Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery
Abstract
1. Introduction
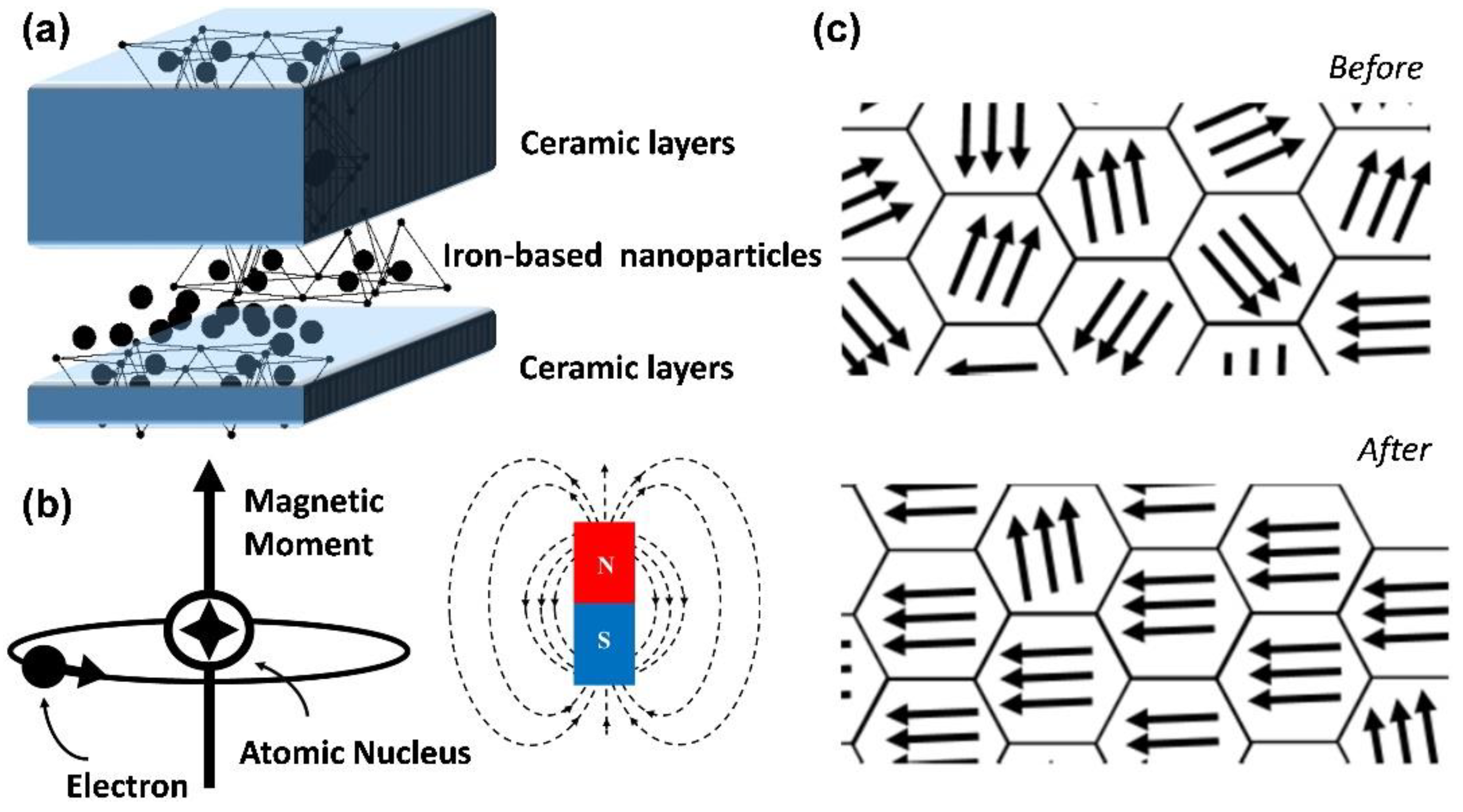
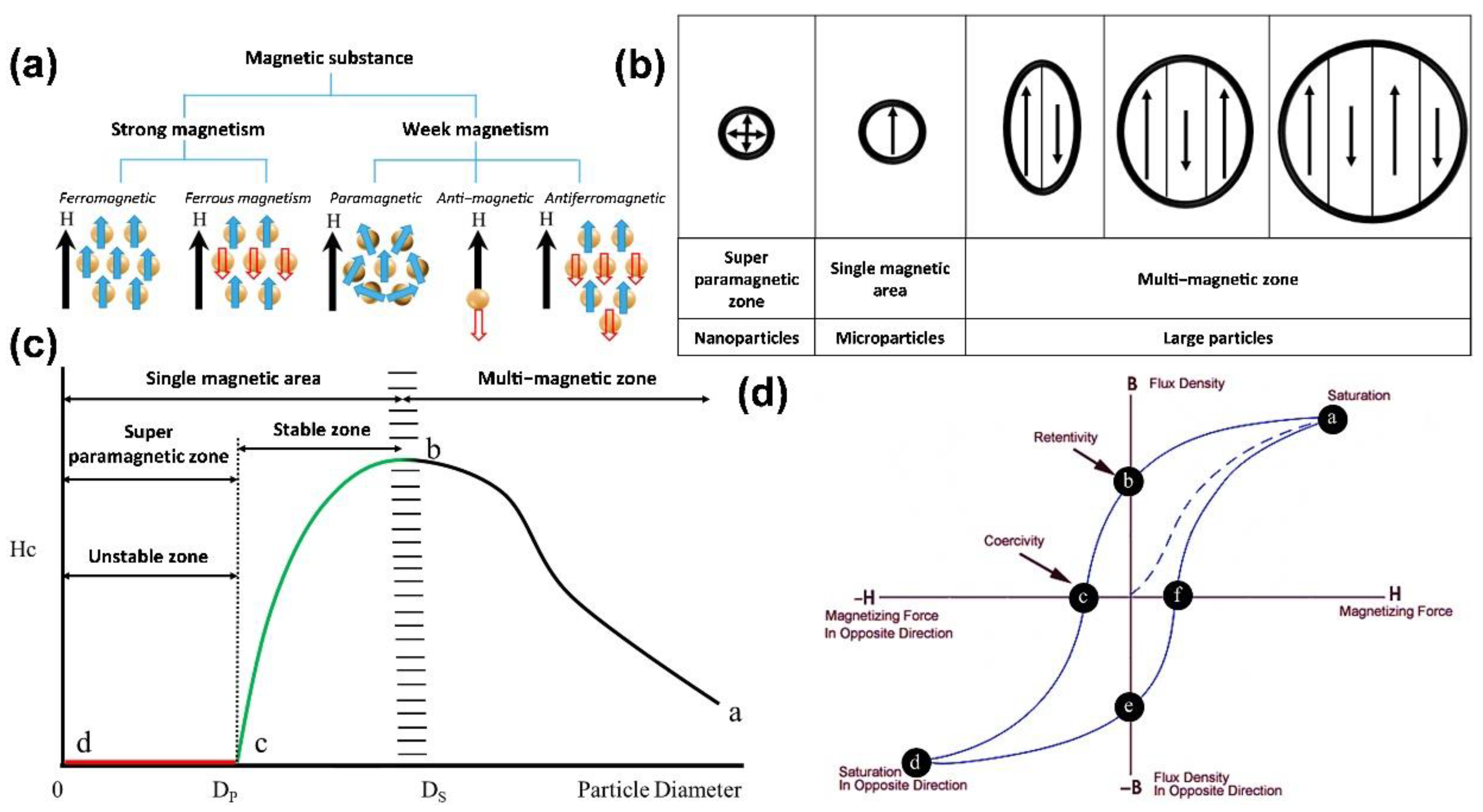
2. Magnetic Properties of Nanoparticles
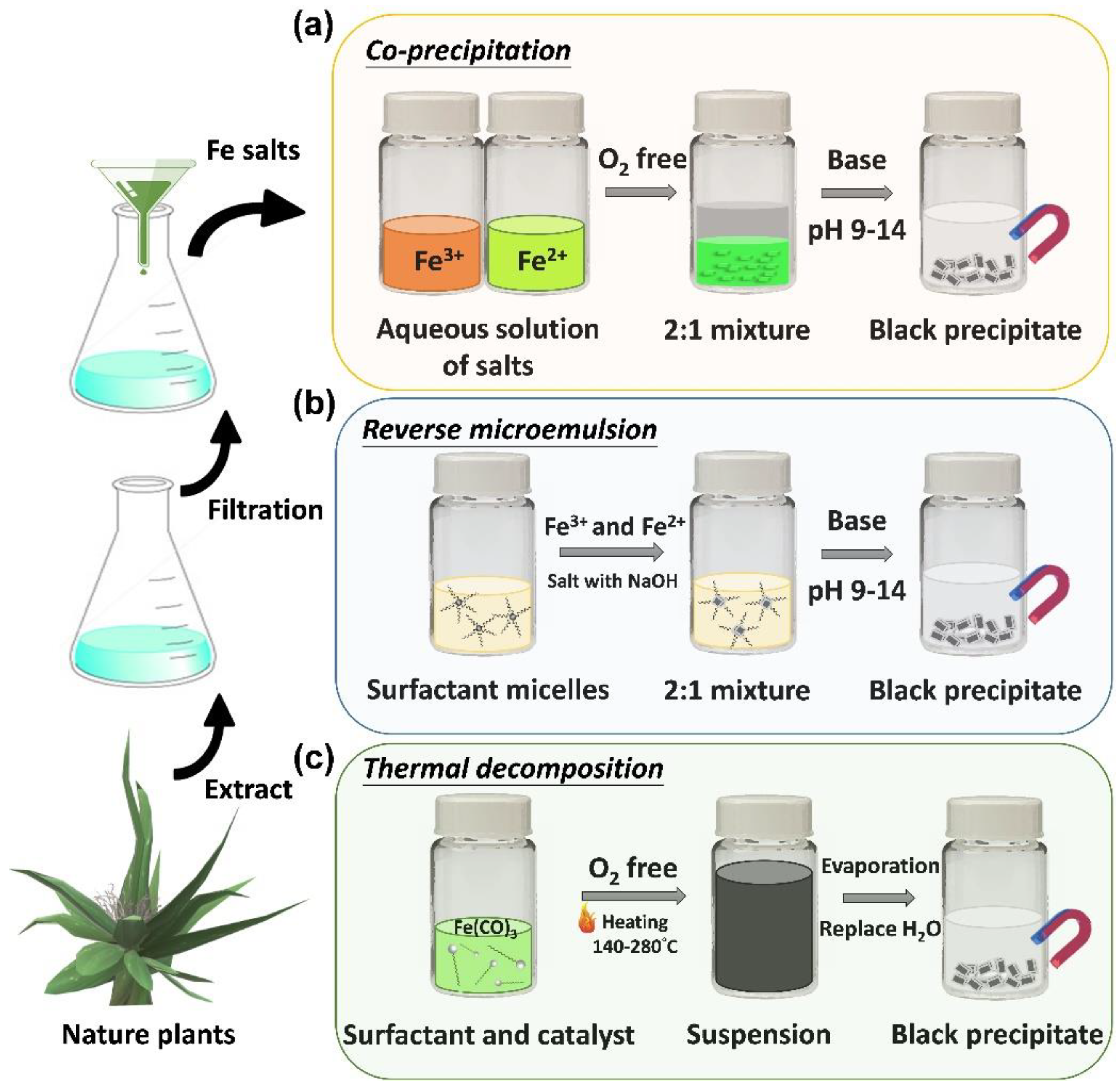
2.1. Properties of Iron Oxide Nanoparticles
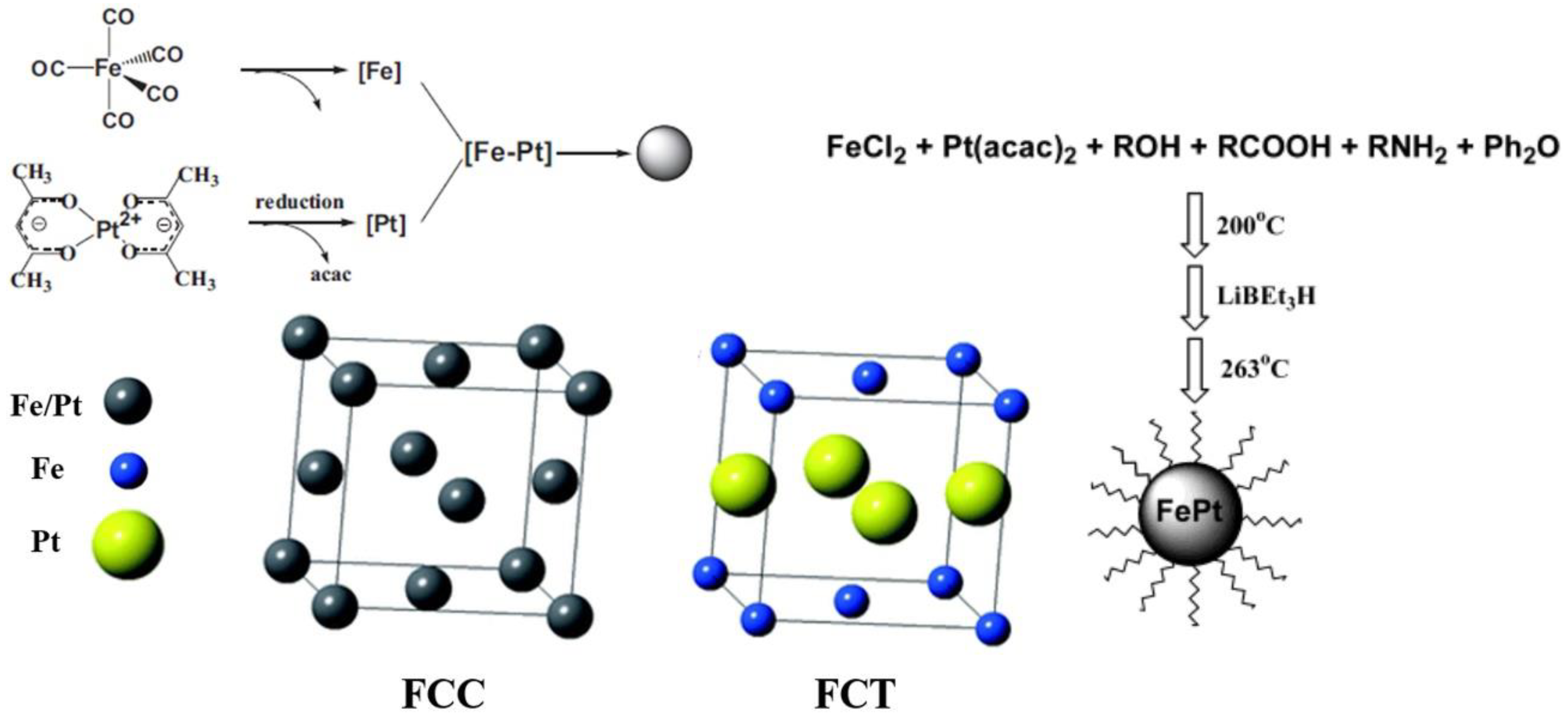
2.2. Properties of Iron–Platinum Nanoparticles
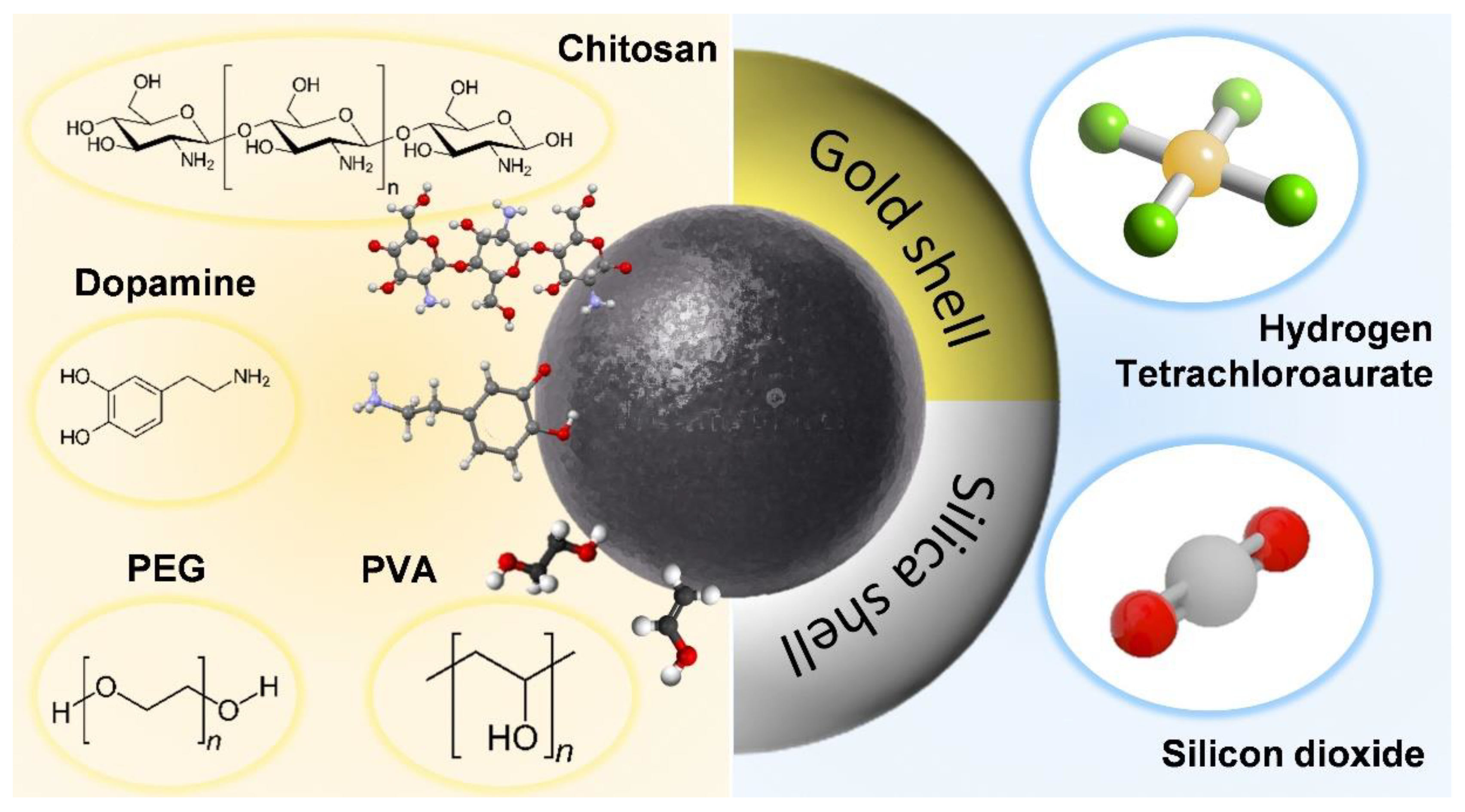
3. Surface Modification of Iron-Based Nanoparticles
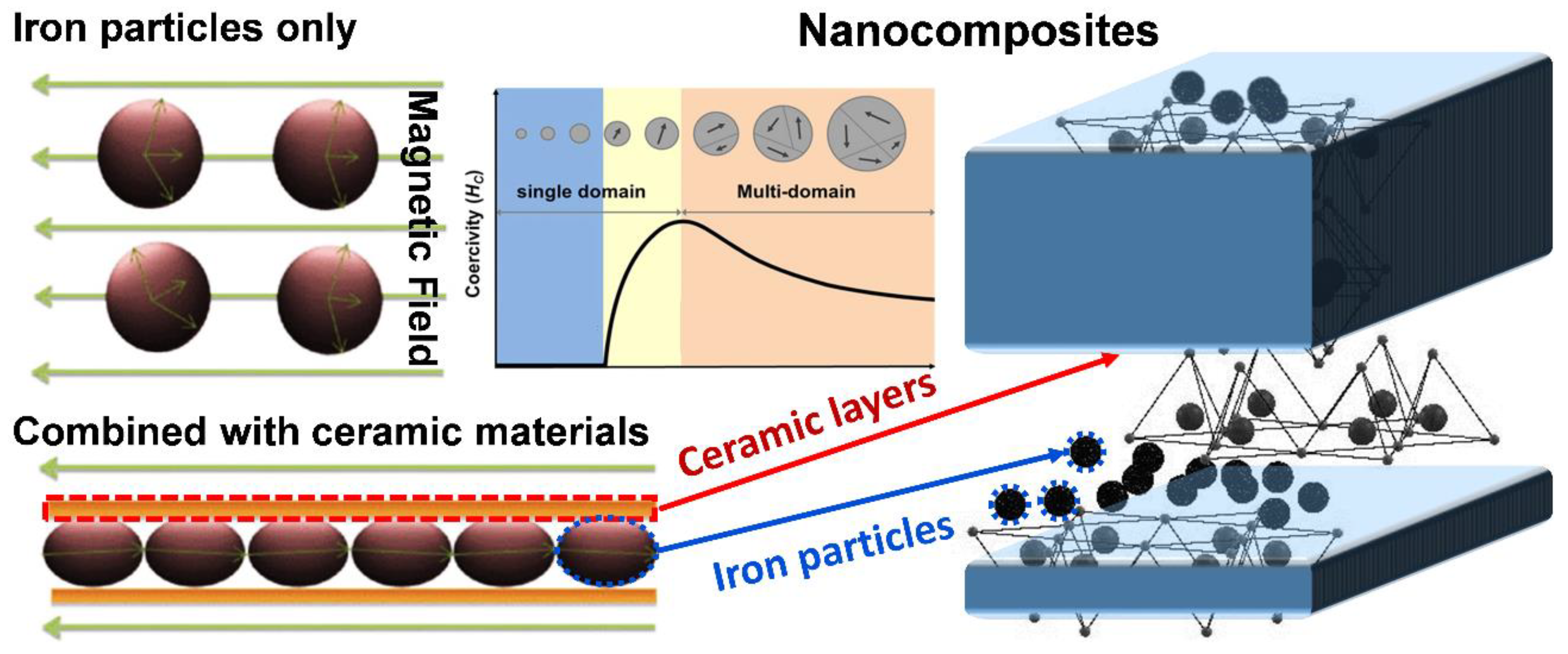
4. Combination of Iron-Based Nanocomposite Particles with Ceramic Materials
4.1. Bioactive Glasses
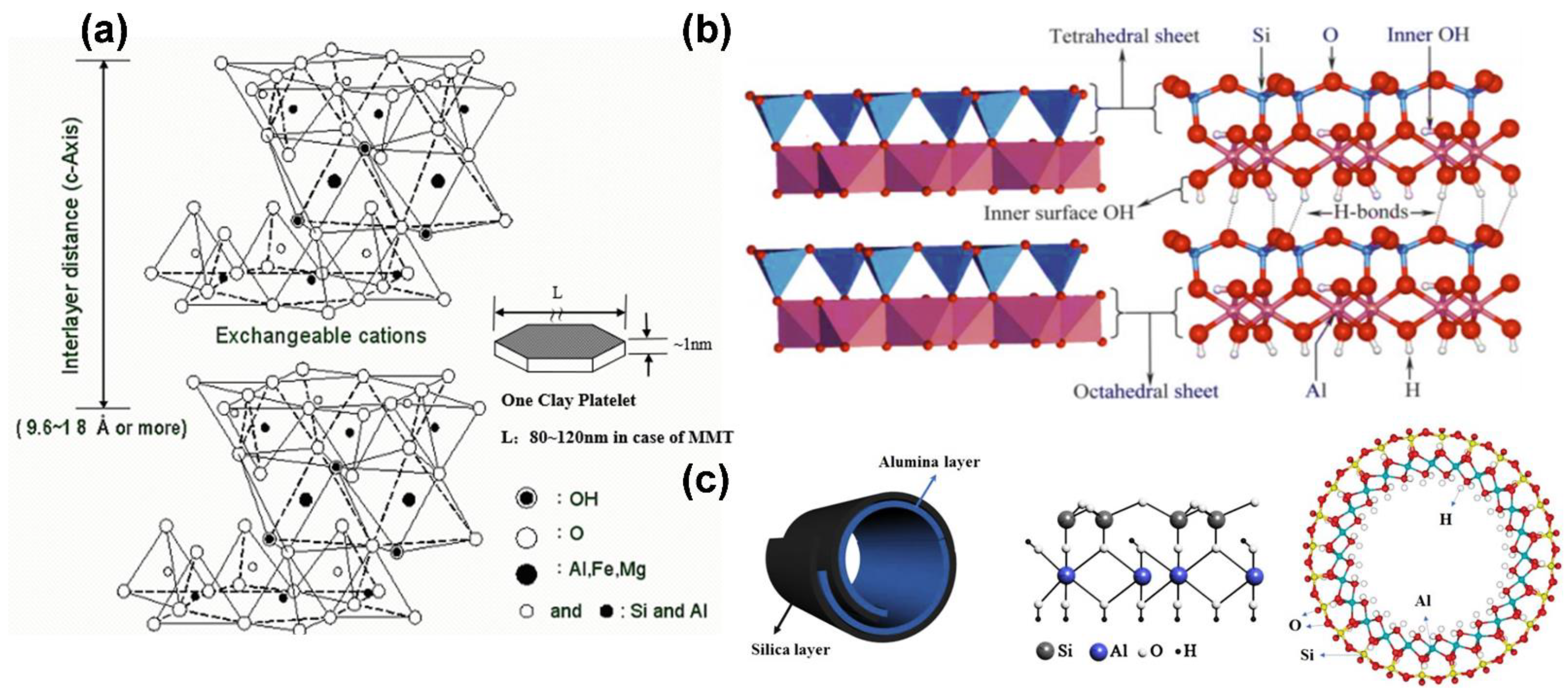
4.2. Biocompatible Nanolayer Ceramics
4.3. Biocompatible Nanotube Ceramics
5. Magnetic Resonance Imaging (MRI) with Ceramic Material Composite Iron Nanoparticles
6. Magnetic Fluid Hyperthermia (MFH) with Ceramic Material Composite Iron Nanoparticles
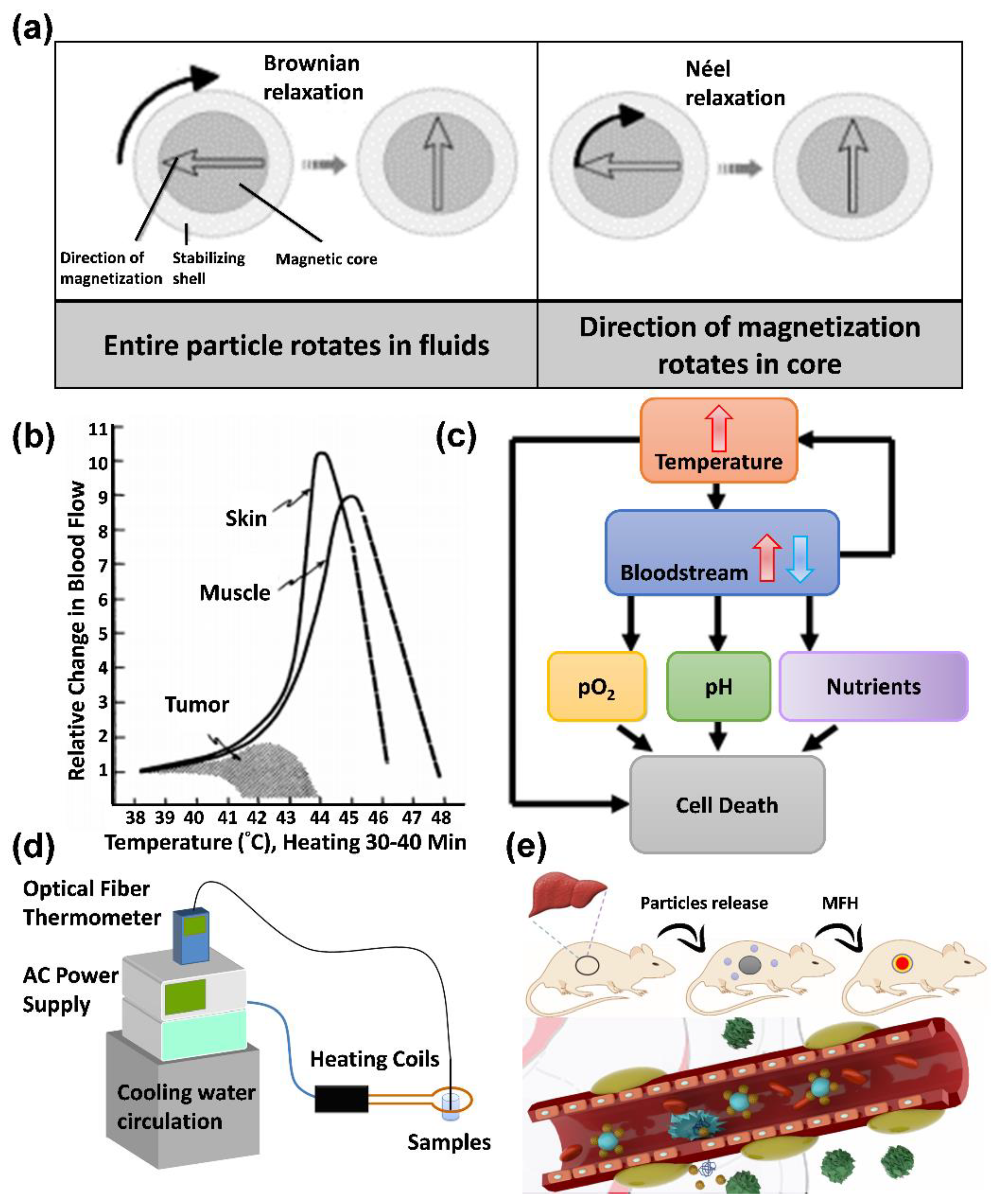
6.1. Principles of MFH
- (A)
- Hysteresis loss: When a material has multiple magnetic domains, the direction of the magnetic moment becomes singular and the same as the magnetic field when an AC magnetic field is applied. When the magnetic-field strength changes, the resulting hysteresis curves do not overlap, which results in heat release;
- (B)
- Néel relaxation: When a material is a single-domain superparamagnetic material, the inner nucleus rotates and overcomes the energy barrier E = KV when an AC magnetic field is applied, where K is the anisotropy constant, and V is the volume of the particle. The thermal energy is released when it returns to the original magnetic moment direction;
- (C)
- Brownian relaxation occurs in materials with multiple or single magnetic domains when an applied magnetic field is applied, which causes the particles to rotate and rub against the external medium and release thermal energy. Therefore, the characteristics of Brownian relaxation are related to the solution viscosity, as shown in Figure 8a.
6.2. Treatment with MFH
7. Drug Delivery with Ceramic Material Composite Iron Nanoparticles
7.1. Cancer Therapy
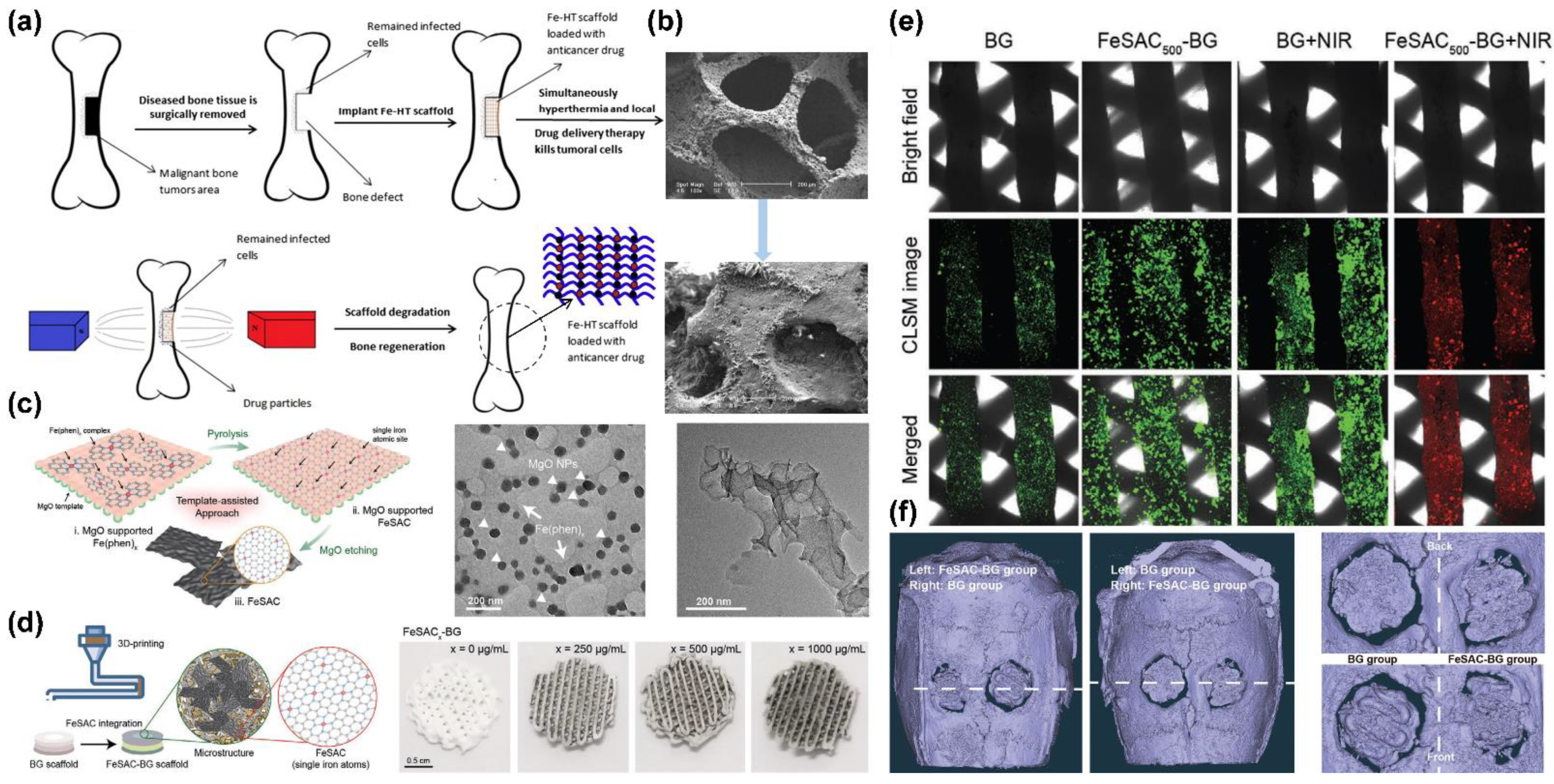
7.1.1. Bone Cancer
7.1.2. Liver Cancer
7.1.3. Breast Cancer
7.2. Promotion of Osteoblast, Fibroblast, and Bone Marrow Mesenchymal Stem Cell Proliferation
7.3. Other Biological Applications Related to Drug Release
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nistico, R.; Cesano, F.; Garello, F. Magnetic Materials and Systems: Domain Structure Visualization and Other Characterization Techniques for the Application in the Materials Science and Biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Bao, Y.P.; Wen, T.L.; Samia, A.C.S.; Khandhar, A.; Krishnan, K.M. Magnetic nanoparticles: Material engineering and emerging applications in lithography and biomedicine. J. Mater. Sci. 2016, 51, 513–553. [Google Scholar] [CrossRef]
- Karimi, Z.; Karimi, L.; Shokrollahi, H. Nano-magnetic particles used in biomedicine: Core and coating materials. Mat. Sci. Eng. C-Mater. 2013, 33, 2465–2475. [Google Scholar] [CrossRef]
- Cohen-Erner, M.; Khandadash, R.; Hof, R.; Shalev, O.; Antebi, A.; Cyjon, A.; Kanakov, D.; Nyska, A.; Goss, G.; Hilton, J.; et al. Fe3O4 Nanoparticles and Paraffin Wax as Phase Change Materials Embedded in Polymer Matrixes for Temperature-Controlled Magnetic Hyperthermia. ACS Appl. Nano Mater. 2021, 4, 11187–11198. [Google Scholar] [CrossRef]
- Ghutepatil, P.R.; Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. APTES (3-aminopropyltriethoxy silane) functionalized MnFe2O4 nanoparticles: A potential material for magnetic fluid hyperthermia. Chem. Pap. 2019, 73, 2189–2197. [Google Scholar] [CrossRef]
- Deka, S.; Saxena, V.; Hasan, A.; Chandra, P.; Pandey, L.M. Synthesis, characterization and in vitro analysis of alpha-Fe2O3-GdFeO3 biphasic materials as therapeutic agent for magnetic hyperthermia applications. Mat. Sci. Eng. C-Mater. 2018, 92, 932–941. [Google Scholar] [CrossRef]
- Choi, H.; An, M.; Eom, W.; Lim, S.W.; Shim, I.B.; Kim, C.S.; Kim, S.J. Crystallographic and Magnetic Properties of the Hyperthermia Material CoFe2O4@AlFe2O4. J. Korean Phys. Soc. 2017, 70, 173–176. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Bear, J.C.; Yu, B.; Blanco-Andujar, C.; McNaughter, P.D.; Southern, P.; Mafina, M.K.; Pankhurst, Q.A.; Parkin, I.P. A low cost synthesis method for functionalised iron oxide nanoparticles for magnetic hyperthermia from readily available materials. Faraday Discuss. 2014, 175, 83–95. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Salamati, H.; Concas, G.; Fernandez, M.S.; Talone, A.; Muscas, G.; Peddis, D. Co-doped MnFe2O4 nanoparticles: Magnetic anisotropy and interparticle interactions. Beilstein J. Nanotech. 2019, 10, 856–865. [Google Scholar] [CrossRef]
- Jalili, H.; Aslibeiki, B.; Varzaneh, A.G.; Chernenko, V.A. The effect of magneto-crystalline anisotropy on the properties of hard and soft magnetic ferrite nanoparticles. Beilstein J. Nanotech. 2019, 10, 1348–1359. [Google Scholar] [CrossRef]
- Zhu, Y.; Kekalo, K.; NDong, C.; Huang, Y.Y.; Shubitidze, F.; Griswold, K.E.; Baker, I.; Zhang, J.X.J. Magnetic-Nanoparticle-Based Immunoassays-on-Chip: Materials Synthesis, Surface Functionalization, and Cancer Cell Screening. Adv. Funct. Mater. 2016, 26, 3953–3972. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Muller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Mat. 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Vurro, F.; Gerosa, M.; Busato, A.; Muccilli, M.; Milan, E.; Gaudet, J.; Goodwill, P.; Mansfield, J.; Negri, A.; Gherlinzoni, F.; et al. Doped Ferrite Nanoparticles Exhibiting Self-Regulating Temperature as Magnetic Fluid Hyperthermia Antitumoral Agents, with Diagnostic Capability in Magnetic Resonance Imaging and Magnetic Particle Imaging. Cancers 2022, 14, 5150. [Google Scholar] [CrossRef]
- Lloyd, S.M.; Lave, L.B.; Matthews, H.S. Life cycle benefits of using nanotechnology to stabilize platinum-group metal particles in automotive catalysts. Environ. Sci. Technol. 2005, 39, 1384–1392. [Google Scholar] [CrossRef]
- Weng, C.J. Nanotechnology copper interconnect processes integrations for high aspect ratio without middle etching stop layer. Mat. Sci. Semicon. Proc. 2010, 13, 56–63. [Google Scholar] [CrossRef]
- Senior, L.; Ratcliffe, S.; Knight, M.; Perriman, A.; Mann, S.; Curnow, P. Silicon Transporters: From Membrane Proteins to Nanotechnology. Protein Sci. 2014, 23, 133. [Google Scholar]
- Lee, J.S.; Cha, J.M.; Yoon, H.Y.; Lee, J.K.; Kim, Y.K. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. 2015, 5, 12135. [Google Scholar]
- Shiroka, T. Introduction to solid state physics. Contemp. Phys. 2020, 61, 221–222. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Manouchehri, I.; Salamati, H. Strongly interacting superspins in Fe3O4 nanoparticles. Curr. Appl. Phys. 2012, 12, 812–816. [Google Scholar] [CrossRef]
- Brollo, M.E.F.; Pinheiro, I.F.; Bassani, G.S.; Varet, G.; Guersoni, V.C.B.; Knobel, M.; Bannwart, A.C.; Muraca, D.; van der Geest, C. Iron Oxide Nanoparticles in a Dynamic Flux: Implications for Magnetic Hyperthermia-Controlled Fluid Viscosity. ACS Appl. Nano Mater. 2021, 4, 13633–13642. [Google Scholar] [CrossRef]
- Sudame, A.; Kandasamy, G.; Maity, D. Single and Dual Surfactants Coated Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Applications. J. Nanosci. Nanotechnol. 2019, 19, 3991–3999. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef] [PubMed]
- Aslibeiki, B.; Kameli, P.; Salamati, H. The role of Ag on dynamics of superspins in MnFe2-xAgxO4 nanoparticles. J. Nanopart. Res. 2013, 15, 1430. [Google Scholar] [CrossRef]
- Ito, A.; Tanaka, K.; Honda, H.; Abe, S.; Yamaguchi, H.; Kobayashi, T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J. Biosci. Bioeng. 2003, 96, 364–369. [Google Scholar] [CrossRef]
- Sun, S.H.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Sun, S.H. Structurally Ordered FePt Nanoparticles and Their Enhanced Catalysis for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2010, 132, 4996–4997. [Google Scholar] [CrossRef]
- Bae, S.Y.; Shin, K.H.; Jeong, J.Y.; Kim, J.G. Feasibility of FePt longitudinal recording media for ultrahigh density recording. J. Appl. Phys. 2000, 87, 6953–6955. [Google Scholar] [CrossRef]
- Rellinghaus, B.; Mohn, E.; Schultz, L.; Gemming, T.; Acet, M.; Kowalik, A.; Kock, B.F. On the L1(0) ordering kinetics in Fe-Pt nanoparticles. IEEE Trans. Magn. 2006, 42, 3048–3050. [Google Scholar] [CrossRef]
- Varanda, L.C.; Jafelicci, M. Self-assembled FePt nanocrystals with large coercivity: Reduction of the fcc-to-L1(0) ordering temperature. J. Am. Chem. Soc. 2006, 128, 11062–11066. [Google Scholar] [CrossRef]
- Lu, L.Y.; Wang, D.; Xu, X.G.; Zhan, Y.; Jiang, Y. Enhancement of Magnetic Properties for FePt Nanoparticles by Rapid Annealing in a Vacuum. J. Phys. Chem. C 2009, 113, 19867–19870. [Google Scholar] [CrossRef]
- Nakaya, M.; Kanehara, M.; Teranishi, T. One-pot synthesis of large FePt nanoparticles from metal salts and their thermal stability. Langmuir 2006, 22, 3485–3487. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Caiulo, N.; Lo, C.C.H.; Tam, K.; Tsang, S.C. Synthesis and fabrication of a thin film containing silica-encapsulated face-centered tetragonal FePt nanoparticles. Adv. Mater. 2006, 18, 2312–2314. [Google Scholar] [CrossRef]
- Burkert, T.; Eriksson, O.; Simak, S.I.; Ruban, A.V.; Sanyal, B.; Nordstrom, L.; Wills, J.M. Magnetic anisotropy of L1(0) FePt and Fe1-xMnxPt. Phys. Rev. B 2005, 71, 13. [Google Scholar] [CrossRef]
- Okamoto, S.; Kikuchi, N.; Kitakami, O.; Miyazaki, T.; Shimada, Y.; Fukamichi, K. Chemical-order-dependent magnetic anisotropy and exchange stiffness constant of FePt (001) epitaxial films. Phys. Rev. B 2002, 66, 024413. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Ehsani, M.H.; Nasirzadeh, F.; Mohammadi, M.A. The effect of interparticle interactions on spin glass and hyperthermia properties of Fe3O4 nanoparticles. Mater. Res. Express 2017, 4, 075051. [Google Scholar] [CrossRef]
- Staunton, J.B.; Ostanin, S.; Razee, S.S.A.; Gyorffy, B.L.; Szunyogh, L.; Ginatempo, B.; Bruno, E. Temperature dependent magnetic anisotropy in metallic magnets from an ab initio electronic structure theory: L1(0)-ordered FePt. Phys. Rev. Lett. 2004, 93, 257204. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Eskandarzadeh, N.; Jalili, H.; Varzaneh, A.G.; Kameli, P.; Orue, I.; Chernenko, V.; Hajalilou, A.; Ferreira, L.P.; Cruz, M.M. Magnetic hyperthermia properties of CoFe2O4 nanoparticles: Effect of polymer coating and interparticle interactions. Ceram. Int. 2022, 48, 27995–28005. [Google Scholar] [CrossRef]
- Rezanezhad, A.; Hajalilou, A.; Eslami, F.; Parvini, E.; Abouzari-Lotf, E.; Aslibeiki, B. Superparamagnetic magnetite nanoparticles for cancer cells treatment via magnetic hyperthermia: Effect of natural capping agent, particle size and concentration. J. Mater. Sci. Mater. Electron. 2021, 32, 24026–24040. [Google Scholar] [CrossRef]
- Limthin, D.; Leepheng, P.; Klamchuen, A.; Phromyothin, D. Enhancement of Electrochemical Detection of Gluten with Surface Modification Based on Molecularly Imprinted Polymers Combined with Superparamagnetic Iron Oxide Nanoparticles. Polymers 2022, 14, 91. [Google Scholar] [CrossRef]
- Lotfi, S.; Aslibeiki, B.; Zarei, M. Efficient Pb (II) removal from wastewater by TEG coated Fe3O4 ferrofluid. J. Water Environ. Nanotechnol. 2021, 6, 109–120. [Google Scholar]
- Stolyar, S.V.; Krasitskaya, V.V.; Frank, L.A.; Yaroslavtsev, R.N.; Chekanova, L.A.; Gerasimova, Y.V.; Volochaev, M.N.; Bairmani, M.S.; Velikanov, D.A. Polysaccharide-coated iron oxide nanoparticles: Synthesis, properties, surface modification. Mater. Lett. 2021, 284, 128920. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Ehsani, M.H.; Salamati, H.; Muscas, G.; Agostinelli, E.; Foglietti, V.; Casciardi, S.; Peddis, D. Solvothermal synthesis of MnFe2O4 nanoparticles: The role of polymer coating on morphology and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 236–244. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskaran, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Dual-Functional Iron Oxide Nanoparticles Coated with Polyvinyl Alcohol/5-Fluorouracil/Zinc-Aluminium-Layered Double Hydroxide for a Simultaneous Drug and Target Delivery System. Polymers 2021, 13, 855. [Google Scholar] [CrossRef]
- Lazzarini, A.; Colaiezzi, R.; Passacantando, M.; D’Orazio, F.; Arrizza, L.; Ferella, F.; Crucianelli, M. Investigation of physico-chemical and catalytic properties of the coating layer of silica-coated iron oxide magnetic nanoparticles. J. Phys. Chem. Solids 2021, 153, 110003. [Google Scholar] [CrossRef]
- Anila, I.; Lahiri, B.B.; Mathew, J.; Philip, J. Synthesis and magneto-structural properties of chitosan coated ultrafine cobalt ferrite nanoparticles for magnetic fluid hyperthermia in viscous medium. Ceram. Int. 2022, 48, 22767–22781. [Google Scholar] [CrossRef]
- Nalluri, L.P.; Popuri, S.R.; Lee, C.H.; Terbish, N. Synthesis of biopolymer coated functionalized superparamagnetic iron oxide nanoparticles for the pH-sensitive delivery of anti-cancer drugs epirubicin and temozolomide. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1039–1052. [Google Scholar] [CrossRef]
- Khani, T.; Alamzadeh, Z.; Sarikhani, A.; Mousavi, M.; Mirrahimi, M.; Tabei, M.; Irajirad, R.; Abed, Z.; Beik, J. Fe3O4@Au core-shell hybrid nanocomposite for MRI-guided magnetic targeted photo-chemotherapy. Laser Med. Sci. 2022, 37, 2387–2395. [Google Scholar] [CrossRef]
- Abdollahi, B.B.; Ghorbani, M.; Hamishehkar, H.; Malekzadeh, R.; Farajollahi, A.R. Synthesis and characterization of actively HER-2 Targeted Fe3O4@Au nanoparticles for molecular radiosensitization of breast cancer. Bioimpacts 2022, 12, 23682. [Google Scholar] [CrossRef]
- Le, T.T.; Nguyen, T.N.L.; Nguyen, H.D.; Phan, T.H.T.; Pham, H.N.; Le, D.G.; Hoang, T.P.; Nguyen, T.Q.H.; Le, T.L.; Tran, L.D. Multimodal Imaging Contrast Property of Nano Hybrid Fe3O4@Ag Fabricated by Seed-Growth for Medicinal Diagnosis. Chemistryselect 2022, 7, e202201374. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif. Cell Nanomed. B 2016, 44, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; El-Fiqi, A.; Park, J.H.; Kim, T.H.; Kim, J.H.; Kim, H.W. Therapeutic bioactive microcarriers: Co-delivery of growth factors and stem cells for bone tissue engineering. Acta Biomater. 2014, 10, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Yu, C.Z.; Zhou, X.F.; Tang, J.W.; Zhao, D.Y. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol-gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Jansen, J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliver Rev. 2007, 59, 234–248. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Hilmas, G.E.; Bal, B.S. Mechanical properties of bioactive glass (13-93) scaffolds fabricated by robotic deposition for structural bone repair. Acta Biomater. 2013, 9, 7025–7034. [Google Scholar] [CrossRef]
- Bi, L.X.; Rahaman, M.N.; Day, D.E.; Brown, Z.; Samujh, C.; Liu, X.; Mohammadkhah, A.; Dusevich, V.; Eick, J.D.; Bonewald, L.F. Effect of bioactive borate glass microstructure on bone regeneration, angiogenesis, and hydroxyapatite conversion in a rat calvarial defect model. Acta Biomater. 2013, 9, 8015–8026. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Colilla, M.; Manzano, M. Bone-regenerative bioceramic implants with drug and protein controlled delivery capability. Prog. Solid State Chem. 2008, 36, 163–191. [Google Scholar] [CrossRef]
- Arcos, D.; Greenspan, D.C.; Vallet-Regi, M. A new quantitative method to evaluate the in vitro bioactivity of melt and sol-gel-derived silicate glasses. J. Biomed. Mater. Res. A 2003, 65A, 344–351. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass (R). J. Mater. Sci.-Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Deng, H.X.; Huang, X.H.; Lu, G.Q.; Qiao, S.Z.; Zhao, D.Y.; Yu, C.Z. Mesoporous bioactive glasses. I. Synthesis and structural characterization. J. Non-Cryst. Solids 2005, 351, 3209–3217. [Google Scholar] [CrossRef]
- Yu, C.Z.; Yan, X.X.; Huang, X.H.; Deng, H.X.; Wang, Y.; Zhang, Z.D.; Qiao, S.Z.; Lu, G.Q.; Zhao, D.Y. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Shang, F.J.; Li, B.; Dong, Y.; Liu, Y.F.; Lohe, M.R.; Hanagata, N.; Kaskel, S. Magnetic mesoporous bioactive glass scaffolds: Preparation, physicochemistry and biological properties. J. Mater. Chem. B 2013, 1, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.Y.; Xia, Y.; Wang, J.; Liang, C.; Yu, L.; Tang, W.; Gu, S.; Xu, S.G. Antibacterial properties and bioactivity of HACC- and HACC-Zein-modified mesoporous bioactive glass scaffolds. J. Mater. Chem. B 2013, 1, 685–692. [Google Scholar] [CrossRef]
- Wu, C.T.; Chang, J.; Fan, W. Bioactive mesoporous calcium-silicate nanoparticles with excellent mineralization ability, osteostimulation, drug-delivery and antibacterial properties for filling apex roots of teeth. J. Mater. Chem. 2012, 22, 16801–16809. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, J.L.; He, Q.J.; Zhang, L.X.; Chen, F.; Chen, Y. An emulsification-solvent evaporation route to mesoporous bioactive glass microspheres for bisphosphonate drug delivery. J. Mater. Sci. 2012, 47, 2256–2263. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Izquierdo-Barba, I.; Colilla, M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Mortera, R.; Onida, B.; Saino, E.; Visai, L.; Verne, E.; Vitale-Brovarone, C. Mesoporous bioactive glass as a multifunctional system for bone regeneration and controlled drug release. J. Appl. Biomater. Funct. 2012, 10, 12–21. [Google Scholar]
- Zhang, J.H.; Zhao, S.C.; Zhu, M.; Zhu, Y.F.; Zhang, Y.D.; Liu, Z.T.; Zhang, C.Q. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.D.; Avery, G.B.; Manock, J.J.; Skrabal, S.A.; Stehman, C.F. Chemical analysis of soils—An environmental chemistry laboratory for undergraduate science majors. J. Chem. Educ. 1999, 76, 1693–1694. [Google Scholar]
- Robinson, C.; Adewunmi, W.; Lindbo, D.; Moebius-Clune, B. Environmental Science, Soil Conservation, and Land Use Management. Know Soil Know Life 2012, 109–138. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.L.; Nicolas, C.; Hernandez, T.; Garcia, C. Soil metaproteomics: A review of an emerging environmental science. Significance, methodology and perspectives. Eur. J. Soil Sci. 2009, 60, 845–859. [Google Scholar] [CrossRef]
- Celik, Y.; Suvaci, E.; Weibel, A.; Peigney, A.; Flahaut, E. Texture development in Fe-doped alumina ceramics via templated grain growth and their application to carbon nanotube growth. J. Eur. Ceram. Soc. 2013, 33, 1093–1100. [Google Scholar] [CrossRef]
- Barcelos, K.A.; Tebaldi, M.L.; do Egito, E.S.T.; Leao, N.M.; Soares, D.C.F. PEG-Iron Oxide Core-Shell Nanoparticles: In situ Synthesis and In Vitro Biocompatibility Evaluation for Potential T-2-MRI Applications. Bionanoscience 2020, 10, 1107–1120. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Restaino, G.; Missere, M.; Positano, V.; Spasiano, A.; Casini, T.; Cossu, A.; Cuccia, L.; Massa, A.; et al. Quantitative T2*MRI for bone marrow iron overload: Normal reference values and assessment in thalassemia major patients. Radiol. Med. 2022, 127, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, E.P.; Moloney, C.; Chaudhuri, T.R.; Clerkin, S.; Behan, K.; Straubinger, R.M.; Crean, J.; Brougham, D.F. Formation of hydrated PEG layers on magnetic iron oxide nanoflowers shows internal magnetisation dynamics and generates high in-vivo efficacy for MRI and magnetic hyperthermia. Acta Biomater. 2022, 152, 393–405. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, S.J.; DeNardo, G.L.; Natarajan, A.; Miers, L.A.; Foreman, A.R.; Gruettner, C.; Adamson, G.N.; Ivkov, R. Thermal dosimetry predictive of efficacy of In-111-ChL6 nanoparticle AMF-induced thermoablative therapy for human breast cancer in mice. J. Nucl. Med. 2007, 48, 437–444. [Google Scholar] [PubMed]
- Aslibeiki, B.; Kameli, P.; Salamati, H. The effect of grinding on magnetic properties of agglomereted MnFe2O4 nanoparticles. J. Magn. Magn. Mater. 2012, 324, 154–160. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P. Magnetic properties of MnFe2O4 nano-aggregates dispersed in paraffin wax. J. Magn. Magn. Mater. 2015, 385, 308–312. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Salamati, H. The effect of dipole-dipole interactions on coercivity, anisotropy constant, and blocking temperature of MnFe2O4 nanoparticles. J. Appl. Phys. 2016, 119, 063901. [Google Scholar] [CrossRef]
- Polevikov, V.; Tobiska, L. On the solution of the steady-state diffusion problem for ferromagnetic particles in a magnetic fluid. Math. Model. Anal. 2008, 13, 233–240. [Google Scholar] [CrossRef]
- Ring, H.L.; Bischof, J.C.; Garwood, M. Use and Safety of Iron Oxide Nanoparticles in MRI and MFH. eMagRes 2019, 8, 265–277. [Google Scholar]
- Ito, A.; Matsuoka, F.; Honda, H.; Kobayashi, T. Heat shock protein 70 gene therapy combined with hyperthermia using magnetic nanoparticles. Cancer Gene Ther. 2003, 10, 918–925. [Google Scholar] [CrossRef][Green Version]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther. 2001, 8, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.S.; Nayef, U.M.; Abdulkadhim, W.K.; Sulaiman, G.M. Supermagnetic Fe3O4-PEG nanoparticles combined with NIR laser and alternating magnetic field as potent anti-cancer agent against human ovarian cancer cells. Mater. Res. Express 2019, 6, 115412. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Y.; Zhou, H.; Sun, Y.K.; Zhang, Y.; Gu, N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- Ebrahimisadr, S.; Aslibeiki, B.; Asadi, R. Magnetic hyperthermia properties of iron oxide nanoparticles: The effect of concentration. Phys. C 2018, 549, 119–121. [Google Scholar] [CrossRef]
- Hilger, I.; Fruhauf, K.; Andra, W.; Hiergeist, R.; Hergt, R.; Kaiser, W.A. Heating potential of iron oxides for therapeutic purposes in interventional radiology. Acad. Radiol. 2002, 9, 198–202. [Google Scholar] [CrossRef]
- Lubbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.S.; Carpino, F.; Zborowski, M. Magnetic Nanoparticle Drug Carriers and Their Study by Quadrupole Magnetic Field-Flow Fractionation. Mol. Pharm. 2009, 6, 1290–1306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Ou, J.; Zhang, Z.H.; Qin, Q.H. Preparation of magnetic and bioactive calcium zinc iron silicon oxide composite for hyperthermia treatment of bone cancer and repair of bone defects. J. Mater. Sci. Mater. Med. 2011, 22, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Fan, W.; Zhu, Y.F.; Gelinsky, M.; Chang, J.; Cuniberti, G.; Albrecht, V.; Friis, T.; Xiao, Y. Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater. 2011, 7, 3563–3572. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Banobre-Lopez, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, Y.Y.; Wang, R.H.; Chen, H.R.; Cai, X.J.; Lu, G.M.; Chu, L.; Xu, C.Y.; Zhang, N.; Wang, Z.G.; et al. A smart, phase transitional and injectable DOX/PLGA-Fe implant for magnetic-hyperthermia-induced synergistic tumor eradication. Acta Biomater. 2016, 29, 298–306. [Google Scholar] [CrossRef]
- Farzin, A.; Fathi, M.; Emadi, R. Multifunctional magnetic nanostructured hardystonite scaffold for hyperthermia, drug delivery and tissue engineering applications. Mater. Sci. Eng. C 2017, 70, 21–31. [Google Scholar] [CrossRef]
- Bretcanu, O.; Miola, M.; Bianchi, C.L.; Marangi, I.; Carbone, R.; Corazzari, I.; Cannas, M.; Verne, E. In Vitro biocompatibility of a ferrimagnetic glass-ceramic for hyperthermia application. Mat. Sci. Eng. C 2017, 73, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Yang, Y.; Ling, Y.; Liu, J.X.; Cai, X.J.; Zhou, X.H.; Tang, X.Z.; Liang, B.; Chen, Y.N.; Chen, H.R.; et al. Injectable and thermally contractible hydroxypropyl methyl cellulose/Fe3O4 for magnetic hyperthermia ablation of tumors. Biomaterials 2017, 128, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Saber-Samandari, S.; Mohammadi-Aghdam, M.; Saber-Samandari, S. A novel magnetic bifunctional nanocomposite scaffold for photothermal therapy and tissue engineering. Int. J. Biol. Macromol. 2019, 138, 810–818. [Google Scholar] [CrossRef]
- Rodrigues, A.F.M.; Torres, P.M.C.; Barros, M.J.S.; Presa, R.; Ribeiro, N.; Abrantes, J.C.C.; Belo, J.H.; Amaral, J.S.; Amaral, V.S.; Banobre-Lopez, M.; et al. Effective production of multifunctional magnetic-sensitive biomaterial by an extrusion-based additive manufacturing technique. Biomed. Mater. 2021, 16, 015011. [Google Scholar] [CrossRef] [PubMed]
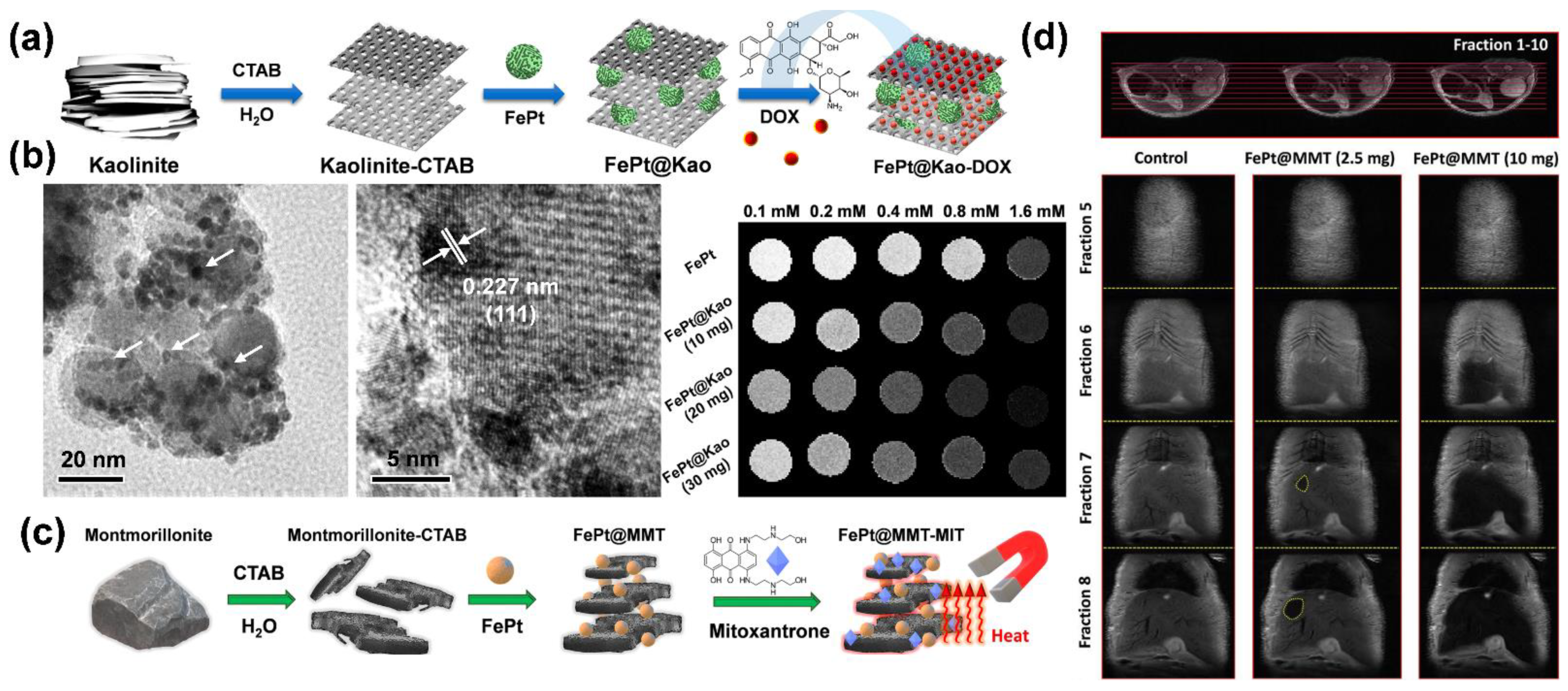
- Chan, M.H.; Hsieh, M.R.; Liu, R.S.; Wei, D.H.; Hsiao, M. Magnetically Guided Theranostics: Optimizing Magnetic Resonance Imaging with Sandwich-Like Kaolinite-Based Iron/Platinum Nanoparticles for Magnetic Fluid Hyperthermia and Chemotherapy. Chem. Mater. 2020, 32, 697–708. [Google Scholar] [CrossRef]
- Borges, R.; Mendonca-Ferreira, L.; Rettori, C.; Pereira, I.S.O.; Baino, F.; Marchi, J. New sol-gel-derived magnetic bioactive glass-ceramics containing superparamagnetic hematite nanocrystals for hyperthermia application. Mater. Sci. Eng. C 2021, 120, 111692. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yang, Q.H.; Huo, M.F.; Lu, D.; Gao, Y.S.; Chen, Y.; Xu, H.X. Engineering Single-Atomic Iron-Catalyst-Integrated 3D-Printed Bioscaffolds for Osteosarcoma Destruction with Antibacterial and Bone Defect Regeneration Bioactivity. Adv. Mater. 2021, 33, 2100150. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.; Lu, C.N.; Chung, Y.L.; Chang, Y.C.; Li, C.H.; Chen, C.L.; Wei, D.H.; Hsiao, M. Magnetically guided theranostics: Montmorillonite-based iron/platinum nanoparticles for enhancing in situ MRI contrast and hepatocellular carcinoma treatment. J. Nanobiotechnol. 2021, 19, 308. [Google Scholar] [CrossRef]
- Borges, R.; Ferreira, L.M.; Rettori, C.; Lourenco, I.M.; Seabra, A.B.; Muller, F.A.; Ferraz, E.P.; Marques, M.M.; Miola, M.; Baino, F.; et al. Superparamagnetic and highly bioactive SPIONS/bioactive glass nanocomposite and its potential application in magnetic hyperthermia. Biomater. Adv. 2022, 135, 112655. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kim, S.; Lee, H.; Kim, J.; Kim, N.; Park, H.J.; Choi, E.K.; Lee, J.S.; Kim, C. A multifunctional mesoporous nanocontainer with an iron oxide core and a cyclodextrin gatekeeper for an efficient theranostic platform. J. Mater. Chem. 2012, 22, 14061–14067. [Google Scholar] [CrossRef]
- Guardado-Alvarez, T.M.; Devi, L.S.; Vabre, J.M.; Pecorelli, T.A.; Schwartz, B.J.; Durand, J.O.; Mongin, O.; Blanchard-Desce, M.; Zink, J.I. Photo-redox activated drug delivery systems operating under two photon excitation in the near-IR. Nanoscale 2014, 6, 4652–4658. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Lin, H.Y.; Chan, M.H. Preparation, characterization, and in vitro evaluation of folate-modified mesoporous bioactive glass for targeted anticancer drug carriers. J. Mater. Chem. B 2013, 1, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Virk, M.S.; Lieberman, J.R. Tumor metastasis to bone. Arthritis Res. Ther. 2007, 9, S5. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Shen, Y.Q.; Tang, J.B.; Fan, M.H.; Van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Charge-Reversal Drug Conjugate for Targeted Cancer Cell Nuclear Drug Delivery. Adv. Funct. Mater. 2009, 19, 3580–3589. [Google Scholar] [CrossRef]
- Chen, B.W.; Chiu, G.W.; He, Y.C.; Huang, C.Y.; Huang, H.T.; Sung, S.Y.; Hsieh, C.L.; Chang, W.C.; Hsu, M.S.; Wei, Z.H.; et al. Extracellular and intracellular intermittent magnetic-fluid hyperthermia treatment of SK-Hep1 hepatocellular carcinoma cells based on magnetic nanoparticles coated with polystyrene sulfonic acid. PLoS ONE 2021, 16, e0245286. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar]
- Mazumder, A.; Dwivedi, A.; Assawapanumat, W.; Saeeng, R.; Sungkarat, W.; Nasongkla, N. In vitro galactose-targeted study of RSPP050-loaded micelles against liver hepatocellular carcinoma. Pharm. Dev. Technol. 2022, 27, 379–388. [Google Scholar] [CrossRef]
- Choi, H.J.; Choi, B.S.; Yu, B.; Li, W.G.; Matsumoto, M.M.; Harris, K.R.; Lewandowski, R.J.; Larson, A.C.; Mouli, S.K.; Kim, D.H. On-demand degradable embolic microspheres for immediate restoration of blood flow during image-guided embolization procedures. Biomaterials 2021, 265, 120408. [Google Scholar] [CrossRef]
- Suleman, M.; Riaz, S. 3D in silico study of magnetic fluid hyperthermia of breast tumor using Fe3O4 magnetic nanoparticles. J. Therm. Biol. 2020, 91, 102635. [Google Scholar] [CrossRef]
- Tseng, C.L.; Chang, K.C.; Yeh, M.C.; Yang, K.C.; Tang, T.P.; Lin, F.H. Development of a dual-functional Pt-Fe-HAP magnetic nanoparticles application for chemo-hyperthermia treatment of cancer. Ceram. Int. 2014, 40, 5117–5127. [Google Scholar] [CrossRef]
- Hayashi, A.; Yokoo, N.; Nakamura, T.; Watanabe, T.; Nagasawa, H.; Kogure, T. Crystallographic characterization of the crossed lamellar structure in the bivalve Meretrix lamarckii using electron beam techniques. J. Struct. Biol. 2011, 176, 91–96. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Gresser, J.D.; Wise, D.L.; Trantolo, D.J. Bioresorbable bone graft substitutes of different osteoconductivities: A histologic evaluation of osteointegration of poly(propylene glycol-co-fumaric acid)-based cement implants in rats. Biomaterials 2000, 21, 757–764. [Google Scholar] [CrossRef]
- Saikia, K.C.; Bhattacharya, T.D.; Bhuyan, S.K.; Talukdar, D.J.; Saikia, S.P.; Jitesh, P. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J. Orthop. 2008, 42, 169–172. [Google Scholar] [CrossRef]
- Singh, A.K.; Hahn, M.A.; Gutwein, L.G.; Rule, M.C.; Knapik, J.A.; Moudgil, B.M.; Grobmyer, S.R.; Brown, S.C. Multi-dye theranostic nanoparticle platform for bioimaging and cancer therapy. Int. J. Nanomed. 2012, 7, 2739–2750. [Google Scholar]
- Rastogi, R.; Gulati, N.; Kotnala, R.K.; Sharma, U.; Jayasundar, R.; Koul, V. Evaluation of folate conjugated pegylated thermosensitive magnetic nanocomposites for tumor imaging and therapy. Colloids. Surf. B Biointerfaces 2011, 82, 160–167. [Google Scholar] [CrossRef]
- Ashokan, A.; Menon, D.; Nair, S.; Koyakutty, M. A molecular receptor targeted, hydroxyapatite nanocrystal based multi-modal contrast agent. Biomaterials 2010, 31, 2606–2616. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, Y.J.; Zhang, K.H.; Wu, J.; Wang, K.W.; Tang, Q.L.; Mo, X.M. Europium-doped amorphous calcium phosphate porous nanospheres: Preparation and application as luminescent drug carriers. Nanoscale Res. Lett. 2011, 6, 67. [Google Scholar] [CrossRef]
- Zuo, Z.Y.; Song, X.M.; Guo, D.Y.; Guo, Z.R.; Niu, Q.F. A dual responsive colorimetric/fluorescent turn-on sensor for highly selective, sensitive and fast detection of Fe3+ ions and its applications. J. Photochem. Photobiol. A Chem. 2019, 382, 111876. [Google Scholar] [CrossRef]
- Xu, C.J.; Sun, S.H. Monodisperse magnetic nanoparticles for biomedical applications. Polym. Int. 2007, 56, 821–826. [Google Scholar] [CrossRef]
| Iron-Based Material | Ceramic | Cell Type | Biological Effect | Material Effect | Year | Ref. |
|---|---|---|---|---|---|---|
| Calcium zinc iron silicon oxide composite | Glass | Bone cancer | Promotes osteoblast proliferation | Supports nascent cell proliferation | 2011 | [96] |
| Fe/mesoporous bioactive glass | Glass | Human bone marrow mesenchymal stem cells | Improves local delivery of drug therapy and killing of infected tissue cells | Intensifies magnetization | 2011 | [97] |
| (Fe2+/Fe3+)-doped hydroxyapatite | Hydroxyapatite | Osteoblast | Lower level of cytotoxicity achieved | Intensifies magnetization | 2012 | [98] |
| Fe3O4 | Magnetic calcium phosphate cement | Breast cancer | Reduces tumor volume | Controlled timing of drug release | 2016 | [99] |
| Fe3+ | Hardystonite | Bone cancer | Enhances drug delivery and killing of tumor cells | Intensifies magnetization | 2017 | [100] |
| Ferrimagnetic | Glass | Fibroblast/bone cancer | Does not substantially affect cell morphology | Supports nascent cell proliferation | 2017 | [101] |
| Fe3O4 | Hydroxypropyl methylcellulose | Breast cancer | Reduces tumor volume | Controlled timing of drug release | 2017 | [102] |
| Fe3O4 | Akermanite | Osteosarcoma | Lower level of cytotoxicity achieved | Controlled timing of drug release | 2019 | [103] |
| Magnetic nanoparticles | Calcium phosphate | Mesenchymal stem cell | Increases metabolic activity and proliferation | Intensifies magnetization | 2020 | [104] |
| FePt | Kaolinite | Hepatocellular carcinoma | Enhances magnetic signal and killing of tumor cells | Intensifies magnetization | 2020 | [105] |
| Hematite nanocrystal | Glass | Fibroblast | Lower level of cytotoxicity achieved | Intensifies magnetization | 2021 | [106] |
| Single-atomic iron catalysts | Glass | Bone marrow mesenchymal stem cell | Efficacious osteosarcoma ablation | Supports nascent cell proliferation | 2021 | [107] |
| FePt | Montmorillonite | Hepatocellular carcinoma | Enhances magnetic signal and killing of tumor cells | Intensifies magnetization | 2021 | [108] |
| Superparamagnetic iron oxide nanoparticles | Glass | Mesenchymal stem cells | Does not affect cell proliferation | Intensifies magnetization | 2022 | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.-H.; Li, C.-H.; Chang, Y.-C.; Hsiao, M. Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery. Pharmaceutics 2022, 14, 2584. https://doi.org/10.3390/pharmaceutics14122584
Chan M-H, Li C-H, Chang Y-C, Hsiao M. Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery. Pharmaceutics. 2022; 14(12):2584. https://doi.org/10.3390/pharmaceutics14122584
Chicago/Turabian StyleChan, Ming-Hsien, Chien-Hsiu Li, Yu-Chan Chang, and Michael Hsiao. 2022. "Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery" Pharmaceutics 14, no. 12: 2584. https://doi.org/10.3390/pharmaceutics14122584
APA StyleChan, M.-H., Li, C.-H., Chang, Y.-C., & Hsiao, M. (2022). Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery. Pharmaceutics, 14(12), 2584. https://doi.org/10.3390/pharmaceutics14122584