The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing
Abstract
:1. Introduction
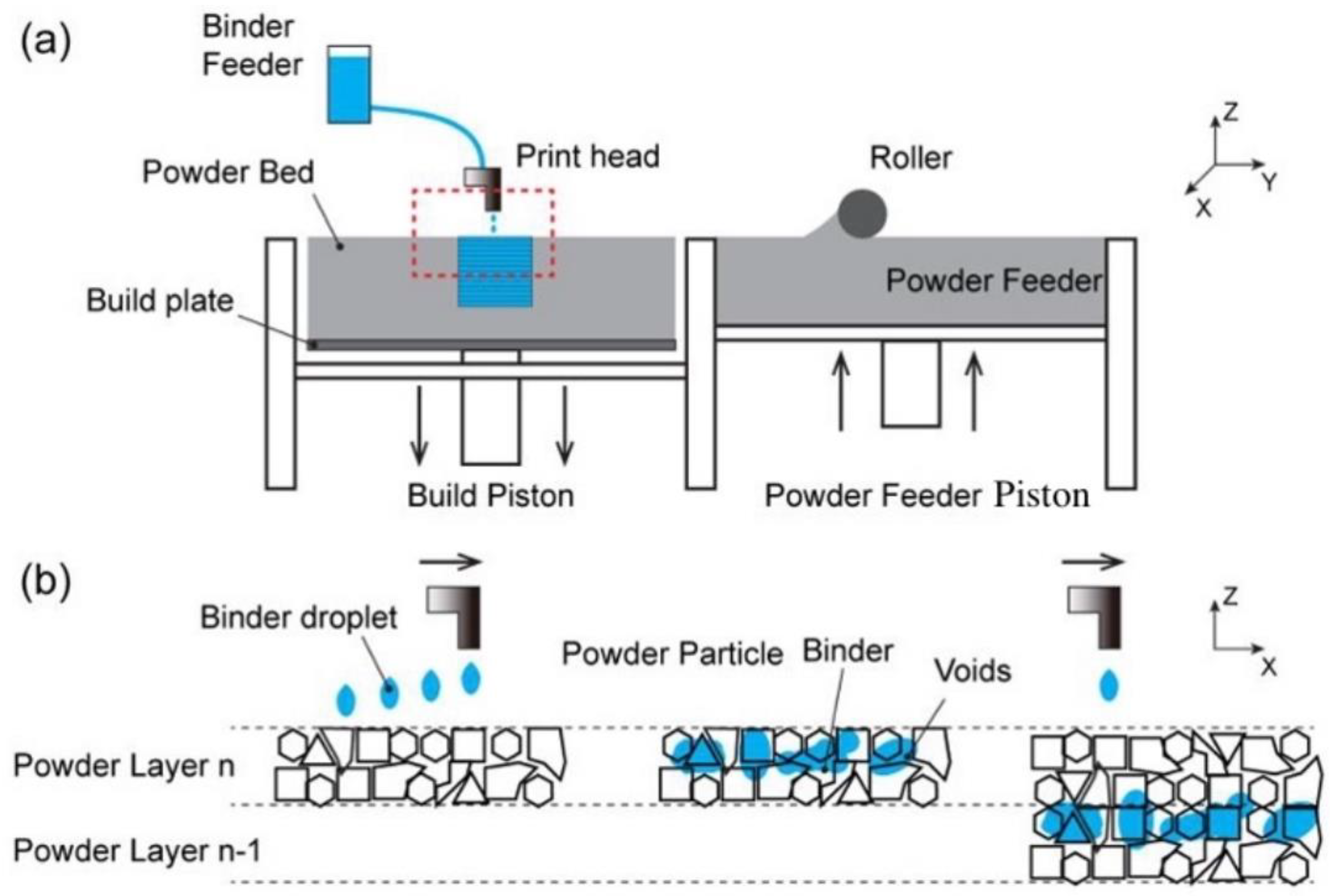
2. Binder Jet 3D Printing
2.1. Factors Affecting the Binder Jet 3D Printing Process
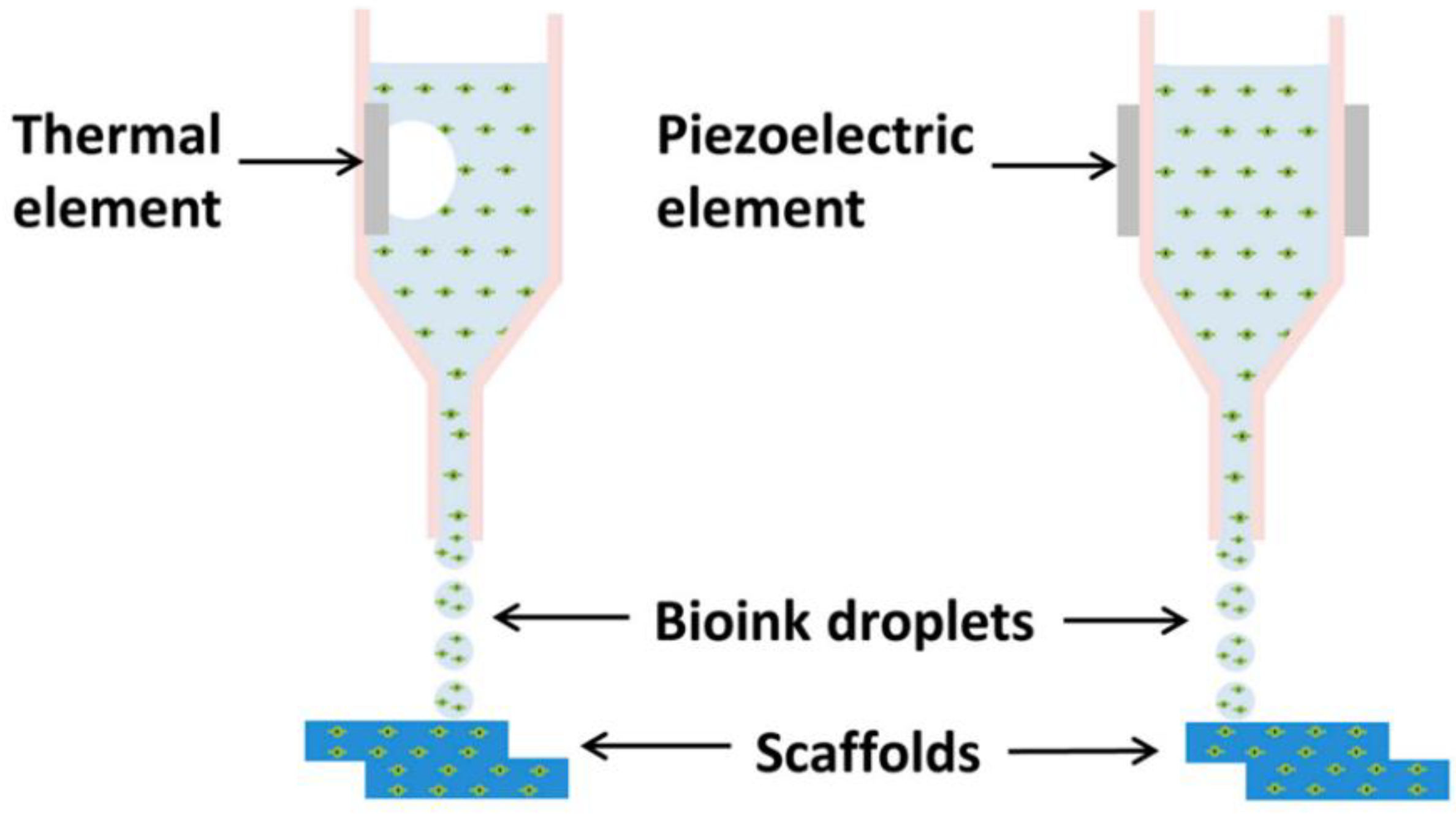
2.1.1. The Print Heads
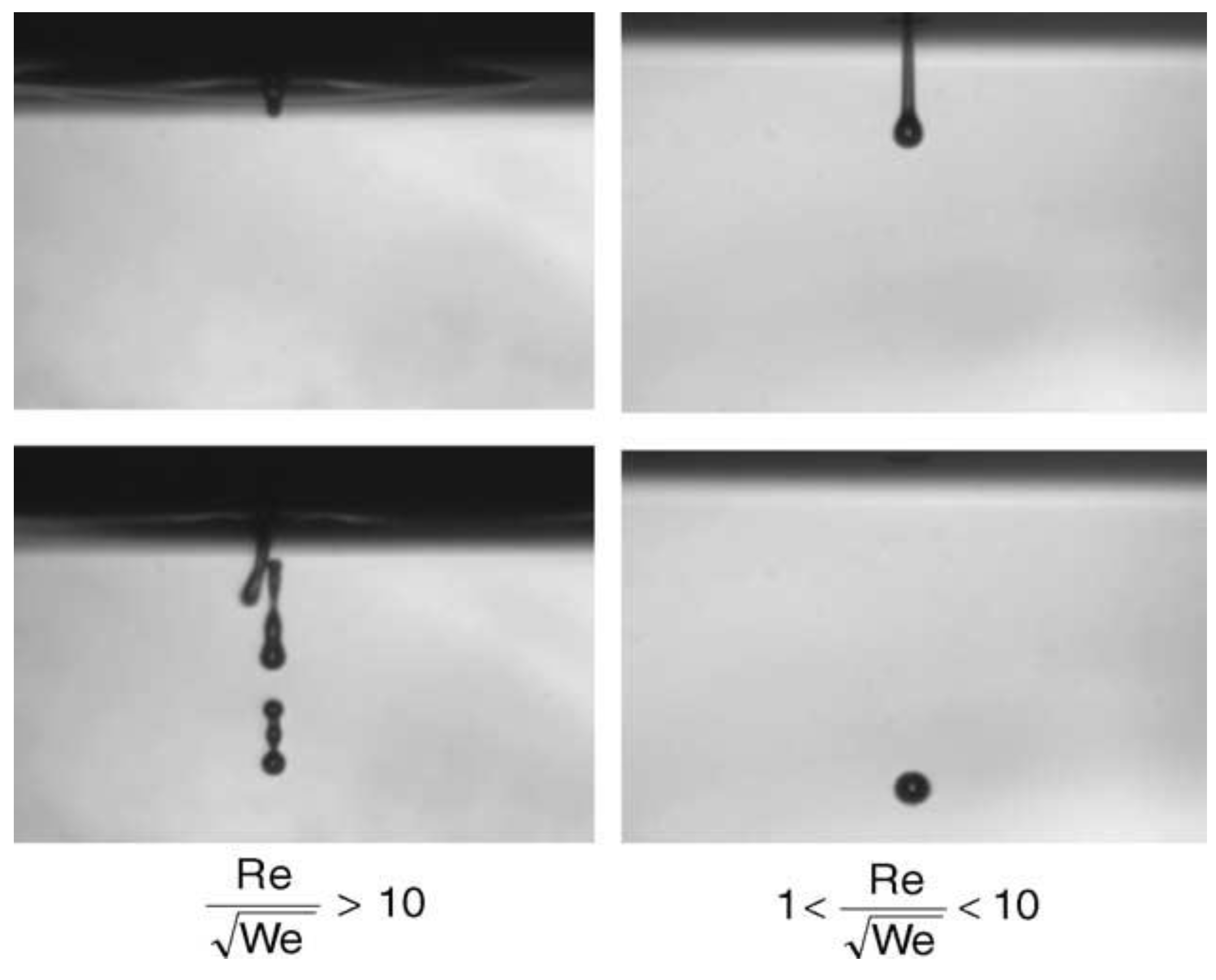
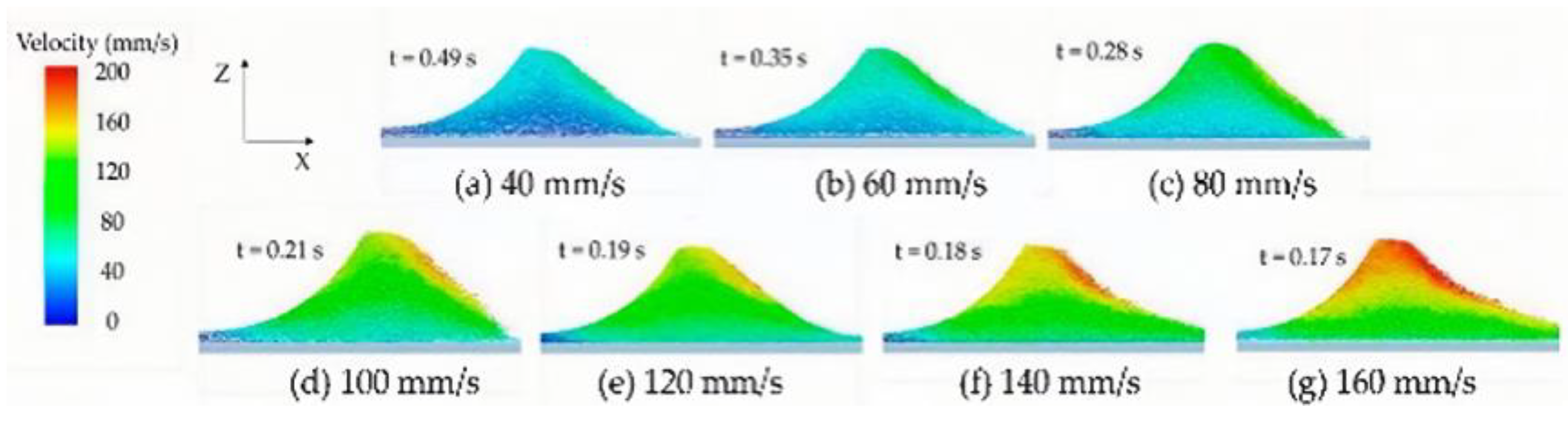
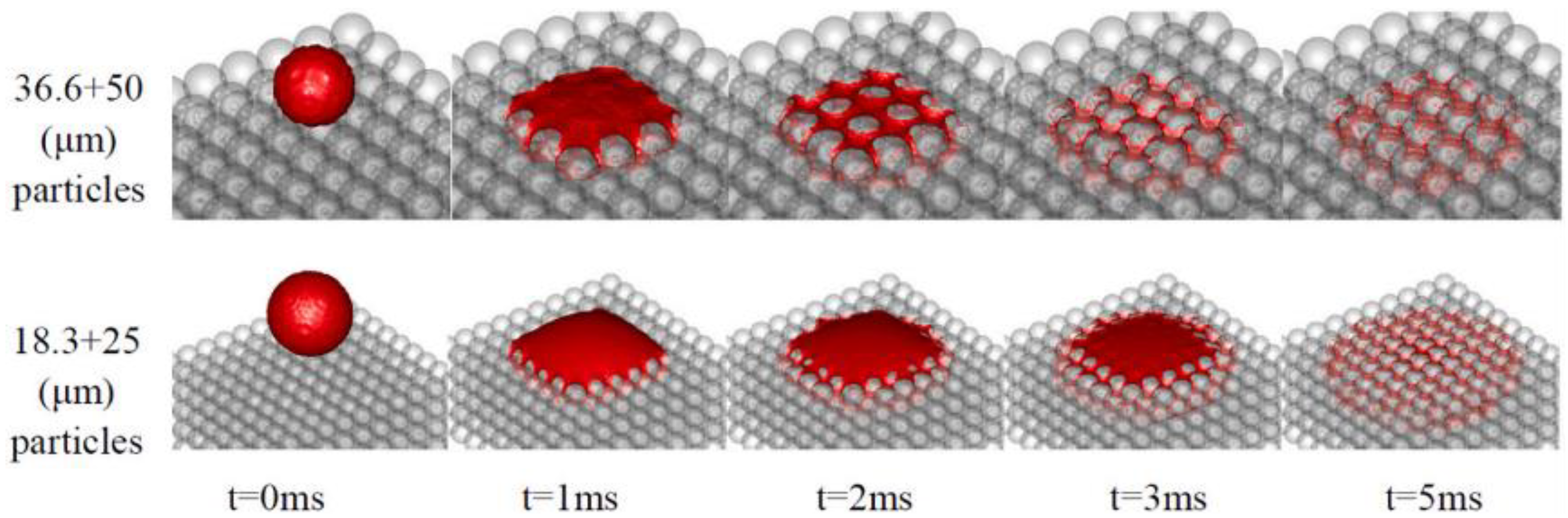
2.1.2. Binder Solution
- (1)
- Calculation of Reynolds number to measure the relative magnitude of fluid inertia force and viscous force:
- (2)
- Calculation of Weber number representing the ratio of inertial force to surface tension effect:
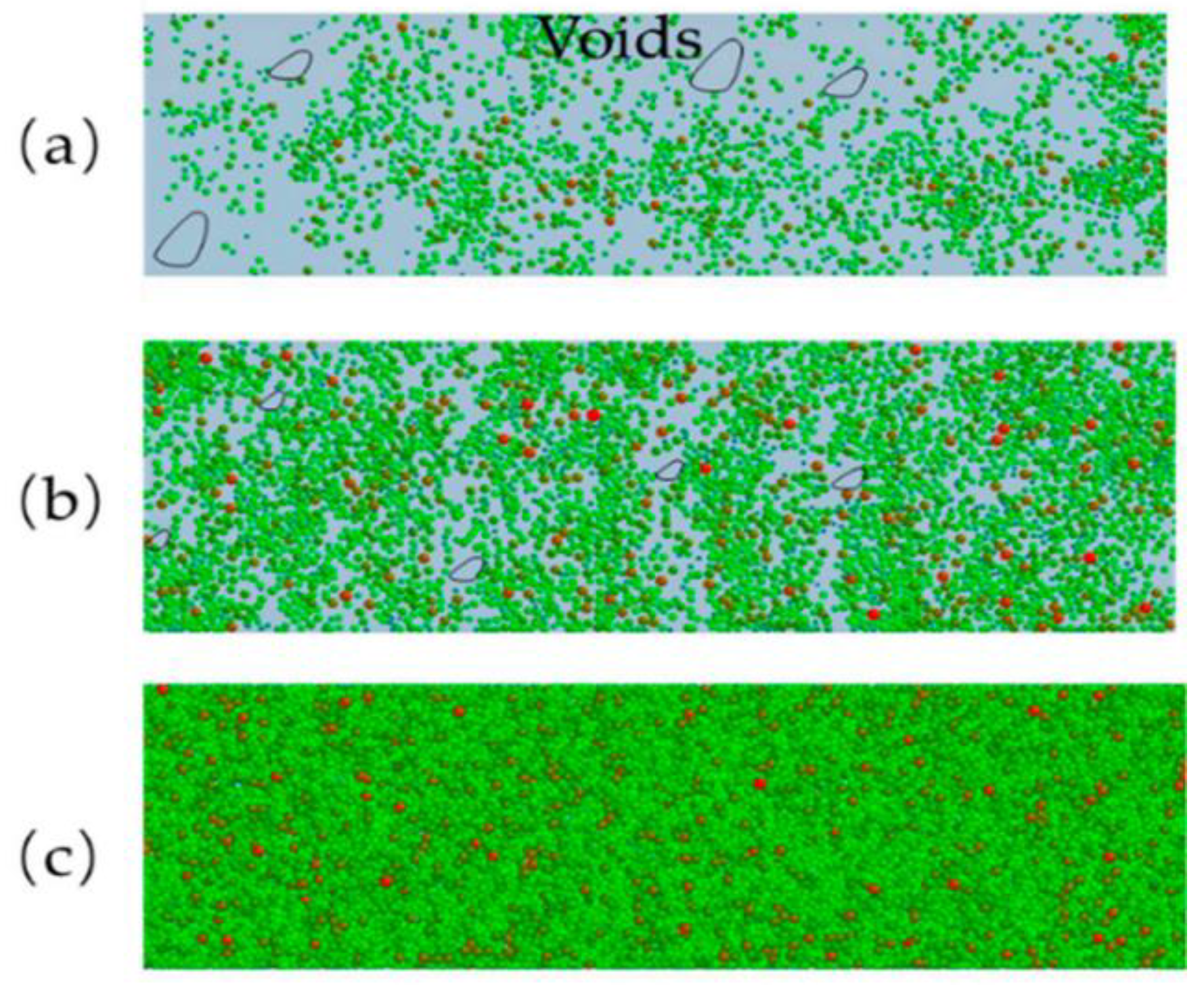
2.1.3. Powder-Specific Properties
- (1)
- powder particle characteristics (including surface morphology and powder particle size and size distribution),
- (2)
- powder flowability,
- (3)
- powder packing density.
2.1.4. Printing Parameters
- (1)
- the thickness of the powder layer;
- (2)
- the pushing speed of the powder roller;
- (3)
- the jetting amount or times of the printing liquid;
- (4)
- the height of the print head from the powder layer.
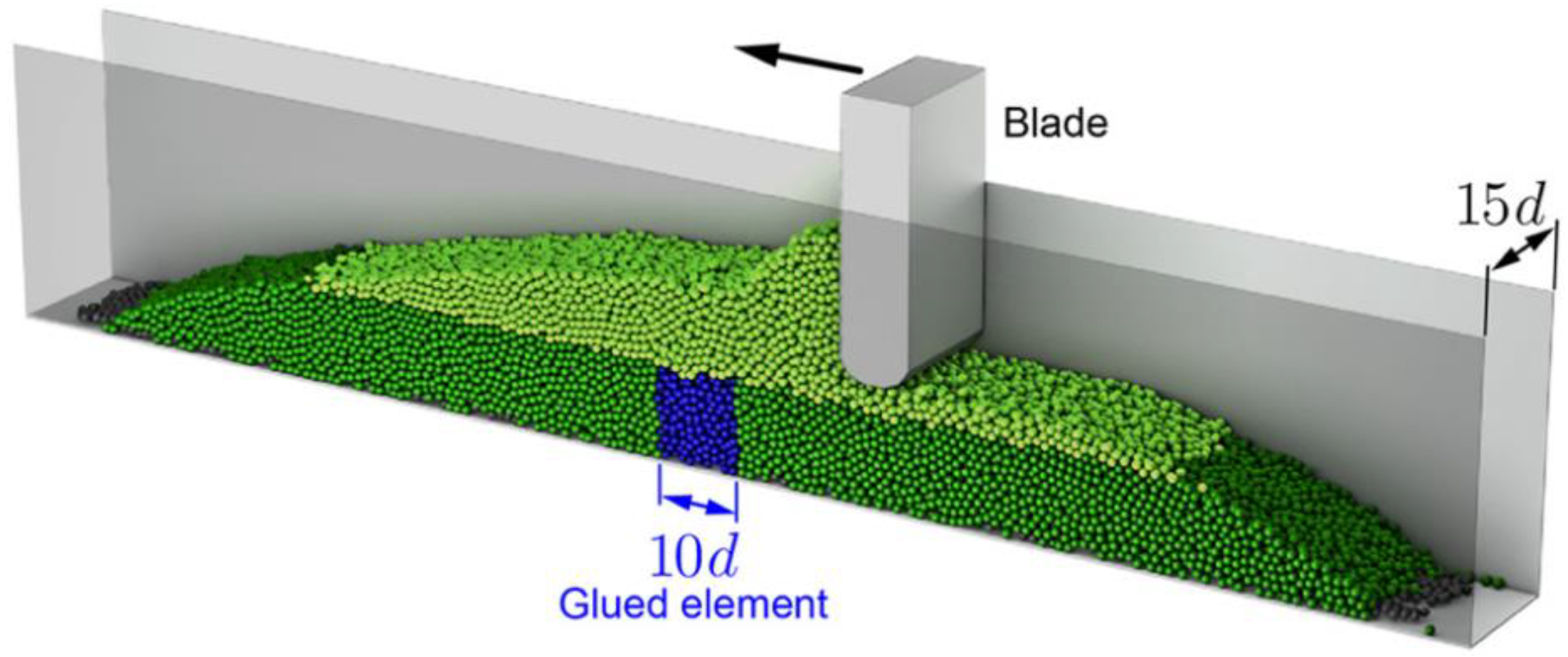
2.2. Simulation Study on Binder Jet 3D Printing Process
3. Applications of Binder Jet 3D Printing in Pharmaceutical Manufacturing
3.1. Orally Rapidly Releasing Dosage Forms
3.2. Sustained-Release Preparations and Controlled-Release Preparations
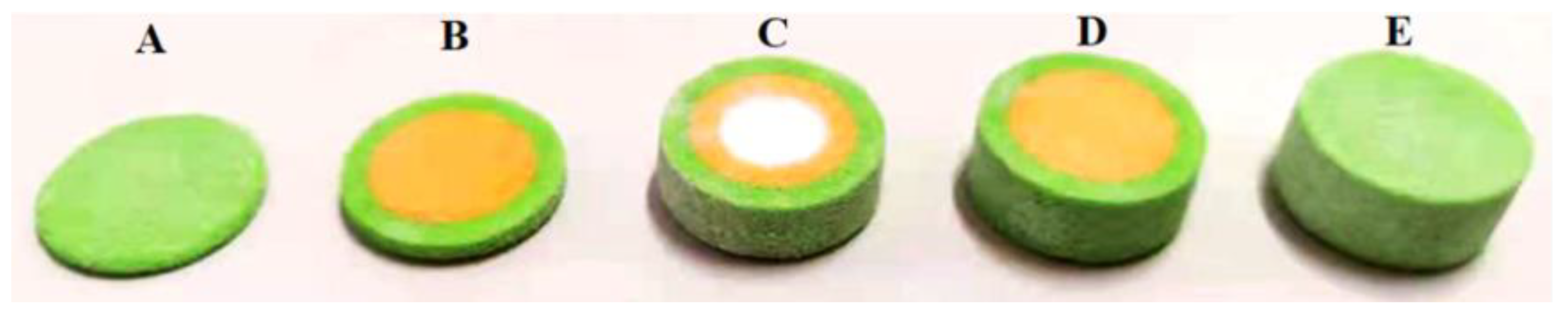
3.3. Fabrication of Dosage Forms with Multiple Drugs
3.4. Preparations for Children
3.5. Implants
| Powder | Binder | Layer Thickness (µm) | Reference |
|---|---|---|---|
| α-tricalcium phosphate(α-TCP) | 10 wt% phosphoric acid solution | 50 | A. Butscher [84] |
| HA/poly(vinyl)alcohol (PVOH) | water-based binder | 100 | Sophie C. Cox [85] |
| SiO2/Zn-O/β-TCP | Not mentioned | 20 | Samit Kumar Nandi [86] |
| Calcium Sulfate hemihydrate | 2-pyrrolidinone solution | 89 | Mitra [87] |
| hydroxyapatite and a-tricalcium phosphate | phosphate buffer, Tween 80 | 89 | Jason A. [88] |
| α-TCP | 2.5 wt% disodium hydrogen phosphate solution | 88 | Ruth [89] |
| HA microsphere | water-based polymeric binder | 90 | Chai [90] |
| MgO/ZnO-TCP | Not mentioned | Not mentioned | Dong-xu Ke [91] |
| Mg–Si-doped TCP | Not mentioned | Not mentioned | SUSMITA BOSE [92] |
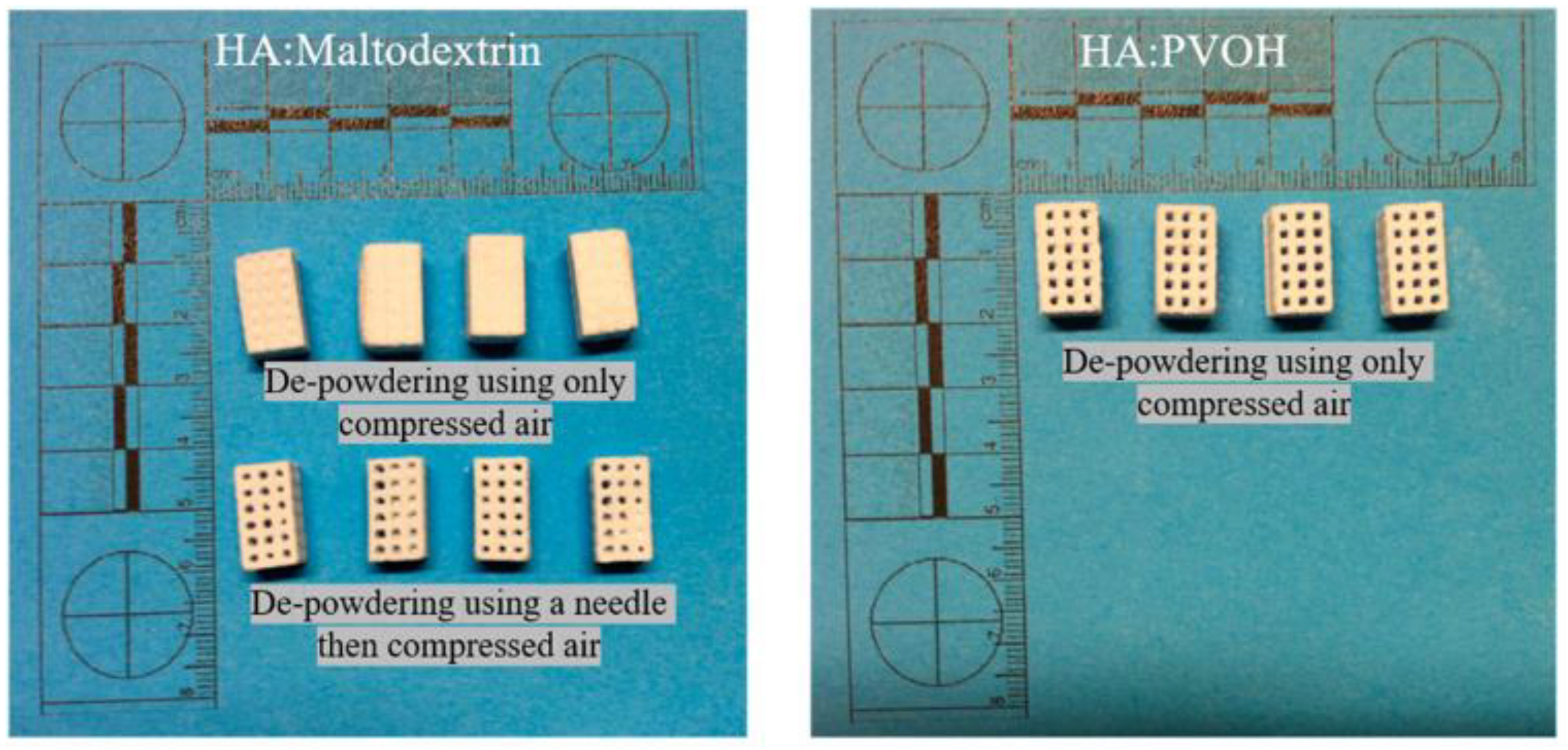
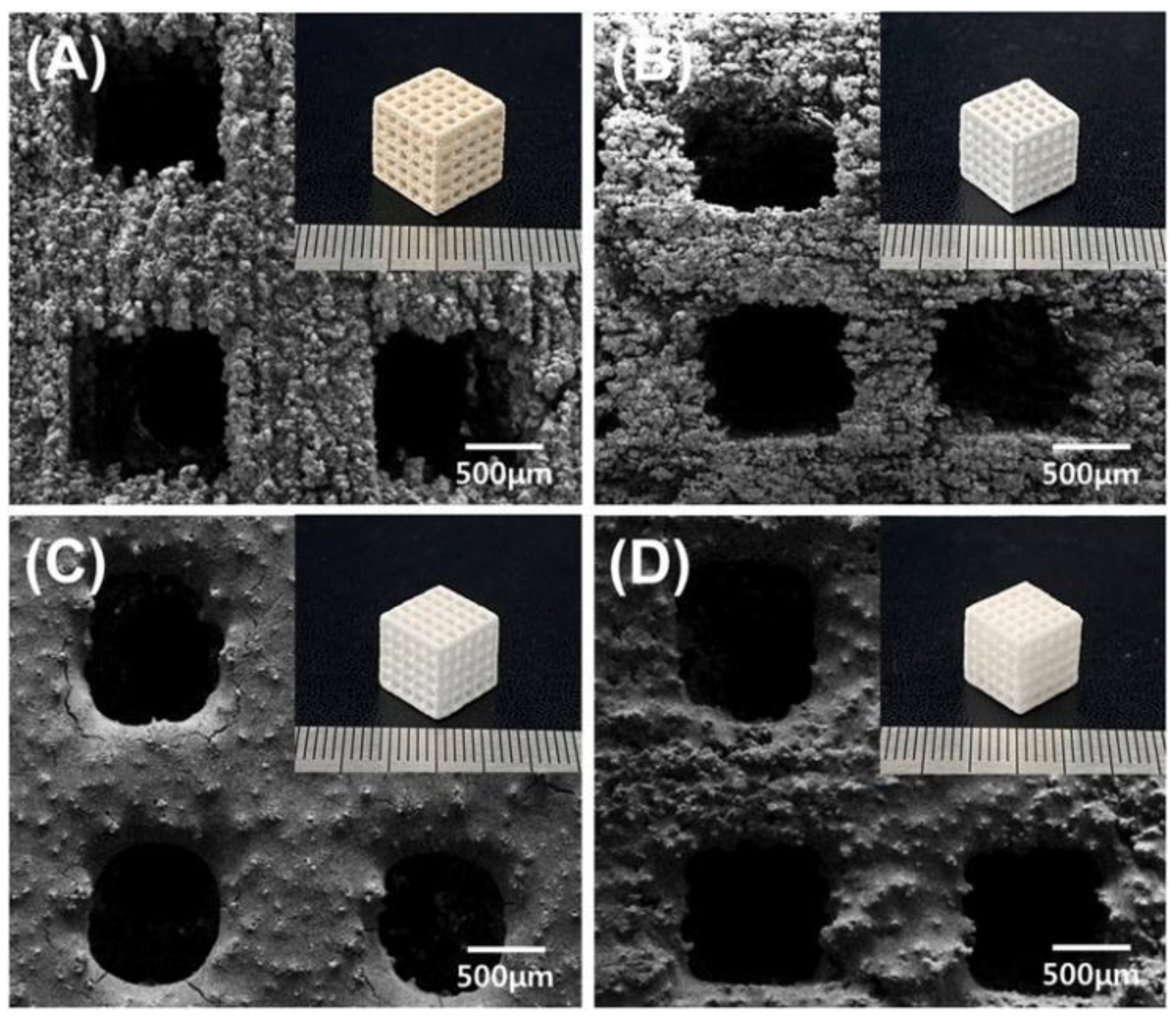
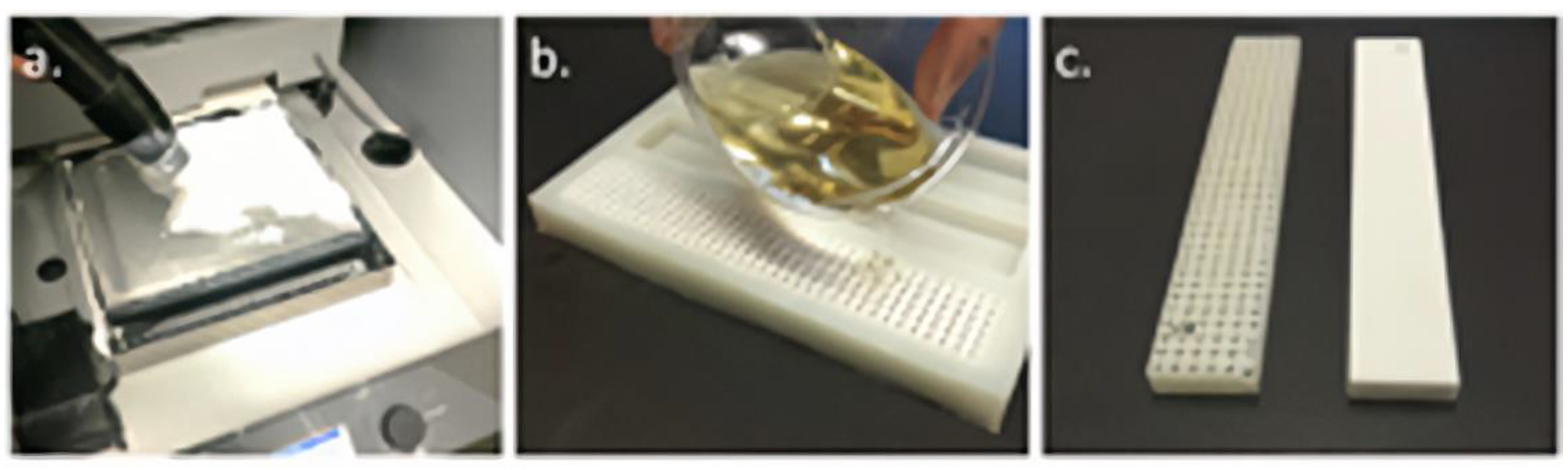
4. Application Prospect and Challenge of Binder Jet 3D Printing Technology
- (1)
- Structural advantages create complex drug delivery systems;
- (2)
- All-in-one manufacturing and small batches of manufacturing;
Challenge
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. The Shape of Things to Come: Emerging Applications of 3D Printing in Healthcare. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 1–19. ISBN 978-3-319-90754-3. [Google Scholar]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Bozkurt, Y.; Karayel, E. 3D Printing Technology; Methods, Biomedical Applications, Future Opportunities and Trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Zastrow, M. The New 3D Printing: Researchers Are Developing Techniques to Print Faster, Bigger and Weirder. Nature 2020, 578, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roopavath, U.K.; Kalaskar, D.M. Introduction to 3D Printing in Medicine. In 3D Printing in Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–20. ISBN 978-0-08-100717-4. [Google Scholar]
- Chen, G.; Wang, X.; Chen, H.; Chen, C. Realization of Rapid Large-Size 3D Printing Based on Full-Color Powder-Based 3DP Technique. Molecules 2020, 25, 2037. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Otero-Espinar, F.J.; Goyanes, Á. 3D Printing of Pharmaceutical Products. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 569–597. ISBN 978-0-12-818411-0. [Google Scholar]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder Jet 3D Printing—Process Parameters, Materials, Properties, Modeling, and Challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Holland, S.; Foster, T.; MacNaughtan, W.; Tuck, C. Design and Characterisation of Food Grade Powders and Inks for Microstructure Control Using 3D Printing. J. Food Eng. 2018, 220, 12–19. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Sanjayan, J. Method of Formulating Geopolymer for 3D Printing for Construction Applications. Mater. Des. 2016, 110, 382–390. [Google Scholar] [CrossRef]
- Oropeza, D.; Roberts, R.; Hart, A.J. A Rapid Development Workflow for Binder Inks for Additive Manufacturing with Application to Polymer and Reactive Binder Ink Formulation. J. Manuf. Process. 2022, 73, 471–482. [Google Scholar] [CrossRef]
- Konta, A.; García-Piña, M.; Serrano, D. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef]
- Guo, Y.; Patanwala, H.S.; Bognet, B.; Ma, A.W.K. Inkjet and Inkjet-Based 3D Printing: Connecting Fluid Properties and Printing Performance. Rapid Prototyp. J. 2017, 23, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Campos, J.C.; Filho, S.C.; Teixeira, M.C.; Martins-Gomes, C.; Zielinska, A.; Carbone, C.; Silva, A.M. 3D Printing in the Design of Pharmaceutical Dosage Forms. Pharm. Dev. Technol. 2019, 24, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised Dosing: Printing a Dose of One’s Own Medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Shirazi, S.F.; Baghayeri, H. Piezo-electric Head Application in a New 3D Printing Design. Rapid Prototyp. J. 2009, 15, 187–191. [Google Scholar] [CrossRef]
- Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, Z.; Charoo, N.A.; Kuttolamadom, M.; Asadi, A.; Khan, M.A. Printing of Personalized Medication Using Binder Jetting 3D Printer. In Precision Medicine for Investigators, Practitioners and Providers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 473–481. ISBN 978-0-12-819178-1. [Google Scholar]
- Holland, S.; Foster, T.; Tuck, C. Creation of Food Structures Through Binder Jetting. In Fundamentals of 3D Food Printing and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–288. ISBN 978-0-12-814564-7. [Google Scholar]
- Zhang, Z.; Jin, Y.; Yin, J.; Xu, C.; Xiong, R.; Christensen, K.; Ringeisen, B.R.; Chrisey, D.B.; Huang, Y. Evaluation of Bioink Printability for Bioprinting Applications. Applied Physics Reviews 2018, 5, 041304. [Google Scholar] [CrossRef]
- Fromm, J.E. Numerical Calculation of the Fluid Dynamics of Drop-on-Demand Jets. IBM J. Res. Dev. 1984, 28, 322–333. [Google Scholar] [CrossRef]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Noguera, R.; Lejeune, M.; Chartier, T. 3D Fine Scale Ceramic Components Formed by Ink-Jet Prototyping Process. J. Eur. Ceram. Soc. 2005, 25, 2055–2059. [Google Scholar] [CrossRef]
- Jang, D.; Kim, D.; Moon, J. Influence of Fluid Physical Properties on Ink-Jet Printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Fang, H.; Ma, Q.; Dong, X. Analysis of Droplet Stability after Ejection from an Inkjet Nozzle. J. Fluid Mech. 2018, 845, 378–391. [Google Scholar] [CrossRef]
- Pardeike, J.; Strohmeier, D.M.; Schrödl, N.; Voura, C.; Gruber, M.; Khinast, J.G.; Zimmer, A. Nanosuspensions as Advanced Printing Ink for Accurate Dosing of Poorly Soluble Drugs in Personalized Medicines. Int. J. Pharm. 2011, 420, 93–100. [Google Scholar] [CrossRef]
- Içten, E.; Giridhar, A.; Taylor, L.S.; Nagy, Z.K.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Melt-Based Dosage Forms. J. Pharm. Sci. 2015, 104, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Wu, D.; Bai, J. Current Status and Prospects of Polymer Powder 3D Printing Technologies. Materials 2020, 13, 2406. [Google Scholar] [CrossRef]
- Chen, Q.; Juste, E.; Lasgorceix, M.; Petit, F.; Leriche, A. Binder Jetting Process with Ceramic Powders: Influence of Powder Properties and Printing Parameters. Open Ceram. 2022, 9, 100218. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle van Der Waals Force in Powder Flowability and Compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Wang, X.; Liu, X. 3D Printing of Silk Powder by Binder Jetting Technique. Addit. Manuf. 2021, 38, 101820. [Google Scholar] [CrossRef]
- Lu, K.; Hiser, M.; Wu, W. Effect of Particle Size on Three Dimensional Printed Mesh Structures. Powder Technol. 2009, 192, 178–183. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Müller, R. Structural and Material Approaches to Bone Tissue Engineering in Powder-Based Three-Dimensional Printing. Acta Biomater. 2011, 7, 907–920. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Roth, C.; Ernstberger, A.; Heuberger, R.; Doebelin, N.; Rudolf von Rohr, P.; Müller, R. Printability of Calcium Phosphate Powders for Three-Dimensional Printing of Tissue Engineering Scaffolds. Acta Biomater. 2012, 8, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Antic, A.; Zhang, J.; Amini, N.; Morton, D.A.V.; Hapgood, K.P. Screening Pharmaceutical Excipient Powders for Use in Commercial 3D Binder Jetting Printers. Advanced Powder Technology 2021, 32, 2469–2483. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Moreland, C. The Effect of Particle Size Distribution on Packing Density. Can. J. Chem. Eng. 1968, 46, 162–167. [Google Scholar] [CrossRef]
- Ziegelmeier, S.; Christou, P.; Wöllecke, F.; Tuck, C.; Goodridge, R.; Hague, R.; Krampe, E.; Wintermantel, E. An Experimental Study into the Effects of Bulk and Flow Behaviour of Laser Sintering Polymer Powders on Resulting Part Properties. J. Mater. Process. Technol. 2015, 215, 239–250. [Google Scholar] [CrossRef]
- Enneti, R.K.; Prough, K.C. Effect of Binder Saturation and Powder Layer Thickness on the Green Strength of the Binder Jet 3D Printing (BJ3DP) WC-12%Co Powders. Int. J. Refract. Met. Hard Mater. 2019, 84, 104991. [Google Scholar] [CrossRef]
- Chen, R.-X.; Wang, Z.-M.; Han, X.-L.; Liu, Z.-C.; Zheng, A.-P. The principle of drop-on-powder 3D printing and its application and challenge in solid preparation. Acta Pharm. Sin. 2020, 55, 2862–2868. [Google Scholar] [CrossRef]
- Wagner, J.J.; Fred Higgs, C. Computation of Hydrodynamic and Capillary Phenomena in Binder Jet Three-Dimensional Printing. J. Tribol. 2021, 143, 051113. [Google Scholar] [CrossRef]
- Wang, Z.N.; Tang, Z.N. Numerical Simulation of Droplet Formation of Piezoelectric Ink-Jet Printing. Adv. Mater. Res. 2010, 174, 191–194. [Google Scholar] [CrossRef]
- Lee, Y.; Nandwana, P.; Simunovic, S. Powder Spreading, Densification, and Part Deformation in Binder Jetting Additive Manufacturing. Prog. Addit. Manuf. 2022, 7, 111–125. [Google Scholar] [CrossRef]
- Maximenko, A.L.; Olumor, I.D.; Maidaniuk, A.P.; Olevsky, E.A. Modeling of Effect of Powder Spreading on Green Body Dimensional Accuracy in Additive Manufacturing by Binder Jetting. Powder Technol. 2021, 385, 60–68. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Y.; Bao, T.; Xu, Y.; Xiao, X.; Jiang, S. Discrete Element Simulation of the Effect of Roller-Spreading Parameters on Powder-Bed Density in Additive Manufacturing. Materials 2020, 13, 2285. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Huang, Y.; Wu, S.; Yang, Y. Binder Jetting Additive Manufacturing: Three-Dimensional Simulation of Micro-Meter Droplet Impact and Penetration into Powder Bed. J. Manuf. Process. 2022, 74, 365–373. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. Binder Jet Printing in Pharmaceutical Manufacturing. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 31, pp. 41–54. ISBN 978-3-319-90754-3. [Google Scholar]
- Tawfeek, H.M.; Hassan, Y.A.; Aldawsari, M.F.; Fayed, M.H. Enhancing the Low Oral Bioavailability of Sulpiride via Fast Orally Disintegrating Tablets: Formulation, Optimization and In Vivo Characterization. Pharmaceuticals 2020, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Mukherjee, R.; Sansare, S.; Halder, A.; Kashi, H.; Ma, A.W.K.; Chaudhuri, B. Impact of Powder-Binder Interactions on 3D Printability of Pharmaceutical Tablets Using Drop Test Methodology. Eur. J. Pharm. Sci. 2021, 160, 105755. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Fang, D.; Sun, H.; Qiao, S.; Pan, W. Exploration and Evaluation of Dynamic Dose-Control Platform for Pediatric Medicine Based on Drop-on-Powder 3D Printing Technology. Int. J. Pharm. 2021, 596, 120201. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Manchanda, A.; Mehta, T.; Ma, A.W.K.; Chaudhuri, B. Formulation Design for Inkjet-Based 3D Printed Tablets. Int. J. Pharm. 2020, 584, 119430. [Google Scholar] [CrossRef]
- Kokott, M.; Lura, A.; Breitkreutz, J.; Wiedey, R. Evaluation of Two Novel Co-Processed Excipients for Direct Compression of Orodispersible Tablets and Mini-Tablets. Eur. J. Pharm. Biopharm. 2021, 168, 122–130. [Google Scholar] [CrossRef]
- Yi, S.; Chen, L. Research Progress and Application Prospect of Preparations Made by 3D Printing. Chin. J. Mod. Appl. Pharm. 2021, 38, 1263–1268. [Google Scholar] [CrossRef]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder Bed 3D-Printing of Highly Loaded Drug Delivery Devices with Hydroxypropyl Cellulose as Solid Binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef]
- O’Donnell, K.P.; Woodward, W.H.H. Dielectric Spectroscopy for the Determination of the Glass Transition Temperature of Pharmaceutical Solid Dispersions. Drug Dev. Ind. Pharm. 2015, 41, 959–968. [Google Scholar] [CrossRef]
- Kozakiewicz-Latała, M.; Nartowski, K.P.; Dominik, A.; Malec, K.; Gołkowska, A.M.; Złocińska, A.; Rusińska, M.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Górniak, A.; et al. Binder Jetting 3D Printing of Challenging Medicines: From Low Dose Tablets to Hydrophobic Molecules. Eur. J. Pharm. Biopharm. 2022, 170, 144–159. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Hong, X.; Han, X.; Liu, B.; Li, X.; Zhang, H.; Gao, J.; Liu, N.; Gao, X.; et al. Taste Masking Study Based on an Electronic Tongue: The Formulation Design of 3D Printed Levetiracetam Instant-Dissolving Tablets. Pharm. Res. 2021, 38, 831–842. [Google Scholar] [CrossRef]
- Kreft, K.; Lavrič, Z.; Stanić, T.; Perhavec, P.; Dreu, R. Influence of the Binder Jetting Process Parameters and Binder Liquid Composition on the Relevant Attributes of 3D-Printed Tablets. Pharmaceutics 2022, 14, 1568. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W.K. Binder-Jet 3D Printing of Indomethacin-Laden Pharmaceutical Dosage Forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Yang, F.; Xu, Y.; Lin, M.-M.; Yu, L.-P.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Oral Disintegrating Patient-Tailored Tablets of Warfarin Sodium Produced by 3D Printing. Drug Dev. Ind. Pharm. 2018, 44, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-X.; Han, X.-L.; Liu, B.-S.; Liu, Y.-B.; Liu, T.; Wang, Z.-M.; Liu, Z.-C.; Zheng, A.-P. Optimization of process parameters of 3D printed clozapine dispersive tablets and establishment of personalized dose model. Acta Pharm. Sin. 2021, 56, 1155–1162. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Ye, X.-C.; Lv, Z.-F.; Chen, Y.-Z. Formulation Optimization of Andrographolide Orally Disintegrating Tablets by 3D Printing Technology. Strait Pharm. J. 2020, 32, 14–17. [Google Scholar]
- Yang, P.; Quan, J. The research situation of sustained−release formulation. J. Pharm. Res. 2017, 36, 44–47. [Google Scholar] [CrossRef]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid Free-Form Fabrication of Drug Delivery Devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of Geometry on Drug Release from 3D Printed Tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Ma, Z.-H.; Zhu, L.-M.; Li, X.-Y.; Yang, X.-L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int J. Pharm. [CrossRef]
- Yu, D.G.; Zhu, L.M.; Branford-White, C.J.; Yang, X.L. Studies on preparation of controlled-release delivery systems with drug gradients using three dimensional printing technique. Chin. Pharm. J. 2006, 41, 1080–1083. [Google Scholar]
- Debnath, S.K.; Debnath, M.; Srivastava, R.; Omri, A. Intervention of 3D Printing in Health Care: Transformation for Sustainable Development. Expert Opin. Drug Deliv. 2021, 18, 1659–1672. [Google Scholar] [CrossRef]
- Stockler, S.; Plecko, B.; Gospe, S.M., Jr.; Coulter-Mackie, M.; Connolly, M.; van Karnebeek, C.; Mercimek-Mahmutoglu, S.; Hartmann, H.; Scharer, G.; Struijs, E.; et al. Pyridoxine dependent epilepsy and antiquitin deficiency: Clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol. Genet. Metab. 2011, 104, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.; Cesario, S. Update on the Treatment of Vitamin B6 Dependent Epilepsies. Expert Rev. Neurother. 2019, 19, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Han, X.; Li, X.; Li, J.; Wang, Z.; Zheng, A. Binder Jet 3D Printing of Compound LEV-PN Dispersible Tablets: An Innovative Approach for Fabricating Drug Systems with Multicompartmental Structures. Pharmaceutics 2021, 13, 1780. [Google Scholar] [CrossRef]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.-B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A Novel Solution to Fabricate 3D Printed Patient-Centred Cardiovascular ‘Polypill’ Architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Vélez, G.; Linsley, C.; Zhu, T.; Wu, W.; Wu, B. Photocurable Bioinks for the 3D Pharming of Combination Therapies. Polymers 2018, 10, 1372. [Google Scholar] [CrossRef] [Green Version]
- Mfoafo, K.A.; Omidian, M.; Bertol, C.D.; Omidi, Y.; Omidian, H. Neonatal and Pediatric Oral Drug Delivery: Hopes and Hurdles. Int. J. Pharm. 2021, 597, 120296. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; Chen, R.; Li, J.; Gao, J.; Zhang, H.; Liu, N.; Gao, X.; Zheng, A. Innovative Color Jet 3D Printing of Levetiracetam Personalized Paediatric Preparations. Asian J. Pharm. Sci. 2021, 16, 374–386. [Google Scholar] [CrossRef]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet Printing of Drug Substances and Use of Porous Substrates-towards Individualized Dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Abu Osman, N.A. Effect of Layer Thickness and Printing Orientation on Mechanical Properties and Dimensional Accuracy of 3D Printed Porous Samples for Bone Tissue Engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lennon, A.; Buchanan, F.; McCarthy, H.O.; Dunne, N. Binder Jetting Additive Manufacturing of Hydroxyapatite Powders: Effects of Adhesives on Geometrical Accuracy and Green Compressive Strength. Addit. Manuf. 2020, 36, 101645. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Li, X.; Wei, Q.; Chai, W.; Wang, S.; Che, Y.; Lu, T.; Zhang, B. 3D Fabrication and Characterization of Phosphoric Acid Scaffold with a HA/β-TCP Weight Ratio of 60:40 for Bone Tissue Engineering Applications. PLoS ONE 2017, 12, e0174870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Banerjee, D.; Robertson, S.; Vahabzadeh, S. Enhanced In Vivo Bone and Blood Vessel Formation by Iron Oxide and Silica Doped 3D Printed Tricalcium Phosphate Scaffolds. Ann. Biomed. Eng. 2018, 46, 1241–1253. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, J.; Han, G.; Kim, D.; Cheon, K.-H.; Lee, H.; Kim, H.-E.; Kim, Y.-J.; Jang, T.-S.; Jung, H.-D. 3D-Printed Biodegradable Composite Scaffolds with Significantly Enhanced Mechanical Properties via the Combination of Binder Jetting and Capillary Rise Infiltration Process. Addit. Manuf. 2021, 41, 101988. [Google Scholar] [CrossRef]
- Tai, B.L.; Kao, Y.-T.; Payne, N.; Zheng, Y.; Chen, L.; Shih, A.J. 3D Printed Composite for Simulating Thermal and Mechanical Responses of the Cortical Bone in Orthopaedic Surgery. Med. Eng. Phys. 2018, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Butscher, A.; Bohner, M.; Doebelin, N.; Hofmann, S.; Müller, R. New Depowdering-Friendly Designs for Three-Dimensional Printing of Calcium Phosphate Bone Substitutes. Acta Biomater. 2013, 9, 9149–9158. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D Printing of Porous Hydroxyapatite Scaffolds Intended for Use in Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Nandi, S.K.; Fielding, G.; Banerjee, D.; Bandyopadhyay, A.; Bose, S. 3D-Printed β-TCP Bone Tissue Engineering Scaffolds: Effects of Chemistry on in Vivo Biological Properties in a Rabbit Tibia Model. J. Mater. Res. 2018, 33, 1939–1947. [Google Scholar] [CrossRef]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Shafiei, S.S.; Mohammadi, S.; Hafezi, M.; Abu Osman, N.A. Structure, Properties, and In Vitro Behavior of Heat-Treated Calcium Sulfate Scaffolds Fabricated by 3D Printing. PLoS ONE 2016, 11, e0151216. [Google Scholar] [CrossRef] [Green Version]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Meißner, R.; Bertol, L.; Rehman, M.A.U.; dos Santos, L.A.L.; Boccaccini, A.R. Bioprinted 3D Calcium Phosphate Scaffolds with Gentamicin Releasing Capability. Ceram. Int. 2019, 45, 7090–7094. [Google Scholar] [CrossRef]
- Chai, W.; Wei, Q.; Yang, M.; Ji, K.; Guo, Y.; Wei, S.; Wang, Y. The Printability of Three Water Based Polymeric Binders and Their Effects on the Properties of 3D Printed Hydroxyapatite Bone Scaffold. Ceram. Int. 2020, 46, 6663–6671. [Google Scholar] [CrossRef]
- Ke, D.; Bose, S. Effects of Pore Distribution and Chemistry on Physical, Mechanical, and Biological Properties of Tricalcium Phosphate Scaffolds by Binder-Jet 3D Printing. Addit. Manuf. 2018, 22, 111–117. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Effect of Chemistry on Osteogenesis and Angiogenesis Towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann. Biomed. Eng. 2017, 45, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D Printing for Enhanced Drug Delivery: Current State-of-the-Art and Challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Lyons, J.G.; Devine, D.M. Additive Manufacturing: Future Challenges. In Polymer-Based Additive Manufacturing; Devine, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 255–264. ISBN 978-3-030-24531-3. [Google Scholar]
- Varghese, R.; Sood, P.; Salvi, S.; Karsiya, J.; Kumar, D. 3D Printing in the Pharmaceutical Sector: Advances and Evidences. Sens. Int. 2022, 3, 100177. [Google Scholar] [CrossRef]
- Mirza, M.A.; Iqbal, Z. 3D Printing in Pharmaceuticals: Regulatory Perspective. Curr. Pharm. Des. 2019, 24, 5081–5083. [Google Scholar] [CrossRef]
- Zheng, F.; Huang, S. Advances in Study on Three-Dimensional Printing in Pharmaceutics. Chin. Herb. Med. 2016, 8, 121–125. [Google Scholar] [CrossRef]
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D Printing of Solid Oral Dosage Forms: Numerous Challenges With Unique Opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef] [PubMed]


| Dosage form | API | Powder | Binder | Reference |
|---|---|---|---|---|
| Instant-Dissolving tablets | Levetiracetam | Microcrystalline cellulose, mannitol, Colloidal silicon dioxide, polyvinyl pyrrolidone | 40% (v/v) isopropanol aqueous solution containing 0.05% (w/w) PVP and 4% (w/w) glycerin | Wang [58] |
| Dispersible tablets | ketoprofen | lactose monohydrate, spray-dried lactose monohydrate, microcrystalline cellulose, mannitol, polyvinyl pyrrolidone grade K 25, silica | Ethanol solution with 10% polyethylene glycol 1500 | Klemen Kreft [59] |
| Fast-Dissolving tablets | Indomethacin | Lactose monohydrate, Kollidon®VA64 (KL) | 5% (w/v) KL in water | Chang [60] |
| Oral disintegrating tablets | Warfarin sodium | D-sucrose, pregelatinized starch, povidone K30, Microcrystalline cellulose, silicon dioxide | 38% (v/v) Ethanol solution | Tian [61] |
| Dispersible tablets | Clozapine | Mannitol, lactose, microcrystalline cellulose, strawberry flavor, Colloidal silicon dioxide | 50% (v/v) Ethanol solution containing 0.3% (w/w) PVP and 4% (w/w) glycerin | CHEN [62] |
| Orally disintegrating tablets | Andrographolide | Sucrose, mannitol, PVP K30, microcrystalline cellulose, aspartame | 30% (v/v) Ethanol solution | HUANG [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, S.; Wu, J.; Duan, S.; Wang, X.; Hong, X.; Han, X.; Li, C.; Kang, D.; Wang, Z.; et al. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics 2022, 14, 2589. https://doi.org/10.3390/pharmaceutics14122589
Chen X, Wang S, Wu J, Duan S, Wang X, Hong X, Han X, Li C, Kang D, Wang Z, et al. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics. 2022; 14(12):2589. https://doi.org/10.3390/pharmaceutics14122589
Chicago/Turabian StyleChen, Xuejun, Shanshan Wang, Jie Wu, Shuwei Duan, Xiaolong Wang, Xiaoxuan Hong, Xiaolu Han, Conghui Li, Dongzhou Kang, Zengming Wang, and et al. 2022. "The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing" Pharmaceutics 14, no. 12: 2589. https://doi.org/10.3390/pharmaceutics14122589
APA StyleChen, X., Wang, S., Wu, J., Duan, S., Wang, X., Hong, X., Han, X., Li, C., Kang, D., Wang, Z., & Zheng, A. (2022). The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics, 14(12), 2589. https://doi.org/10.3390/pharmaceutics14122589




