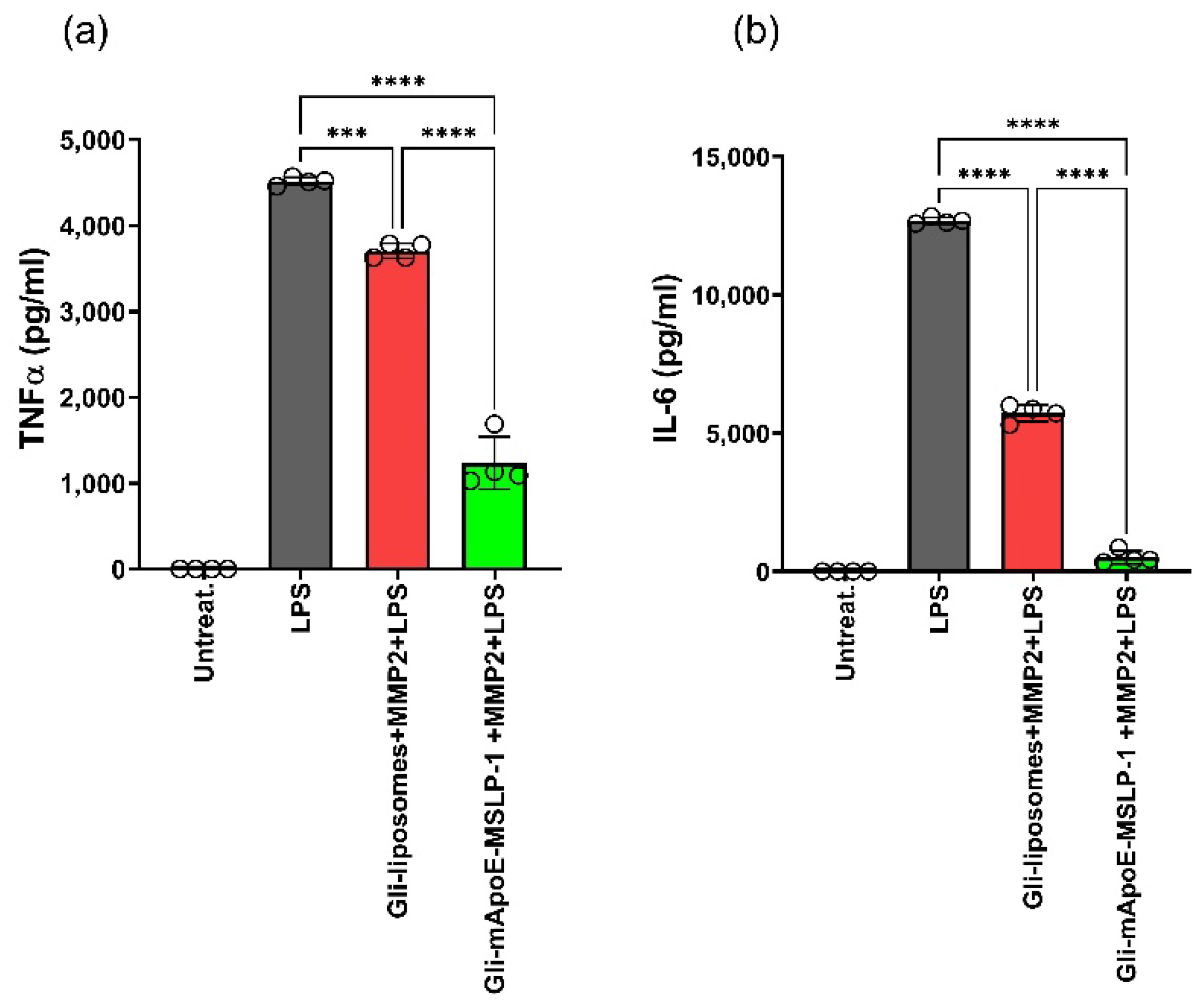
3.5. Effects on LPS-Induced Cytokine Release by Glibenclamide-Loaded mApoE-MSLP1-Liposomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Graff, O.; Chen, C. Quantifying the probability of pharmacological success to inform compound progression decisions. PLoS ONE 2020, 15, e0240234. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Girelli, M. Intracisternal delivery of PEG-coated gold nanoparticles results in high brain penetrance and long-lasting stability. J. Nanobiotechnol. 2019, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Keren-Shaul, H. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Wan Mohamad, W.B.; Tun Fizi, A. Efficacy and safety of single versus multiple daily doses of glibenclamide in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2000, 49, 93–99. [Google Scholar] [CrossRef]
- Kurland, D.B.; Tosun, C. Glibenclamide for the treatment of acute CNS injury. Pharmaceuticals 2013, 6, 1287–1303. [Google Scholar] [CrossRef]
- Chen, M.; Dong, Y.; Simard, J.M. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J. Neurosci. 2003, 23, 8568–8577. [Google Scholar] [CrossRef]
- Simard, J.M.; Geng, Z. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009, 29, 317–330. [Google Scholar] [CrossRef]
- Thompson, E.M.; Pishko, G.L. Inhibition of SUR1 decreases the vascular permeability of cerebral metastases. Neoplasia 2013, 15, 535–543. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H. Glibenclamide ameliorates cerebral edema and improves outcomes in a rat model of status epilepticus. Neuropharmacology 2017, 121, 1–11. [Google Scholar] [CrossRef]
- Jha, R.M.; Bell, J. Role of Sulfonylurea Receptor 1 and Glibenclamide in Traumatic Brain Injury: A Review of the Evidence. Int. J. Mol. Sci. 2020, 21, 409. [Google Scholar] [CrossRef]
- Woo, S.K.; Tsymbalyuk, N. SUR1-TRPM4 channels, not KATP, mediate brain swelling following cerebral ischemia. Neurosci. Lett. 2020, 718, 134729. [Google Scholar] [CrossRef]
- Nakayama, S.; Taguchi, N. Glibenclamide and Therapeutic Hypothermia Have Comparable Effect on Attenuating Global Cerebral Edema Following Experimental Cardiac Arrest. Neurocritical Care 2018, 29, 119–127. [Google Scholar] [CrossRef]
- Tomiyama, Y.; Brian, J.E. Cerebral blood flow during hemodilution and hypoxia in rats: Role of ATP-sensitive potassium channels. Stroke 1999, 30, 1942–1948. [Google Scholar] [CrossRef]
- Lahmann, C.; Kramer, H.B.; Ashcroft, F.M. Systemic Administration of Glibenclamide Fails to Achieve Therapeutic Levels in the Brain and Cerebrospinal Fluid of Rodents. PLoS ONE 2015, 10, e0134476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arrua, E.C.; Hartwig, O. Surfactant-Free Glibenclamide Nanoparticles: Formulation, Characterization and Evaluation of Interactions with Biological Barriers. Pharm. Res. 2021, 38, 1081–1092. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Ashley, J.D.; Stefanick, J.F. Liposomal carfilzomib nanoparticles effectively target multiple myeloma cells and demonstrate enhanced efficacy in vivo. J. Control. Release 2014, 196, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, S.S.; Lim, K.; Wang-Gillam, A. Nanoliposomal irinotecan plus fluorouracil and folinic acid: A new treatment option in metastatic pancreatic cancer. Exp. Rev. Anticancer Ther. 2016, 16, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.I.; Giofrè, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kohane, D. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y. Development of a hypoxia-triggered and hypoxic radiosensitized liposome as a doxorubicin carrier to promote synergetic chemo-/radio-therapy for glioma. Biomaterials 2017, 121, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Zhang, L.; Robertson, C.R. Structural requirements for a lipoamino acid in modulating the anticonvulsant activities of systemically active galanin analogues. J. Med. Chem. 2009, 52, 1310–1316. [Google Scholar] [CrossRef]
- Abdul-Gader, A.; Miles, A.J.; Wallace, B.A. A reference dataset for the analyses of membrane protein secondary structures and transmembrane residues using circular dichroism spectroscopy. Bioinformatics 2011, 27, 1630–1636. [Google Scholar] [CrossRef]
- Formicola, B.; D’Aloia, A. Differential Exchange of Multifunctional Liposomes Between Glioblastoma Cells and Healthy Astrocytes via Tunneling Nanotubes. Front. Bioeng. Biotechnol. 2019, 7, 403. [Google Scholar] [CrossRef]
- Re, F.; Cambianica, I. Functionalization with ApoE-derived peptides enhances the interaction with brain capillary endothelial cells of nanoliposomes binding amyloid-beta peptide. J. Biotechnol. 2011, 156, 341–346. [Google Scholar] [CrossRef]
- Rizzuto, M.A.; Dal Magro, R. H-Ferritin nanoparticle-mediated delivery of antibodies across a BBB in vitro model for treatment of brain malignancies. Biomater. Sci. 2021, 9, 2032–2042. [Google Scholar] [CrossRef]
- Zorec, B.; Zupančič, Š. Combinations of nanovesicles and physical methods for enhanced transdermal delivery of a model hydrophilic drug. Eur. J. Pharm. Biopharm. 2018, 127, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Duraj, S. Integrity of liposomes in presence of various formulation excipients, when dispersed in aqueous media and in hydrogels. Colloids Surf. B Biointerfaces 2008, 61, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bana, L.; Minniti, S. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood-brain-barrier: Implications for therapy of Alzheimer disease. Nanomedicine 2014, 10, 1583–1590. [Google Scholar] [CrossRef]
- Formicola, B.; Dal Magro, R. The synergistic effect of chlorotoxin-mApoE in boosting drug-loaded liposomes across the BBB. J. Nanobiotechnol. 2019, 17, 115. [Google Scholar] [CrossRef]
- Mancini, S.; Minniti, S. The hunt for brain Aβ oligomers by peripherally circulating multi-functional nanoparticles: Potential therapeutic approach for Alzheimer disease. Nanomedicine 2016, 12, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, R.; Simonelli, S. The Extent of Human Apolipoprotein A-I Lipidation Strongly Affects the β-Amyloid Efflux Across the Blood-Brain Barrier in vitro. Front. Neurosci. 2019, 13, 419. [Google Scholar] [CrossRef]
- de Ceglia, R.; Chaabane, L. Down-sizing of neuronal network activity and density of presynaptic terminals by pathological acidosis are efficiently prevented by Diminazene Aceturate. Brain Behav. Immun. 2015, 45, 263–276. [Google Scholar] [CrossRef]
- Zhu, L.; Kate, P.; Torchilin, V.P. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano 2012, 6, 3491–3498. [Google Scholar] [CrossRef]
- Terada, T.; Iwai, M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J. Control. Release 2006, 111, 333–342. [Google Scholar] [CrossRef]
- Chen, E.I.; Kridel, S.J. A unique substrate recognition profile for matrix metalloproteinase-2. J. Biol. Chem. 2002, 277, 4485–4491. [Google Scholar] [CrossRef]
- Balmert, S.C.; Zmolek, A.C. Positive Charge of “Sticky” Peptides and Proteins Impedes Release From Negatively Charged PLGA Matrices. J. Mater. Chem. B 2015, 3, 4723–4734. [Google Scholar] [CrossRef]
- Masum, S.M.; Li, S. Effect of positively charged short peptides on stability of cubic phases of monoolein/dioleoylphosphatidic acid mixtures. Langmuir 2005, 21, 5290–5297. [Google Scholar] [CrossRef]
- Orpiszewski, J.; Benson, M.D. Induction of beta-sheet structure in amyloidogenic peptides by neutralization of aspartate: A model for amyloid nucleation. J. Mol. Biol. 1999, 289, 413–428. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Z. Size-adaptable and ligand (biotin)-sheddable nanocarriers equipped with avidin scavenging technology for deep tumor penetration and reduced toxicity. J. Control. Release 2020, 320, 142–158. [Google Scholar] [CrossRef]
- Kozlov, L.V.; Bichucher, A.M. Determination of protease activity in blood and microorganisms. Biochem. Mosc. Suppl. Ser. B 2008, 2, 381–384. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci 2022, 23, 4153. [Google Scholar] [CrossRef]
- Pizzocri, M.; Re, F. Radiation and adjuvant drug-loaded liposomes target glioblastoma stem cells and trigger in-situ immune response. Neuro Oncol. Adv. 2021, 3, vdab076. [Google Scholar] [CrossRef]
- Mancini, S.; Balducci, C. Multifunctional liposomes delay phenotype progression and prevent memory impairment in a presymptomatic stage mouse model of Alzheimer disease. J. Control. Release 2017, 258, 121–129. [Google Scholar] [CrossRef]
- Re, F.; Cambianica, I. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomedicine 2011, 7, 551–559. [Google Scholar] [CrossRef]
- Balducci, C.; Mancini, S. Multifunctional liposomes reduce brain β-amyloid burden and ameliorate memory impairment in Alzheimer’s disease mouse models. J. Neurosci. 2014, 34, 14022–14031. [Google Scholar] [CrossRef]
- Taiarol, L.; Bigogno, C. Givinostat-Liposomes: Anti-Tumor Effect on 2D and 3D Glioblastoma Models and Pharmacokinetics. Cancers 2022, 14, 2978. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lang, J. Designing Liposomes To Suppress Extracellular Matrix Expression To Enhance Drug Penetration and Pancreatic Tumor Therapy. ACS Nano 2017, 11, 8668–8678. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P. Colloidal stability of liposomes. AIMS Mater. Sci. 2019, 6, 200–213. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Narisawa, S.; Nagata, M. An organic acid-induced sigmoidal release system for oral controlled-release preparations. Pharm. Res. 1994, 11, 111–116. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Calpena, A.C. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- More, M.P.; Deshmukh, P.K. Development of amine-functionalized superparamagnetic iron oxide nanoparticles anchored graphene nanosheets as a possible theranostic agent in cancer metastasis. Drug Deliv. Transl. Res. 2020, 10, 862–877. [Google Scholar] [CrossRef]
- Makar, T.K.; Gerzanich, V. Silencing of Abcc8 or inhibition of newly upregulated Sur1-Trpm4 reduce inflammation and disease progression in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2015, 12, 210. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giofrè, S.; Renda, A.; Sesana, S.; Formicola, B.; Vergani, B.; Leone, B.E.; Denti, V.; Paglia, G.; Groppuso, S.; Romeo, V.; et al. Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions. Pharmaceutics 2022, 14, 2402. https://doi.org/10.3390/pharmaceutics14112402
Giofrè S, Renda A, Sesana S, Formicola B, Vergani B, Leone BE, Denti V, Paglia G, Groppuso S, Romeo V, et al. Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions. Pharmaceutics. 2022; 14(11):2402. https://doi.org/10.3390/pharmaceutics14112402
Chicago/Turabian StyleGiofrè, Sabrina, Antonio Renda, Silvia Sesana, Beatrice Formicola, Barbara Vergani, Biagio Eugenio Leone, Vanna Denti, Giuseppe Paglia, Serena Groppuso, Valentina Romeo, and et al. 2022. "Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions" Pharmaceutics 14, no. 11: 2402. https://doi.org/10.3390/pharmaceutics14112402
APA StyleGiofrè, S., Renda, A., Sesana, S., Formicola, B., Vergani, B., Leone, B. E., Denti, V., Paglia, G., Groppuso, S., Romeo, V., Muzio, L., Balboni, A., Menegon, A., Antoniou, A., Amenta, A., Passarella, D., Seneci, P., Pellegrino, S., & Re, F. (2022). Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions. Pharmaceutics, 14(11), 2402. https://doi.org/10.3390/pharmaceutics14112402









