Curcumin-Loaded Oil-Free Self-Assembled Micelles Inhibit the Influenza A Virus Activity and the Solidification of Curcumin-Loaded Micelles for Pharmaceutical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
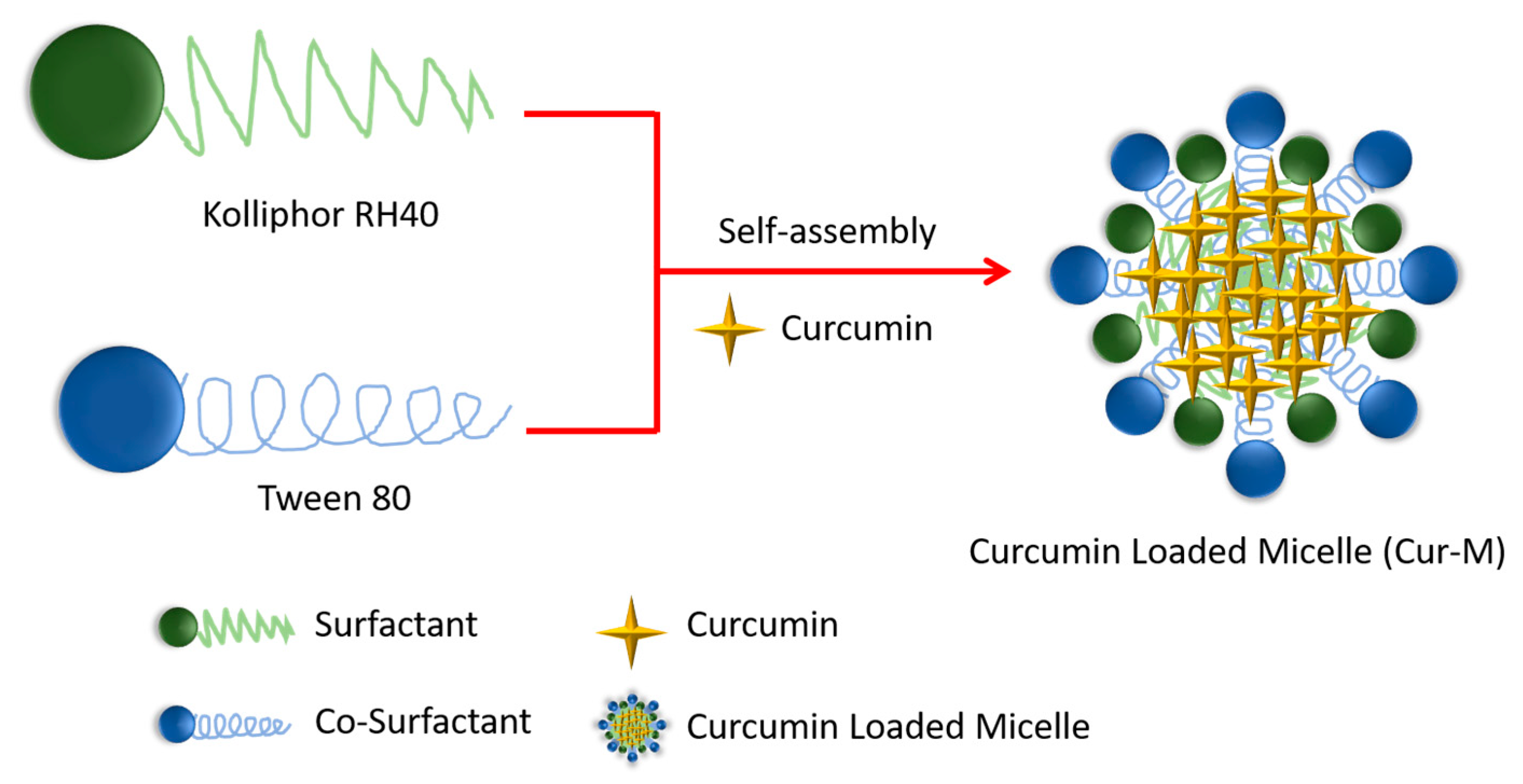
2.2. Preparation of Cur-M
2.3. Characterization of Particle Size and Morphology of Cur-M
2.4. Drug-Loading Rate and Entrapment Efficiency Measurements of Cur-M
2.5. In Vitro Cytotoxicity
2.6. Virus Infection and Plaque Assay
2.7. Time-of-Drug-Addition Assays during Virus Infection
2.8. Preparation of Curcumin-Loaded Solid Self-Assembly Micelles (Cur-SM)
2.9. Water Solubility/Dispersity Study of Cur-M and Cur-SM
2.10. Stability of Self-Assembled Curcumin-Loaded Micelles in Different pH Buffers
2.11. In Vitro Release Profile of Self-Assembled Curcumin-Loaded Micelles
2.12. Statistical Analysis
3. Results and Discussions
3.1. The Cur-M Emulsifying Capability by Different Surfactants and Co-Surfactants
3.2. Characterization of Cur-M
3.3. In Vitro Cytotoxicity and Anti-Influenza Activity of Cur-M
3.4. Investigation of the Anti-Influenza Efficacy of the Cur-M
3.5. Preparation of Cur-SM and Morphological Changes
3.6. Water Solubility/Dispersity Study of Cur-M and Cur-SM
3.7. Stability of Formulated Curcumin in Different pH Conditions
3.8. In Vitro Release Profile of Curcumin from Cur-M and Cur-SM
3.9. Pharmaceutical Formulations of Tablet and Capsule Formation Using Cur-SM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morens, D.M.; Taubenberger, J.K.; Taubenberger, J.K. Influenza: The Mother of All Pandemics. Emerg. Infect. Dis. 1918, 12, 15–22. [Google Scholar]
- Longini, I.M., Jr.; Halloran, M.E.; Nizam, A.; Yang, Y. Containing Pandemic Influenza with Antiviral Agents. Am. J. Epidemiol. 2004, 159, 623–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenforde, M.W.; Talbot, H.K.; Trabue, C.H.; Gaglani, M.; McNeal, T.M.; Monto, A.S.; Martin, E.T.; Zimmerman, R.K.; Silveira, F.P.; Middleton, D.B. Influenza Vaccine Effectiveness against Hospitalization in the United States, 2019–2020. J. Infect. Dis. 2021, 224, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-K.; Minakuchi, M.; Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Kuo, K.-K.; Lin, Y.-C.; Saito, S.; Lin, C.-S.; Yokoyama, K.K. Redox Control in the Pathophysiology of Influenza Virus Infection. BMC Microbiol. 2020, 20, 214. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Macfarlane, K.; Cragan, J.D.; Williams, J.; Henderson, Z.; the Pandemic Influenza and Pregnancy Working Group. Pandemic Influenza and Pregnant Women: Summary of a Meeting of Experts. Am. J. Public Health 2009, 99, S248–S254. [Google Scholar] [CrossRef]
- Da Silva Antonio, A.; Wiedemann, L.S.M.; Veiga-Junior, V.F. Natural Products’ Role against COVID-19. Rsc Adv. 2020, 10, 23379–23393. [Google Scholar] [CrossRef]
- Mousa, H.A.-L. Prevention and Treatment of Influenza, Influenza-like Illness, and Common Cold by Herbal, Complementary, and Natural Therapies. J. Evid. Based. Complement. Altern. Med. 2017, 22, 166–174. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Arshad, L.; Haque, M.A.; Abbas Bukhari, S.N.; Jantan, I. An Overview of Structure—Activity Relationship Studies of Curcumin Analogs as Antioxidant and Anti-Inflammatory Agents. Future Med. Chem. 2017, 9, 605–626. [Google Scholar] [CrossRef]
- Ausili, A.; Gómez-Murcia, V.; Candel, A.M.; Beltrán, A.; Torrecillas, A.; He, L.; Jiang, Y.; Zhang, S.; Teruel, J.A.; Gómez-Fernández, J.C. A Comparison of the Location in Membranes of Curcumin and Curcumin-Derived Bivalent Compounds with Potential Neuroprotective Capacity for Alzheimer’s Disease. Colloids Surf. B 2021, 199, 111525. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed Res. Int. 2014, 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- More, S.K.; Pawar, A.P. Preparation, Optimization and Preliminary Pharmacokinetic Study of Curcumin Encapsulated Turmeric Oil Microemulsion in Zebra Fish. Eur. J. Pharm. Sci. 2020, 155, 105539. [Google Scholar] [CrossRef] [PubMed]
- Delshadi, R.; Bahrami, A.; McClements, D.J.; Moore, M.D.; Williams, L. Development of Nanoparticle-Delivery Systems for Antiviral Agents: A Review. J. Control. Release 2021, 331, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Shien, J.-H.; Tiley, L.; Chiou, S.-S.; Wang, S.-Y.; Chang, T.-J.; Lee, Y.-J.; Chan, K.-W.; Hsu, W.-L. Curcumin Inhibits Influenza Virus Infection and Haemagglutination Activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Ou, J.; Mizushina, Y.; Wang, S.; Chuang, D.; Nadar, M.; Hsu, W. Structure–Activity Relationship Analysis of Curcumin Analogues on Anti-influenza Virus Activity. FEBS J. 2013, 280, 5829–5840. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of Enveloped Viruses Infectivity by Curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Lin, H.-Y.; Chen, H.-C.; Yu, M.-W.; Lee, M.-H. Stability and Characterisation of Phospholipid-Based Curcumin-Encapsulated Microemulsions. Food Chem. 2009, 116, 923–928. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. PH-Responsive Hyaluronic Acid-Based Nanoparticles for Targeted Curcumin Delivery and Enhanced Cancer Therapy. Colloids Surf. B 2021, 198, 111455. [Google Scholar] [CrossRef]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric Nanoparticle-Encapsulated Curcumin (“Nanocurcumin”): A Novel Strategy for Human Cancer Therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Shieh, M.-J.; Peng, C.-L.; Lou, P.-J.; Chiu, C.-H.; Tsai, T.-Y.; Hsu, C.-Y.; Yeh, C.-Y.; Lai, P.-S. Non-Toxic Phototriggered Gene Transfection by PAMAM-Porphyrin Conjugates. J. Control. Release 2008, 129, 200–206. [Google Scholar] [CrossRef]
- Jian, Y.-S.; Chen, C.-W.; Lin, C.-A.; Yu, H.-P.; Lin, H.-Y.; Liao, M.-Y.; Wu, S.-H.; Lin, Y.-F.; Lai, P.-S. Hyaluronic Acid–Nimesulide Conjugates as Anticancer Drugs against CD44-Overexpressing HT-29 Colorectal Cancer in Vitro and in Vivo. Int. J. Nanomed. 2017, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A.; Ho, H.-M.; Venkatesan, P.; Huang, C.-Y.; Cheng, Y.-J.; Lin, Y.-H.; Lin, H.-Y.; Chen, T.-Y.; Huang, M.-H.; Lai, P.-S. Hyaluronic Acid-Glycine-Cholesterol Conjugate-Based Nanoemulsion as a Potent Vaccine Adjuvant for T Cell-Mediated Immunity. Pharmaceutics 2021, 13, 1569. [Google Scholar] [CrossRef] [PubMed]
- Nicolau Costa, K.M.; Sato, M.R.; Barbosa, T.L.A.; Rodrigues, M.G.F.; Medeiros, A.C.D.; Damasceno, B.P.G.d.L.; Oshiro-Júnior, J.A. Curcumin-Loaded Micelles Dispersed in Ureasil-Polyether Materials for a Novel Sustained-Release Formulation. Pharmaceutics 2021, 13, 675. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.S.; dos Santos, D.M.; Almeida, A.; Marchiori, L.; Campana-Filho, S.P.; Ribeiro, S.J.L.; Sarmento, B. N-(2-Hydroxy)-Propyl-3-Trimethylammonium, o-Mysristoyl Chitosan Enhances the Solubility and Intestinal Permeability of Anticancer Curcumin. Pharmaceutics 2018, 10, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on PH-Sensitive Biocompatible Poly (2-Vinyl Pyridine)-b-Poly (Ethylene Oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Shieh, M.-J.; Hsu, C.-Y.; Huang, L.-Y.; Chen, H.-Y.; Huang, F.-H.; Lai, P.-S. Reversal of Doxorubicin-Resistance by Multifunctional Nanoparticles in MCF-7/ADR Cells. J. Control. Release 2011, 152, 418–425. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted Delivery of Curcumin in Breast Cancer Cells via Hyaluronic Acid Modified Mesoporous Silica Nanoparticle to Enhance Anticancer Efficiency. Colloids Surf. B 2021, 197, 111404. [Google Scholar] [CrossRef]
- Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef]
- Peng, C.-L.; Shieh, M.-J.; Tsai, M.-H.; Chang, C.-C.; Lai, P.-S. Self-Assembled Star-Shaped Chlorin-Core Poly (ε-Caprolactone)–Poly (Ethylene Glycol) Diblock Copolymer Micelles for Dual Chemo-Photodynamic Therapies. Biomaterials 2008, 29, 3599–3608. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Nieh, M.-P.; Lai, P.-S. Facile Self-Assembly of Porphyrin-Embedded Polymeric Vesicles for Theranostic Applications. Chem. Commun. 2012, 48, 9343–9345. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent Advances in Encapsulation of Curcumin in Nanoemulsions: A Review of Encapsulation Technologies, Bioaccessibility and Applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.H.M.; Colangelo, H.; Kee, T.W. Encapsulation of Curcumin in Cationic Micelles Suppresses Alkaline Hydrolysis. Langmuir 2008, 24, 5672–5675. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Wang, X.; Yang, Q.; Niu, Y.; Chai, S.; Chen, Z.; An, X.; Shen, W. Spectrometric Study on the Interaction of Dodecyltrimethylammonium Bromide with Curcumin. Langmuir 2011, 27, 14112–14117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y. Effects of Length and Unsaturation of the Alkyl Chain on the Hydrophobic Binding of Curcumin with Tween Micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef]
- Lee, W.-H.; Bebawy, M.; Loo, C.-Y.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Fabrication of Curcumin Micellar Nanoparticles with Enhanced Anti-Cancer Activity. J. Biomed. Nanotechnol. 2015, 11, 1093–1105. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.; Hung, Y.; Mou, C. Size Effect on Cell Uptake in Well-suspended, Uniform Mesoporous Silica Nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and Evaluation of Self-Microemulsifying Liquid and Pellet Formulations of Curcumin, and Absorption Studies in Rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef]
- Song, L.; Shen, Y.; Hou, J.; Lei, L.; Guo, S.; Qian, C. Polymeric Micelles for Parenteral Delivery of Curcumin: Preparation, Characterization and in Vitro Evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 25–32. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Curcumin-Loaded Ultradeformable Nanovesicles as a Potential Delivery System for Breast Cancer Therapy. Colloids Surf. B 2018, 167, 63–72. [Google Scholar] [CrossRef]
- Khalil, N.M.; do Nascimento, T.C.F.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of Curcumin-Loaded PLGA and PLGA–PEG Blend Nanoparticles after Oral Administration in Rats. Colloids Surf. B 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chiu, Y.-H.; Li, Y.-S.; Lin, E.-Y.; Hsieh, D.-K.; Lee, C.-H.; Huang, M.-H.; Chuang, H.-M.; Lin, S.-Z.; Harn, H.-J. Integration of PEG 400 into a Self-Nanoemulsifying Drug Delivery System Improves Drug Loading Capacity and Nasal Mucosa Permeability and Prolongs the Survival of Rats with Malignant Brain Tumors. Int. J. Nanomed. 2019, 14, 3601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Peng, X.; Araki, Y.; Fang, Y.; Zeng, Y.; Kosol, R.; Yang, G.; Tsubaki, N. Fabrication of a CuZn-Based Catalyst Using a Polyethylene Glycol Surfactant and Supercritical Drying. Catal. Sci. Technol. 2020, 10, 8410–8420. [Google Scholar] [CrossRef]
- Buyuktimkin, T. Apparent Molal Volumes and Hydration Numbers from Viscosity Studies for Microemulsions with a Nonionic Surfactant Derived from Castor Oil and a Series of Polar Oils. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125244. [Google Scholar] [CrossRef]
- Singh, B.; Khurana, L.; Bandyopadhyay, S.; Kapil, R.; Katare, O.O.P. Development of Optimized Self-Nano-Emulsifying Drug Delivery Systems (SNEDDS) of Carvedilol with Enhanced Bioavailability Potential. Drug Deliv. 2011, 18, 599–612. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.J.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and Immunomodulatory Activity of Curcumin: A Case for Prophylactic Therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral Nano-curcumin Formulation Efficacy in Management of Mild to Moderate Hospitalized Coronavirus Disease-19 Patients: An Open Label Nonrandomized Clinical Trial. Phyther. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Pawlak, S.A.; Dalrymple, D.M.; Nider, C.J.; Trombetta, L.D.; Serajuddin, A. Development of Solid SEDDS, IV: Effect of Adsorbed Lipid and Surfactant on Tableting Properties and Surface Structures of Different Silicates. Pharm. Res. 2013, 30, 3170–3185. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, C.; Sahoo, S.K. The in Vitro Stability and in Vivo Pharmacokinetics of Curcumin Prepared as an Aqueous Nanoparticulate Formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef]
- Posǎ, M.; Ćirin, D. Mixed Micelles of Sodium Salts of Bile Acids and Tween 40: Effect of the Steroid Skeleton on the Coefficient of Interaction in Mixed Micelles. Ind. Eng. Chem. Res. 2012, 51, 14722–14728. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Liu, B.; Pan, H.; Liu, H. Establishment and Optimization of Voriconazole/HS15/SBE-β-CD Complex System: Based on Micellization Thermodynamics. J. Mol. Liq. 2021, 321, 114453. [Google Scholar] [CrossRef]






| Cur (mg) | Surfactant | Co-Surfactant | Water Phase | Mean Diameter (nm)/(PDI) | |
|---|---|---|---|---|---|
| 1 | 10 | PEG400 (100 mg) | - | 158.5/(0.192) | |
| 2 | 10 | PEG400 (500 mg) | - | 219.1/(0.349) | |
| 3 | 10 | PEG400 (100 mg) | RH40 (50 mg) | - | 115.0/(0.297) |
| 4 | 10 | PEG400 (100 mg) | PG (50 mg) | - | 322.6/(0.340) |
| 5 | 10 | PEG400 (100 mg) | RH40 (75 mg) | Tween 80 (10 mg) | 13.43/(0.429) |
| 6 | 50 | RH40 (175 mg) | Tween80 (10 mg) | - | 84.27/(0.268) |
| 7 | 50 | RH40 (175 mg) | PEG400 (10 mg) | - | 5949/(1.0) |
| Micelle | 0 | RH40 (140 mg) | Tween80 (10 mg) | - | 10.27/(0.110) |
| Cur-M | 10 | RH40 (140 mg) | Tween80 (10 mg) | - | 13.55/(0.144) |
| Cur-SM * | 10 | RH40 (140 mg) | Tween80 (10 mg) | 13.49/(0.047) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-Z.; Chang, H.-M.; Hsu, W.-L.; Venkatesan, P.; Lin, M.H.-C.; Lai, P.-S. Curcumin-Loaded Oil-Free Self-Assembled Micelles Inhibit the Influenza A Virus Activity and the Solidification of Curcumin-Loaded Micelles for Pharmaceutical Applications. Pharmaceutics 2022, 14, 2422. https://doi.org/10.3390/pharmaceutics14112422
Li C-Z, Chang H-M, Hsu W-L, Venkatesan P, Lin MH-C, Lai P-S. Curcumin-Loaded Oil-Free Self-Assembled Micelles Inhibit the Influenza A Virus Activity and the Solidification of Curcumin-Loaded Micelles for Pharmaceutical Applications. Pharmaceutics. 2022; 14(11):2422. https://doi.org/10.3390/pharmaceutics14112422
Chicago/Turabian StyleLi, Cun-Zhao, Hui-Min Chang, Wei-Li Hsu, Parthiban Venkatesan, Martin Hsiu-Chu Lin, and Ping-Shan Lai. 2022. "Curcumin-Loaded Oil-Free Self-Assembled Micelles Inhibit the Influenza A Virus Activity and the Solidification of Curcumin-Loaded Micelles for Pharmaceutical Applications" Pharmaceutics 14, no. 11: 2422. https://doi.org/10.3390/pharmaceutics14112422
APA StyleLi, C.-Z., Chang, H.-M., Hsu, W.-L., Venkatesan, P., Lin, M. H.-C., & Lai, P.-S. (2022). Curcumin-Loaded Oil-Free Self-Assembled Micelles Inhibit the Influenza A Virus Activity and the Solidification of Curcumin-Loaded Micelles for Pharmaceutical Applications. Pharmaceutics, 14(11), 2422. https://doi.org/10.3390/pharmaceutics14112422






