Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes
Abstract
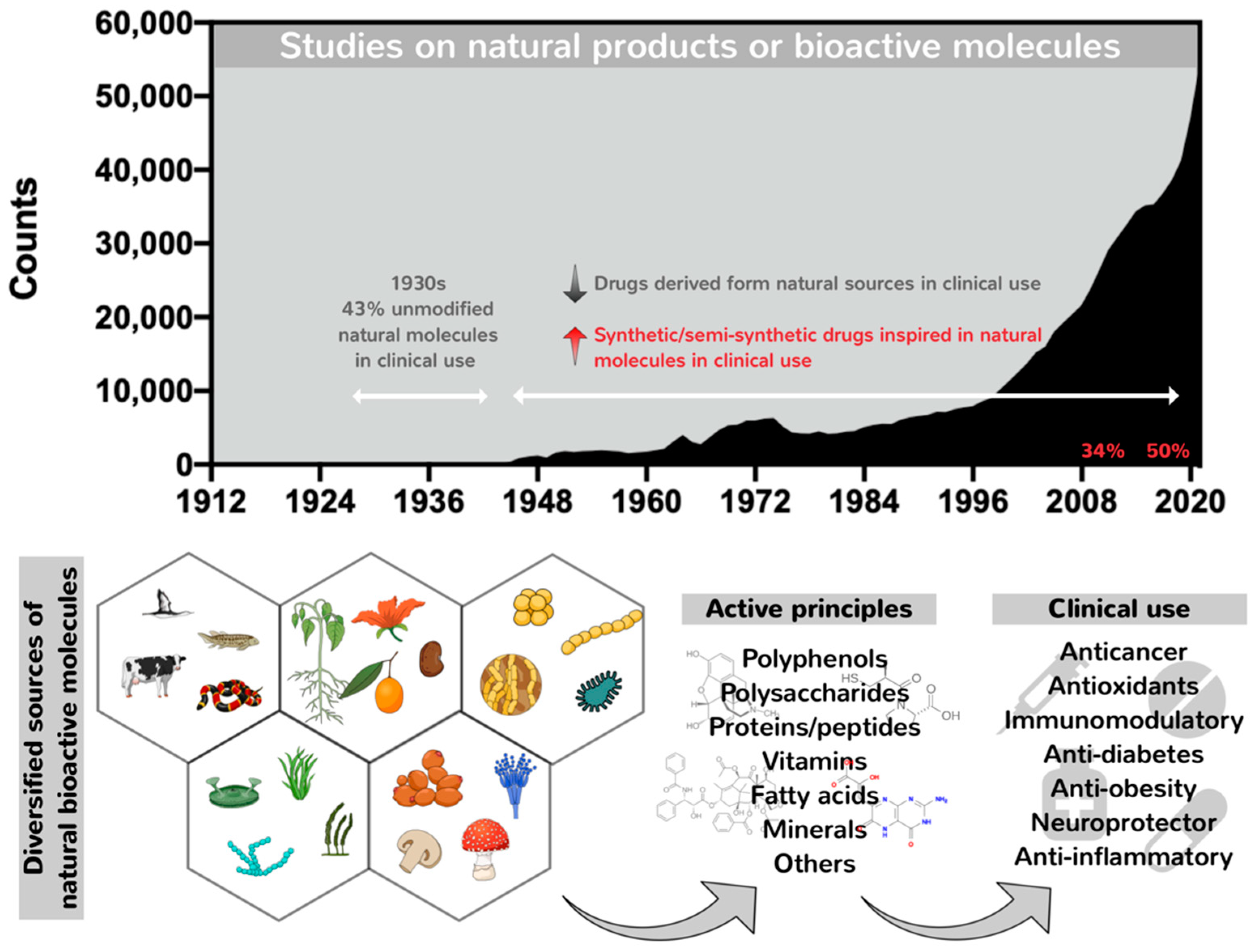
1. A General Overview of the Remarkable Role of Nature in Providing Potential Pharmacological Compounds
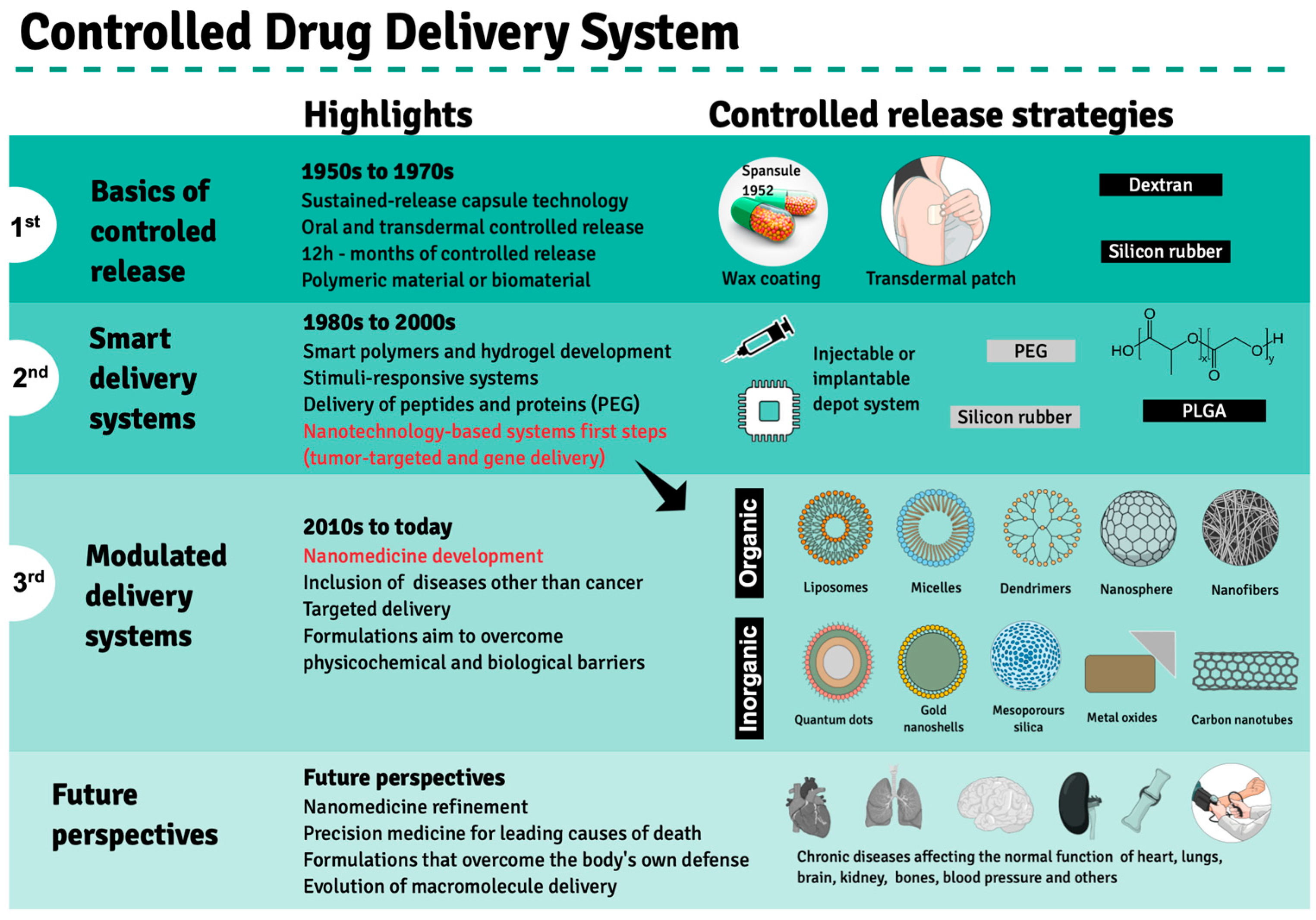
2. Evolution of Drug Delivery Systems and the Emergence of Nanotechnology in Clinical Treatments
2.1. Challenges and Successful Strategies for the Therapeutic Use of Proteins and Peptides
2.2. Nano-Delivery Strategies for Protein and Peptide Drugs
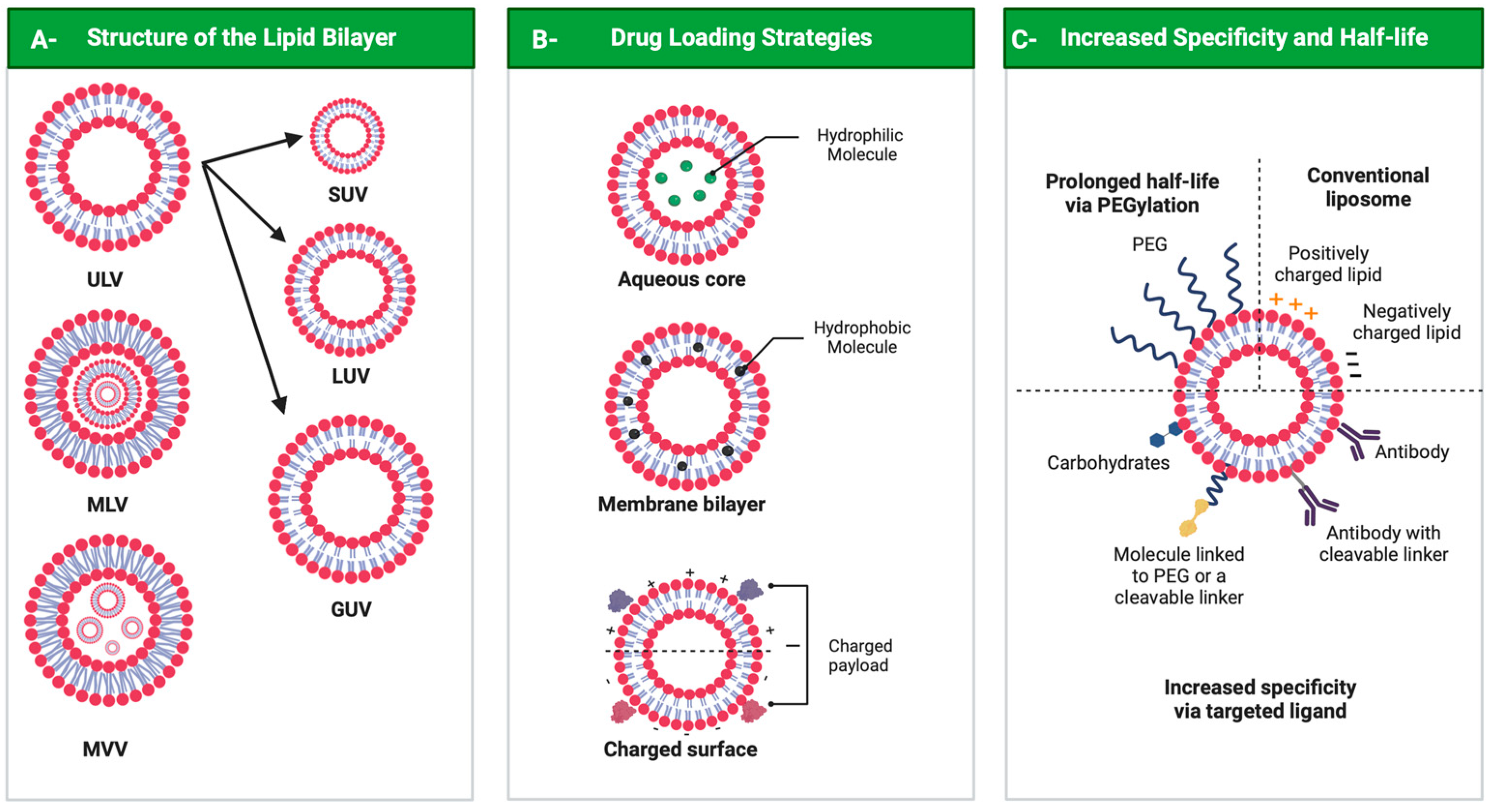
3. Liposomal Formulation Performance: Characteristics, Functionalization and Internalization
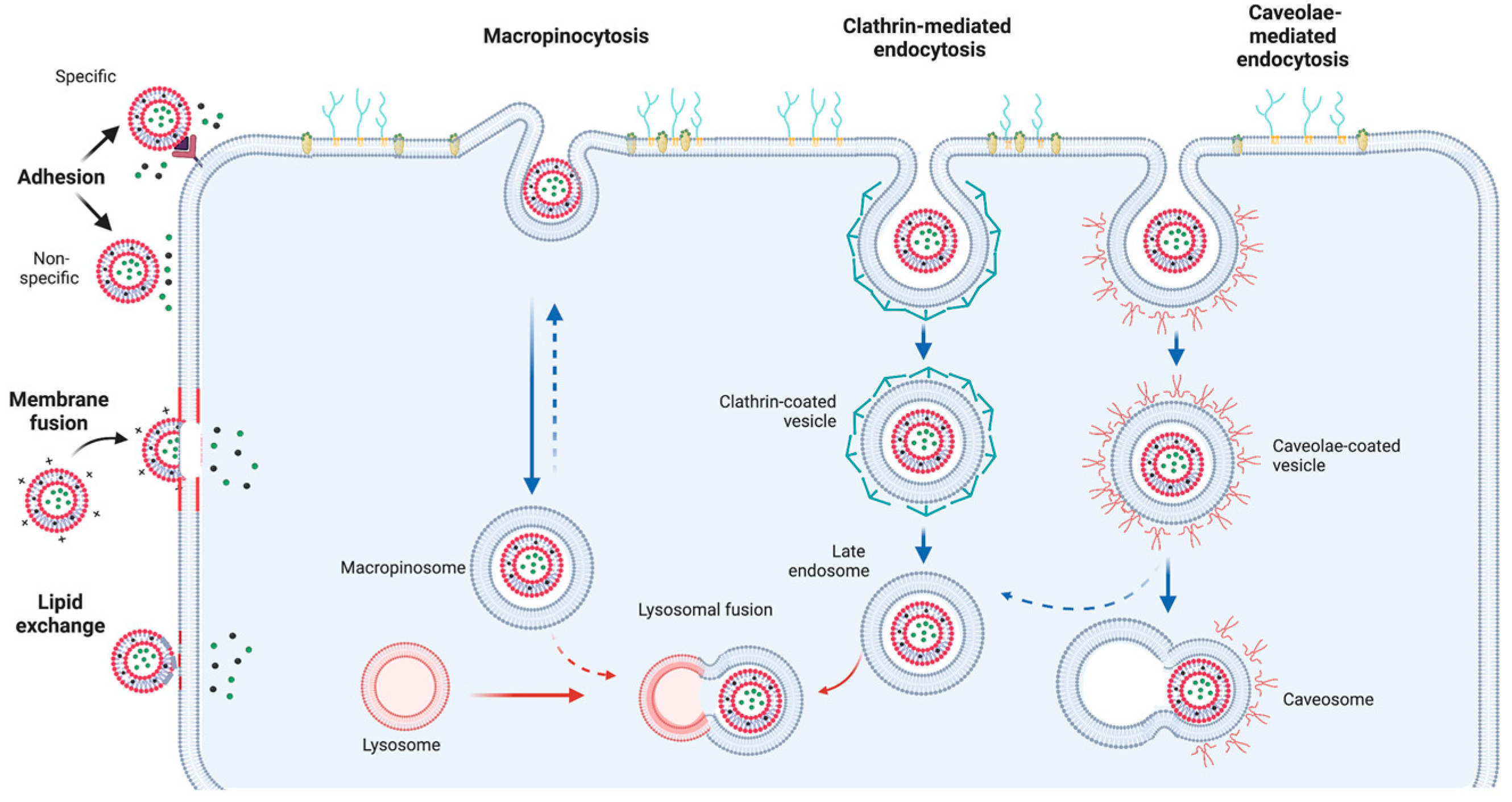
3.1. Liposome Functionalization
3.2. Liposome Internalization and Delivery Mechanism
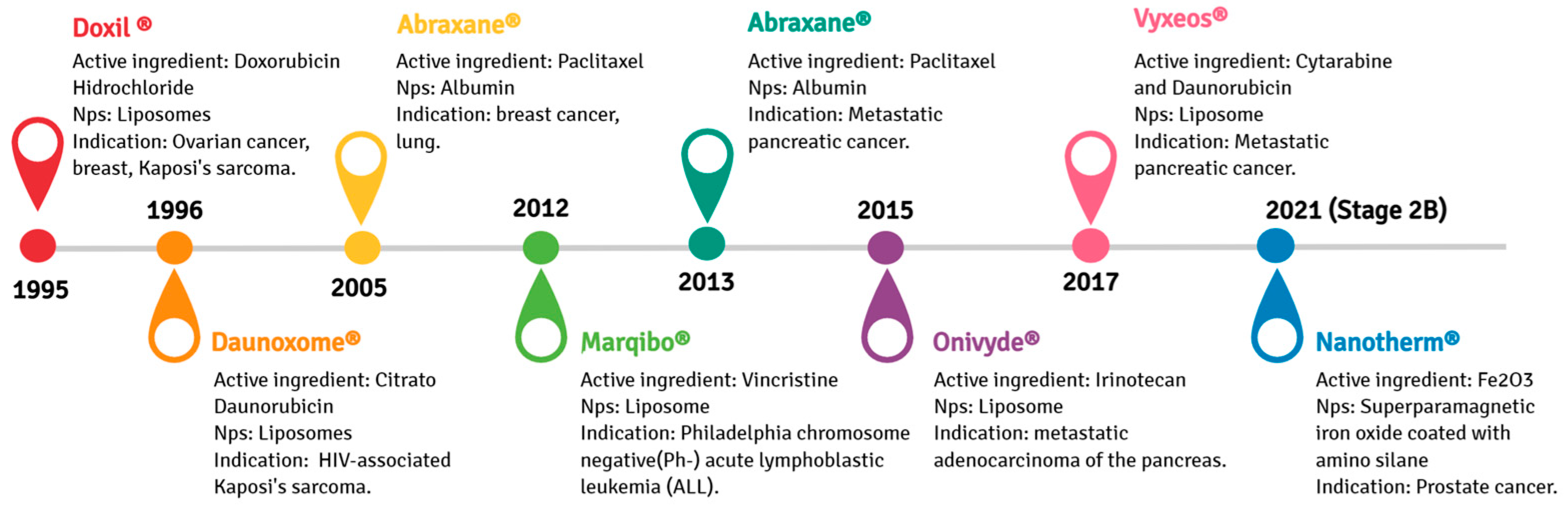
3.3. Liposomal Formulations in Antitumorigenic Drugs and Vaccines: Marketed or Phase III Clinical Trial Products
3.4. Limitations concerning the Application of Nano-Liposomes in Drug Delivery
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Masuram, S.; Schiöth, H.B. The Druggable Genome: Evaluation of Drug Targets in Clinical Trials Suggests Major Shifts in Molecular Class and Indication. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release Off. J. Control. Release Soc. 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. [Google Scholar]
- Baranowska, M.; Suliborska, K.; Todorovic, V.; Kusznierewicz, B.; Chrzanowski, W.; Sobajic, S.; Bartoszek, A. Interactions between bioactive components determine antioxidant, cytotoxic and nutrigenomic activity of cocoa powder extract. Free. Radic. Biol. Med. 2020, 154, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, Y.; Zhou, X.; Bi, H.; Yang, B. Structure identification of soybean peptides and their immunomodulatory activity. Food Chem. 2021, 359, 129970. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Wu, S.-J.; Tung, Y.-J.; Ng, L.-T. Anti-diabetic effects of Grifola frondosa bioactive compound and its related molecular signaling pathways in palmitate-induced C2C12 cells. J. Ethnopharmacol. 2020, 260, 112962. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, I.; Sharma, M.; Mehta, M.; Badyal, S.; Sharma, V.; Sharma, I.; Singh, H.; Sistla, S. Bioactive compounds and probiotics–a ray of hope in COVID-19 management. Food Sci. Hum. Wellness 2021, 10, 131–140. [Google Scholar] [CrossRef]
- Siu, F.Y.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 2018, 13, 4133. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, J. (−)-Epigallocatechin-3-gallate provides neuroprotection via AMPK activation against traumatic brain injury in a mouse model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2209–2220. [Google Scholar] [CrossRef]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern drug delivery strategies applied to natural active compounds. Expert Opin. Drug Deliv. 2017, 14, 755–768. [Google Scholar] [CrossRef]
- Reza Rezaie, H.; Esnaashary, M.; Aref arjmand, A.; Öchsner, A. The History of Drug Delivery Systems. In A Review of Biomaterials and Their Applications in Drug Delivery; Reza Rezaie, H., Esnaashary, M., Aref arjmand, A., Öchsner, A., Eds.; Springer: Singapore, 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Dai Phung, C.; Tran, T.H.; Nguyen, H.T.; Jeong, J.-H.; Yong, C.S.; Kim, J.O. Current developments in nanotechnology for improved cancer treatment, focusing on tumor hypoxia. J. Control. Release 2020, 324, 413–429. [Google Scholar] [CrossRef]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, D.; Banerjee, M.; Datta, A.; Kalia, K.; Dhar, S.; Yavagal, D.R.; Bhattacharya, P. Nanotechnology in the diagnosis and treatment of stroke. Drug Discov. Today 2021, 26, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Schlichtmann, B.W.; Hepker, M.; Palanisamy, B.N.; John, M.; Anantharam, V.; Kanthasamy, A.G.; Narasimhan, B.; Mallapragada, S.K. Nanotechnology-mediated therapeutic strategies against synucleinopathies in neurodegenerative disease. Curr. Opin. Chem. Eng. 2021, 31, 100673. [Google Scholar] [CrossRef]
- Bhavana, V.; Thakor, P.; Singh, S.B.; Mehra, N.K. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020, 261, 118336. [Google Scholar] [CrossRef]
- Anand, K.; Vadivalagan, C.; Joseph, J.S.; Singh, S.K.; Gulati, M.; Shahbaaz, M.; Abdellattif, M.H.; Prasher, P.; Gupta, G.; Chellappan, D.K. A novel nano therapeutic using convalescent plasma derived exosomal (CPExo) for COVID-19: A combined hyperactive immune modulation and diagnostics. Chem. -Biol. Interact. 2021, 344, 109497. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- McNeil, S.E. Unique benefits of nanotechnology to drug delivery and diagnostics. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–8. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodriguez-Serrano, F.; Peran, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Rojas-Aguirre, Y.; Aguado-Castrejón, K.; González-Méndez, I. La nanomedicina y los sistemas de liberación de fármacos:¿ la (r) evolución de la terapia contra el cáncer? Educ. Química 2016, 27, 286–291. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. New advances in nanotechnology-based diagnosis and therapeutics for breast cancer: An assessment of active-targeting inorganic nanoplatforms. Bioconjugate Chem. 2017, 28, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 2020, 112, 110924. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Shirode, A.B.; Sylvester, P.W.; Nazzal, S. Preparation, characterization, and anticancer effects of simvastatin–tocotrienol lipid nanoparticles. Int. J. Pharm. 2010, 389, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Safwat, S.; Hathout, R.M.; Ishak, R.A.; Mortada, N.D. Augmented simvastatin cytotoxicity using optimized lipid nanocapsules: A potential for breast cancer treatment. J. Liposome Res. 2017, 27, 1–10. [Google Scholar] [CrossRef]
- Kumar, N.; Salar, R.K.; Prasad, M.; Ranjan, K. Synthesis, characterization and anticancer activity of vincristine loaded folic acid-chitosan conjugated nanoparticles on NCI-H460 non-small cell lung cancer cell line. Egypt. J. Basic Appl. Sci. 2018, 5, 87–99. [Google Scholar] [CrossRef]
- Bai, L.; Fei, W.-D.; Gu, Y.-Y.; He, M.; Du, F.; Zhang, W.-Y.; Yang, L.-L.; Liu, Y.-J. Liposomes encapsulated iridium (III) polypyridyl complexes enhance anticancer activity in vitro and in vivo. J. Inorg. Biochem. 2020, 205, 111014. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Liu, X.; Liu, L.; Fang, Y.; Rao, R.; Ren, Y.; Yang, X.; Liu, W. Enhanced anticancer effect of doxorubicin by TPGS-coated liposomes with Bcl-2 siRNA-corona for dual suppression of drug resistance. Asian J. Pharm. Sci. 2020, 15, 646–660. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Mousazadeh, H.; Ghareghomi, S.; Dadashpour, M.; Babazadeh, M.; Zarghami, N. In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. J. Drug Deliv. Sci. Technol. 2021, 61, 102318. [Google Scholar] [CrossRef]
- Torres-Pérez, S.A.; del Pilar Ramos-Godínez, M.; Ramón-Gallegos, E. Glycosylated one-step PAMAM dendrimers loaded with methotrexate for target therapy in breast cancer cells MDA-MB-231. J. Drug Deliv. Sci. Technol. 2020, 58, 101769. [Google Scholar] [CrossRef]
- He, H.; Yuan, Q.; Bie, J.; Wallace, R.L.; Yannie, P.J.; Wang, J.; Lancina III, M.G.; Zolotarskaya, O.Y.; Korzun, W.; Yang, H. Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: Use of this platform to modulate atherosclerosis. Transl. Res. 2018, 193, 13–30. [Google Scholar] [CrossRef]
- Gao, G.; Chen, R.; He, M.; Li, J.; Wang, L.; Sun, T. Gold nanoclusters for Parkinson’s disease treatment. Biomaterials 2019, 194, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kuen, C.Y.; Fakurazi, S.; Othman, S.S.; Masarudin, M.J. Increased loading, efficacy and sustained release of silibinin, a poorly soluble drug using hydrophobically-modified chitosan nanoparticles for enhanced delivery of anticancer drug delivery systems. Nanomaterials 2017, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Zhao, X.; Ma, M.; Zhu, G.; Yin, L. Resveratrol-loaded albumin nanoparticles with prolonged blood circulation and improved biocompatibility for highly effective targeted pancreatic tumor therapy. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [PubMed]
- Cancino, J.; Marangoni, V.S.; Zucolotto, V. Nanotechnology in medicine: Concepts and concerns. Química Nova 2014, 37, 521–526. [Google Scholar] [CrossRef]
- Society, A.C. Types and Phases of Clinical Trials. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know/phases-of-clinical-trials.html (accessed on 7 October 2022).
- Presant, C.A.; Scolaro, M.; Kennedy, P.; Blayney, D.; Flanagan, B.; Lisak, J.; Presant, J. Liposomal daunorubicin treatment of HIV-associated Kaposi’s sarcoma. Lancet 1993, 341, 1242–1243. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Martin, F.J.; Working, P.; Volberding, P.A.; Russell, J.; Newman, M.; Amantea, M.A.; Kaplan, L.D. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: Pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J. Clin. Pharmacol. 1996, 36, 55–63. [Google Scholar] [CrossRef]
- Khawar, I.A.; Kim, J.H.; Kuh, H.-J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control. Release 2015, 201, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.N.; Granai, C.O.; Rose, P.G.; Hainsworth, J.; Lopez, A.; Weissman, C.; Rosales, R.; Sharpington, T. Phase II Study of Liposomal Doxorubicin in Platinum- and Paclitaxel-Refractory Epithelial Ovarian Cancer. J. Clin. Oncol. 2000, 18, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanomedicines for cancer therapy: An update of FDA approved and those under various stages of development. SOJ Pharm. Pharm. Sci. 2014, 1, 13. [Google Scholar]
- Guarneri, V.; Dieci, M.V.; Conte, P. Enhancing intracellular taxane delivery: Current role and perspectives of nanoparticle albumin-bound paclitaxel in the treatment of advanced breast cancer. Expert Opin. Pharmacother. 2012, 13, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef]
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Swaminathan, J.; Ehrhardt, C. Liposomal delivery of proteins and peptides. Expert Opin. Drug Deliv. 2012, 9, 1489–1503. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnurch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef]
- Jain, D.; Mahammad, S.S.; Singh, P.P.; Kodipyaka, R. A review on parenteral delivery of peptides and proteins. Drug Dev. Ind. Pharm. 2019, 45, 1403–1420. [Google Scholar] [CrossRef]
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, H.M.; Danquah, M.K. Protein and Peptide Biopharmaceuticals: An Overview. Protein Pept. Lett. 2017, 24, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, P.; Zhang, L.; Yang, M.; Nielsen, H.M.; Müllertz, A.; Mu, H. Solid lipid particles for oral delivery of peptide and protein drugs I–Elucidating the release mechanism of lysozyme during lipolysis. Eur. J. Pharm. Biopharm. 2013, 85, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; McClements, D.J. Lactase (β-galactosidase) encapsulation in hydrogel beads with controlled internal pH microenvironments: Impact of bead characteristics on enzyme activity. Food Hydrocoll. 2017, 67, 85–93. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.L.; McClements, D.J. Recent advances in encapsulation, protection, and oral delivery of bioactive proteins and peptides using colloidal systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Administration strategies for proteins and peptides. Int. J. Pharm. 2014, 477, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. The.r Drug Carrier Syst. 2013, 30, 293–329. [Google Scholar] [CrossRef]
- Moncalvo, F.; Martinez Espinoza, M.I.; Cellesi, F. Nanosized Delivery Systems for Therapeutic Proteins: Clinically Validated Technologies and Advanced Development Strategies. Front. Bioeng. Biotechnol. 2020, 8, 89. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Niu, Z.; Conejos-Sánchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Guindani, C.; Feuser, P.E.; Cordeiro, A.P.; de Meneses, A.C.; Possato, J.C.; da Silva Abel, J.; Machado-de-Avila, R.A.; Sayer, C.; de Araújo, P.H.H. Bovine serum albumin conjugation on poly (methyl methacrylate) nanoparticles for targeted drug delivery applications. J. Drug Deliv. Sci. Technol. 2020, 56, 101490. [Google Scholar] [CrossRef]
- Wei, M.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Xu, Y.; Wu, C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015, 10, 5123. [Google Scholar]
- Balcão, V.M.; Costa, C.I.; Matos, C.M.; Moutinho, C.G.; Amorim, M.; Pintado, M.E.; Gomes, A.P.; Vila, M.M.; Teixeira, J.A. Nanoencapsulation of bovine lactoferrin for food and biopharmaceutical applications. Food Hydrocoll. 2013, 32, 425–431. [Google Scholar] [CrossRef]
- Tian, M.; Han, J.; Ye, A.; Liu, W.; Xu, X.; Yao, Y.; Li, K.; Kong, Y.; Wei, F.; Zhou, W. Structural characterization and biological fate of lactoferrin-loaded liposomes during simulated infant digestion. J. Sci. Food Agric. 2019, 99, 2677–2684. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, W.; Liu, C.; Singh, H. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J. Dairy Sci. 2013, 96, 2061–2070. [Google Scholar] [CrossRef]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer Ther. 2005, 4, 612–624. [Google Scholar] [CrossRef]
- Kanwar, R.K.; Kanwar, J.R. Immunomodulatory lactoferrin in the regulation of apoptosis modulatory proteins in cancer. Protein Pept. Lett. 2013, 20, 450–458. [Google Scholar]
- Pan, W.-R.; Chen, P.-W.; Chen, Y.-L.; Hsu, H.-C.; Lin, C.-C.; Chen, W.-J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef]
- Wu, H.; Gao, Y.; Li, S.; Bao, X.; Wang, J.; Zheng, N. Lactoferrin Alleviated AFM1-Induced Apoptosis in Intestinal NCM 460 Cells through the Autophagy Pathway. Foods 2021, 11, 23. [Google Scholar] [CrossRef]
- Ataide, J.A.; Cefali, L.C.; Figueiredo, M.C.; Braga, L.E.D.O.; Ruiz, A.L.T.G.; Foglio, M.A.; Oliveira-Nascimento, L.; Mazzola, P.G. In vitro performance of free and encapsulated bromelain. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.C.; Vericimo, M.A.; Dashevskiy, A.; Pereira, P.R.; Paschoalin, V.M. Liposomal taro lectin nanocapsules control human glioblastoma and mammary adenocarcinoma cell proliferation. Molecules 2019, 24, 471. [Google Scholar] [CrossRef]
- Corrêa, A.C.; Pereira, P.R.; Paschoalin, V.M. Preparation and characterization of nanoliposomes for the entrapment of bioactive hydrophilic globular proteins. JoVE (J. Vis. Exp.) 2019, 150, e59900. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.R.; Meagher, J.L.; Winter, H.C.; Goldstein, I.J.; Paschoalin, V.M.; Silva, J.T.; Stuckey, J.A. High-resolution crystal structures of Colocasia esculenta tarin lectin. Glycobiology 2017, 27, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.R.; Winter, H.C.; Verícimo, M.A.; Meagher, J.L.; Stuckey, J.A.; Goldstein, I.J.; Paschoalin, V.M.; Silva, J.T. Structural analysis and binding properties of isoforms of tarin, the GNA-related lectin from Colocasia esculenta. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2015, 1854, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Ma, X.; Hoag, S.; Wang, F.; Ibrahim, A.; Godoy-Ruiz, R.; Weber, D.J.; Fulton, A.M. An Extract of Taro (Colocasia esculenta) Mediates Potent Inhibitory Actions on Metastatic and Cancer Stem Cells by Tumor Cell-Autonomous and Immune-Dependent Mechanisms. Breast Cancer: Basic Clin. Res. 2021, 15, 11782234211034937. [Google Scholar] [CrossRef]
- Kundu, N.; Campbell, P.; Hampton, B.; Lin, C.Y.; Ma, X.; Ambulos, N.; Zhao, X.F.; Goloubeva, O.; Holt, D.; Fulton, A.M. Antimetastatic activity isolated from Colocasia esculenta (taro). Anticancer Drugs 2012, 23, 200–211. [Google Scholar] [CrossRef]
- Yasin, U.; Bilal, M.; Bashir, H.; Amirzada, M.I.; Sumrin, A.; Asad, M.H.H.B. Preparation and nanoencapsulation of lectin from lepidium sativum on chitosan-tripolyphosphate nanoparticle and their cytotoxicity against hepatocellular carcinoma cells (HepG2). BioMed Res. Int. 2020, 2020, 7251346. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Budak, G.; Celiktas, M.S.; Sevimli-Gur, C.; Yesil-Celiktas, O. Anticancer activities of bioactive peptides derived from rice husk both in free and encapsulated form in chitosan. J. Ind. Eng. Chem. 2021, 103, 381–391. [Google Scholar] [CrossRef]
- Kapoor, M.; Lee, S.L.; Tyner, K.M. Liposomal drug product development and quality: Current US experience and perspective. AAPS J. 2017, 19, 632–641. [Google Scholar] [CrossRef]
- Euliss, L.E.; DuPont, J.A.; Gratton, S.; DeSimone, J. Imparting size, shape, and composition control of materials for nanomedicine. Chem. Soc. Rev. 2006, 35, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Greige-Gerges, H.; Stainmesse, S.; Fessi, H.; Charcosset, C. Effect of composition, hydrogenation of phospholipids and lyophilization on the characteristics of eugenol-loaded liposomes prepared by ethanol injection method. Food Biosci. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Tsuji, T.; Morita, S.-y.; Ikeda, Y.; Terada, T. Enzymatic fluorometric assays for quantifying all major phospholipid classes in cells and intracellular organelles. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for drug delivery. J. Biotechnol. Biomater 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazù, S.; Kiselev, M.A. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Han, F.; Han, J. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Adv. Colloid Interface Sci. 2019, 263, 52–67. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Frezard, F. Liposomes: From biophysics to the design of peptide vaccines. Braz. J. Med. Biol. Res. 1999, 32, 181–189. [Google Scholar] [CrossRef]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, characterization and applications of liposomes: State of the art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Zawada, Z.H. Vesicles with a double bilayer. Cell. Mol. Biol. Lett. 2004, 9, 589–602. [Google Scholar] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.G.; Ferreira, V.F.; da Silva, F.D.C.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics 2022, 14, 1307. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, K.-E.; Kim, J.-J.; Lim, S.-H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef]
- Sharifi, F.; Zhou, R.; Lim, C.; Jash, A.; Abbaspourrad, A.; Rizvi, S.S. Generation of liposomes using a supercritical carbon dioxide eductor vacuum system: Optimization of process variables. J. CO2 Util. 2019, 29, 163–171. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- Lu, R.-M.; Chen, M.-S.; Chang, D.-K.; Chiu, C.-Y.; Lin, W.-C.; Yan, S.-L.; Wang, Y.-P.; Kuo, Y.-S.; Yeh, C.-Y.; Lo, A. Targeted drug delivery systems mediated by a novel Peptide in breast cancer therapy and imaging. PLoS ONE 2013, 8, e66128. [Google Scholar] [CrossRef]
- Maranhão, R.C.; Vital, C.G.; Tavoni, T.M.; Graziani, S.R. Clinical experience with drug delivery systems as tools to decrease the toxicity of anticancer chemotherapeutic agents. Expert Opin. Drug Deliv. 2017, 14, 1217–1226. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Caddeo, C.; Pons, R.; Carbone, C.; Fernàndez-Busquets, X.; Cardia, M.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr. Polym. 2017, 157, 1853–1861. [Google Scholar] [CrossRef]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: Comparison with conventional liposomes. AAPS PharmSciTech 2012, 13, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Mura, S.; Sinico, C.; Fadda, A.M.; Vila, A.; Molina, F. Development and characterization of liposomes containing glycols as carriers for diclofenac. Colloids Surf. Physicochem. Eng. Asp. 2009, 342, 53–58. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Perry, J.L.; Reuter, K.G.; Kai, M.P.; Herlihy, K.P.; Jones, S.W.; Luft, J.C.; Napier, M.; Bear, J.E.; DeSimone, J.M. PEGylated PRINT nanoparticles: The impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012, 12, 5304–5310. [Google Scholar] [CrossRef]
- Nogueira, E.; Loureiro, A.; Nogueira, P.; Freitas, J.; Almeida, C.R.; Härmark, J.; Hebert, H.; Moreira, A.; Carmo, A.M.; Preto, A. Liposome and protein based stealth nanoparticles. Faraday Discuss. 2013, 166, 417–429. [Google Scholar] [CrossRef]
- Dams, E.T.; Laverman, P.; Oyen, W.J.; Storm, G.; Scherphof, G.L.; Van der Meer, J.W.; Corstens, F.H.; Boerman, O.C. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [Google Scholar]
- Ishida, T.; Kiwada, H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int. J. Pharm. 2008, 354, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lila, A.S.A.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ambegia, E.; Ansell, S.; Cullis, P.; Heyes, J.; Palmer, L.; MacLachlan, I. Stabilized plasmid–lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2005, 1669, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.S.; Saxon, D.; Wong, F.M.; Lim, H.J.; Wang, Z.; Bally, M.B.; Choi, L.S.; Cullis, P.R.; Mayer, L.D. Comparison of different hydrophobic anchors conjugated to poly (ethylene glycol): Effects on the pharmacokinetics of liposomal vincristine. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1998, 1372, 272–282. [Google Scholar] [CrossRef]
- Abra, R.; Bankert, R.; Chen, F.; Egilmez, N.; Huang, K.; Saville, R.; Slater, J.; Sugano, M.; Yokota, S. The next generation of liposome delivery systems: Recent experience with tumor-targeted, sterically-stabilized immunoliposomes and active-loading gradients. J. Liposome Res. 2002, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cattel, L.; Ceruti, M.; Dosio, F. From conventional to stealth liposomes: A new frontier in cancer chemotherapy. J. Chemother. 2004, 16, 94–97. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fathi, S.; Oyelere, A.K. Liposomal drug delivery systems for targeted cancer therapy: Is active targeting the best choice? Future Med. Chem. 2016, 8, 2091–2112. [Google Scholar] [CrossRef] [PubMed]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B: Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef]
- Drummond, D.C.; Zignani, M.; Leroux, J.-C. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 2000, 5, 409–460. [Google Scholar] [CrossRef]
- Karanth, H.; Murthy, R. pH-Sensitive liposomes-principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, W.; Gu, Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J. Control. Release 2014, 194, 1–19. [Google Scholar] [CrossRef]
- Li, S.; Goins, B.; Zhang, L.; Bao, A. Novel multifunctional theranostic liposome drug delivery system: Construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjugate Chem. 2012, 23, 1322–1332. [Google Scholar] [CrossRef]
- El-Sayed, A.; Harashima, H. Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef]
- El-Sayed, A.; Futaki, S.; Harashima, H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 2009, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Kogure, K.; Akita, H.; Harashima, H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006, 58, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.; Grabowska, A.; Stolnik, S. Pathways of cellular internalisation of liposomes delivered siRNA and effects on siRNA engagement with target mRNA and silencing in cancer cells. Sci. Rep. 2018, 8, 1–9. [Google Scholar]
- Pagano, R.E.; Weinstein, J.N. Interactions of liposomes with mammalian cells. Annu. Rev. Biophys. Bioeng. 1978, 7, 435–468. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; McAllister, L.; Mausolf, S.; Gyorffy, E. Liposome-cell interactions A study of the interactions of liposomes containing entrapped anti-cancer drugs with the EMT6, S49 and AE1 (transport-deficient) cell lines. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1981, 643, 346–362. [Google Scholar] [CrossRef]
- Knoll, G.; Burger, K.; Bron, R.; van Meer, G.; Verkleij, A.J. Fusion of liposomes with the plasma membrane of epithelial cells: Fate of incorporated lipids as followed by freeze fracture and autoradiography of plastic sections. J. Cell Biol. 1988, 107, 2511–2521. [Google Scholar] [CrossRef]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091. [Google Scholar] [CrossRef]
- Sandra, A.; Pagano, R. Liposome-cell interactions. Studies of lipid transfer using isotopically asymmetric vesicles. J. Biol. Chem. 1979, 254, 2244–2249. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Working, P.; Dayan, A. Pharmacological-toxicological expert report CAELYXTM:(stealth® liposomal doxorubicin HCl). Hum. Exp. Toxicol. 1996, 15, 751–785. [Google Scholar] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Lasic, D.D.; Frederik, P.; Stuart, M.; Barenholz, Y.; McIntosh, T. Gelation of liposome interior A novel method for drug encapsulation. FEBS Lett. 1992, 312, 255–258. [Google Scholar] [CrossRef]
- Lasic, D.; Čeh, B.; Stuart, M.; Guo, L.; Frederik, P.; Barenholz, Y. Transmembrane gradient driven phase transitions within vesicles: Lessons for drug delivery. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1995, 1239, 145–156. [Google Scholar] [CrossRef]
- Gabizon, A.A. Liposome circulation time and tumor targeting: Implications for cancer chemotherapy. Adv. Drug Deliv. Rev. 1995, 16, 285–294. [Google Scholar] [CrossRef]
- Miller, K.; Cortes, J.; Hurvitz, S.A.; Krop, I.E.; Tripathy, D.; Verma, S.; Riahi, K.; Reynolds, J.G.; Wickham, T.J.; Molnar, I. HERMIONE: A randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Borys, N.; Dewhirst, M.W. Drug development of lyso-thermosensitive liposomal doxorubicin: Combining hyperthermia and thermosensitive drug delivery. Adv. Drug Deliv. Rev. 2021, 178, 113985. [Google Scholar] [CrossRef]
- Chen, J.; He, C.-Q.; Lin, A.-H.; Gu, W.; Chen, Z.-P.; Li, W.; Cai, B.-C. Thermosensitive liposomes with higher phase transition temperature for targeted drug delivery to tumor. Int. J. Pharm. 2014, 475, 408–415. [Google Scholar] [CrossRef]
- Mo, S.; Coussios, C.-C.; Seymour, L.; Carlisle, R. Ultrasound-enhanced drug delivery for cancer. Expert Opin. Drug Deliv. 2012, 9, 1525–1538. [Google Scholar] [CrossRef]
- Puri, A. Phototriggerable liposomes: Current research and future perspectives. Pharmaceutics 2013, 6, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Regenold, M.; Bannigan, P.; Evans, J.C.; Waspe, A.; Temple, M.J.; Allen, C. Turning down the heat: The case for mild hyperthermia and thermosensitive liposomes. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102484. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Martin, F.J. Advantages of liposomal delivery systems for anthracyclines. Semin. Oncol. 2004, 31 (Suppl. 13), 5–15. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hong, K.; Park, J.W.; Kirpotin, D.B. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006, 66, 3271–3277. [Google Scholar] [CrossRef]
- Rani, R.; Raina, N.; Khan, A.; Choudhary, M.; Gupta, M. Liposomal-Based Pharmaceutical Formulations–Current Landscape, Limitations and Technologies for Industrial Scale-Up. In Micro-and Nanotechnologies-Based Product Development; CRC Press: Boca Raton, FL, USA, 2021; pp. 209–224. [Google Scholar]
- Aquino, A.; Paschoalin, V.M.F.; Tessaro, L.L.G.; Raymundo-Pereira, P.A.; Conte-Junior, C.A. Updating the use of nano-biosensors as promising devices for the diagnosis of coronavirus family members: A systematic review. J. Pharm. Biomed. Anal. 2022, 211, 114608. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.C.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. -Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Ansell, S.M.; Du, X. Novel Lipids and Lipid Nanoparticle Formulations for Delivery of Nucleic Acids. U.S. Patent US10166298B2, 1 January 2019. [Google Scholar]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Hyodo, K.; Suzuki, T.; Tanaka, Y.; Kikuchi, H.; Ishihara, H. Biodegradable lipid nanoparticles induce a prolonged RNA interference-mediated protein knockdown and show rapid hepatic clearance in mice and nonhuman primates. Int. J. Pharm. 2017, 519, 34–43. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, H. Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
| Nano-Delivery System | Therapeutic Agent | Loading Mechanism | Pathology | Biological Assay | Pharmacological Response | Ref. |
|---|---|---|---|---|---|---|
| Nanostructured lipid carriers (NLCs) | Tocotrienol/ Simvastatin | Core co-encapsulation | Mammary adenocarcinoma | In vitro (+SA lineage) | Improved anti-proliferative TRF and SIM effect upon encapsulation | [37] |
| Solid lipid nanoparticles (SLN) | Linalool | Encapsulation | Hepatocarcinoma Lung adenocarcinoma | In vitro (HepG2 and A549 cell lineages) | Improved cytotoxic effect on human lung- and liver-derived tumor cells (A549 and HepG2) at > 1.0 mM in a dose/time-dependent manner | [38] |
| Lipid nano-capsules | Simvastatin | Encapsulation | Breast carcinoma | In vitro (MCF-7 lineage) | Increased cytotoxicity at IC50 = 1.4 ± 0.02 mg/mL | [39] |
| Folic acid-chitosan | Vincristine | Encapsulation | Non-small-cell lung cancer (NSCLC) | In vitro (NCI-H460 lineage) | Anticancer activity at a 4:25 formulation against non-small-cell lung cancer (NCI-H460). | [40] |
| Liposomes | (III) complexes | Encapsulation | Several types of cancer | In vitro (HepG2; HTC-116; HeLa; A549; BEL-7402; SGC-7901; Eca-109; B-16 and human liver cell L02) In vivo (mice) | Ir-1-Lipo and Ir-2-Lipo induced apoptosis at 55.6% and 69.3% levels. Improved anticancer activity against A549 cells; Ir-2-Lipo effectively inhibited tumor growth in a murine model | [41] |
| PGS-coated cationic liposomes with Bcl-2 siRNA-corona | Doxorubicin (Dox) | Electrostatic adsorption | Hepatocellular carcinoma | In vitro (Bel7402 sensitive cells and Bel7402/5-FU MDR cells) In vivo (mice) | 7-fold improved anticancer effect by apoptosis induction and tumor growth inhibition compared to free Dox | [42] |
| Poly lactic-co-glycolic acid (PLGA) nanofibers (NFs) | Metformin | Encapsulation | Lung adenocarcinoma | In vitro (A549 cell lineage) | Significant cytotoxicity against A549 cells by apoptosis induction | [43] |
| Polyamidoamine (PAMAM) dendrimers | Methotrexate (MTX) and D-glucose (GLU) | Encapsulation | Breast cancer | In vitro (MDA MB-231 lineage | OS-PAMAM-MTX-GLU displaying higher anticancer potential compared to free MTX after a 4 h exposure without significantly affecting healthy human HaCat cells | [44] |
| Polyamidoamine (PAMAM) dendrimers | Liver-x-receptor (LXR) | Specific receptor binding | Atherosclerosis | In vitro (mouse peritoneal macrophages) In vivo (mice) | mDNP-LXR-L-mediated delivery reduced in the expression of metalloproteinase 9 (MMP-9); followed by plaque size reduction and decreased necrosis | [45] |
| Gold nanoclusters (AuNCs) | N-isobutyryl-L-cysteine (L-NIBC) | Au-S bond | Parkinson’s disease | In vitro (PC12 and SH-SY5Y lineages) In vivo (mice) | AuNCs exhibited superior neuroprotective effects in 1-metil-4-phenilyridine (MPP+) lesioned cell and 1-methyl-4-phenylpyridine (MPTP) induced mouse PD models | [46] |
| Therapeutic Indication | Marketed Protein and Peptide Drugs | Active Principle | Delivery Strategy | Administration Route |
|---|---|---|---|---|
| Cancer | 1- Lazertinib (leclaza®) 2- Pegaspargase (Oncaspar®) 3- Mepact® | 1- EGFR-tyrosine kinase inhibitor 2- L-asparaginase 3- Muramyl tripeptide phosphatidyl ethanolamine | 1- Amino acid modification 2- Polymeric nanoparticle (PEG) 3- Liposome encapsulation | 1- Oral 2- IM/IV 3- IV |
| Diabetes | 1- Insulin degludec Tresiba® 2- Lixisenatide (Adlyxin®) | 1- Insulin 2- Glucagon-like peptide-1 receptor agonist | 1- Amino acid modification 2- Amino acid modification and amidation | 1- SC 2- SC |
| Immune modulation | 1- Belatacept (Nulojix®) 2- Pegfilgrastim (Neulasta®) 3- Sandimmune Neoral® | 1- CTLA4 antibody 2- G-CSF 3- Cyclosporine A | 1- Amino acid substitution 2- Polymeric nanoparticle (PEG) 3- Lipid-based formulation | 1- IV 2- on-body injection 3- oral |
| Infection | 1- Bezlotoxumab (Zinplava®) 2- Ibalizumab-uiyk (Trogarzo®) 3- Peginterferon-α2a Pegasys® | 1- Monoclonal antibody against Clostridium difficile toxins A and B 2- Monoclonal antibody CD4-directed 3- Interferon-α2a | 1- Natural 2- Natural 3- Polymeric nanoparticle (PEG) | 1- IV 2- IV 3- IV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, R.V.; Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes. Pharmaceutics 2022, 14, 2808. https://doi.org/10.3390/pharmaceutics14122808
Cardoso RV, Pereira PR, Freitas CS, Paschoalin VMF. Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes. Pharmaceutics. 2022; 14(12):2808. https://doi.org/10.3390/pharmaceutics14122808
Chicago/Turabian StyleCardoso, Raiane Vieira, Patricia Ribeiro Pereira, Cyntia Silva Freitas, and Vania Margaret Flosi Paschoalin. 2022. "Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes" Pharmaceutics 14, no. 12: 2808. https://doi.org/10.3390/pharmaceutics14122808
APA StyleCardoso, R. V., Pereira, P. R., Freitas, C. S., & Paschoalin, V. M. F. (2022). Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes. Pharmaceutics, 14(12), 2808. https://doi.org/10.3390/pharmaceutics14122808








