Design of Reservoirs Enabling Stress-Induced Sequential Release Systems
Abstract
:1. Introduction
2. Methods and Materials
2.1. Finite Element Analysis
2.2. Device Preparation
2.3. Device Replication
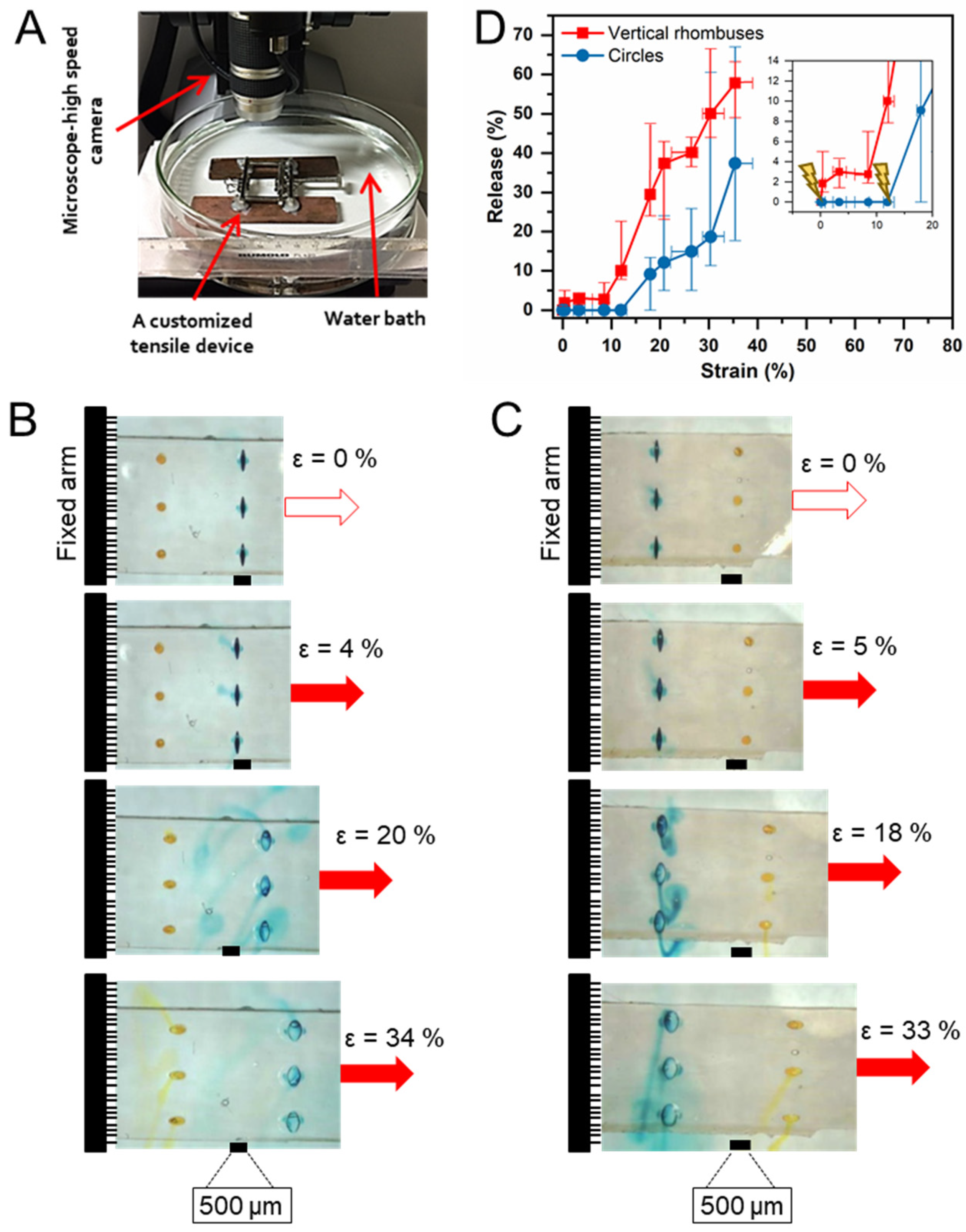
2.4. Device Loading and Coating
2.5. Device Characterization
3. Results and Discussion
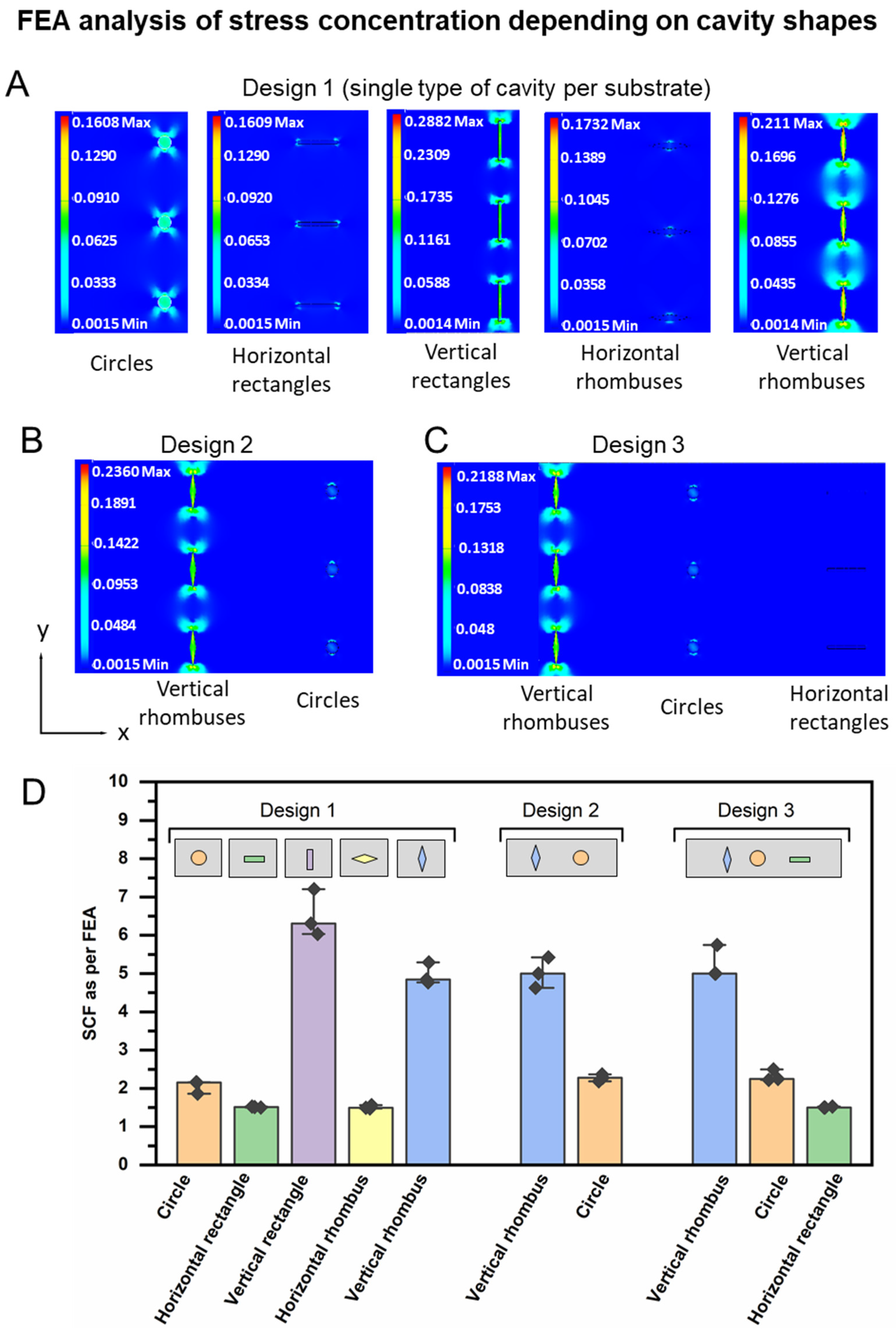
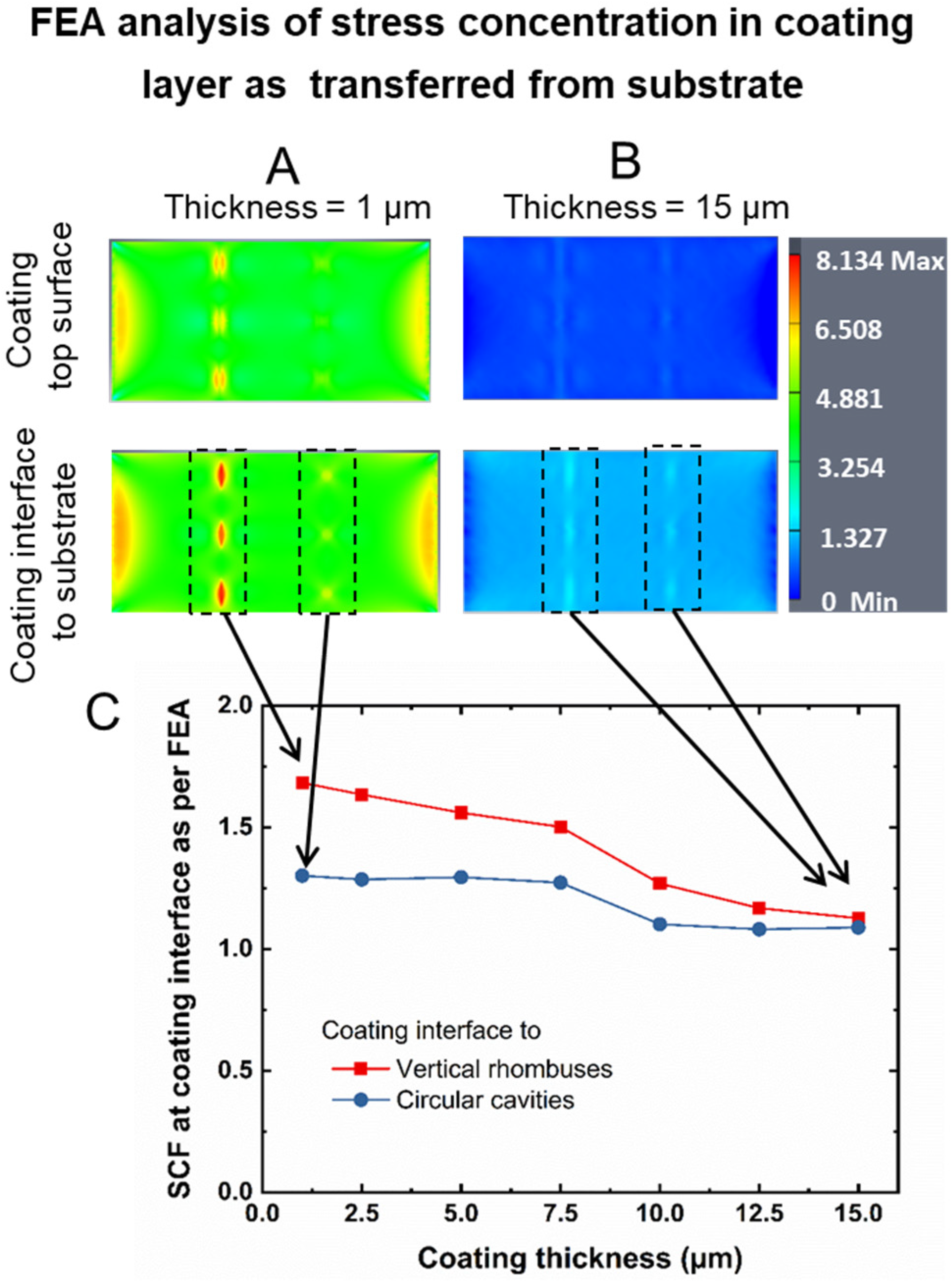
3.1. Device Design and FEA Analysis
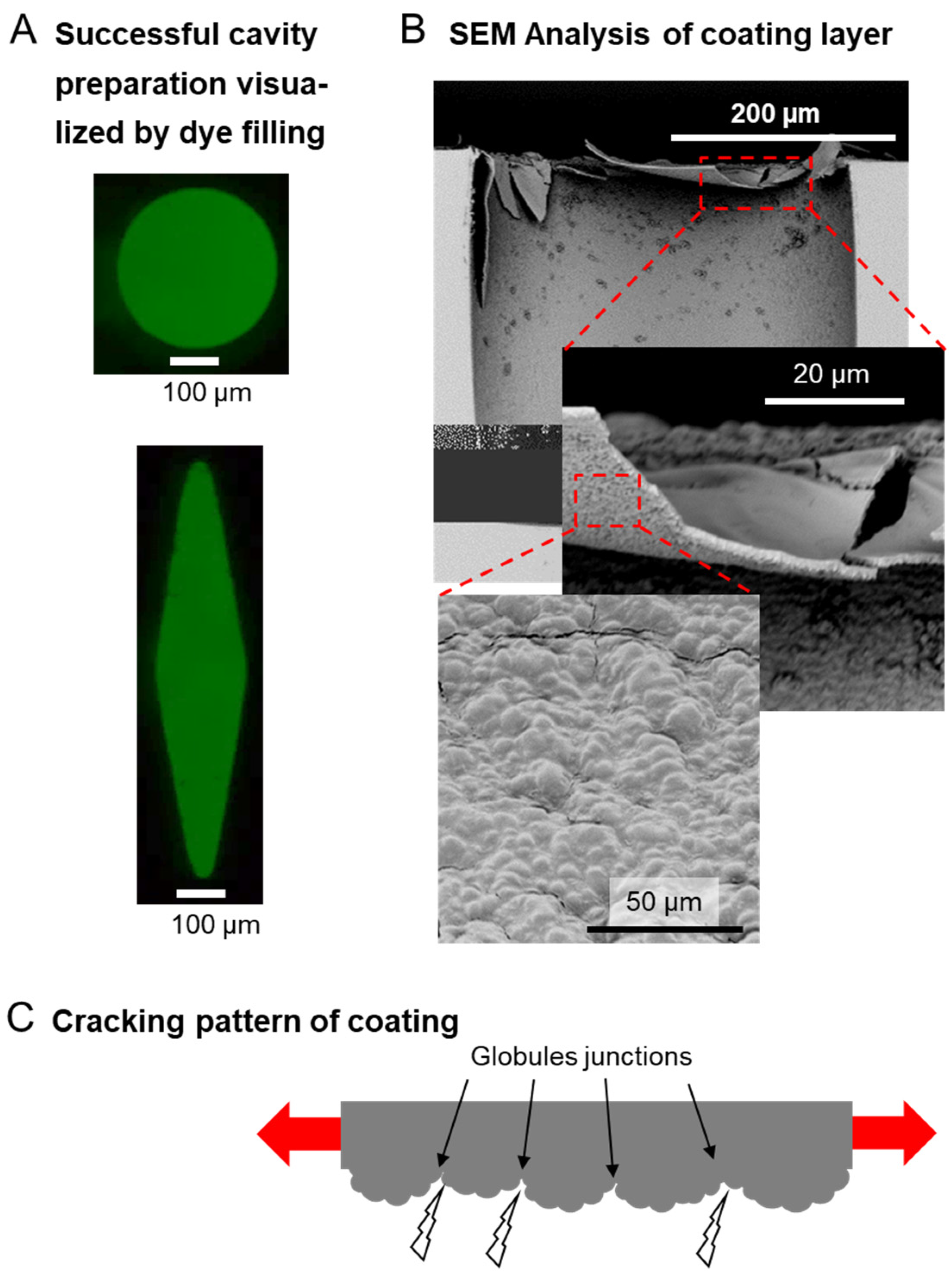
3.2. Device Fabrication and Coating Strategy
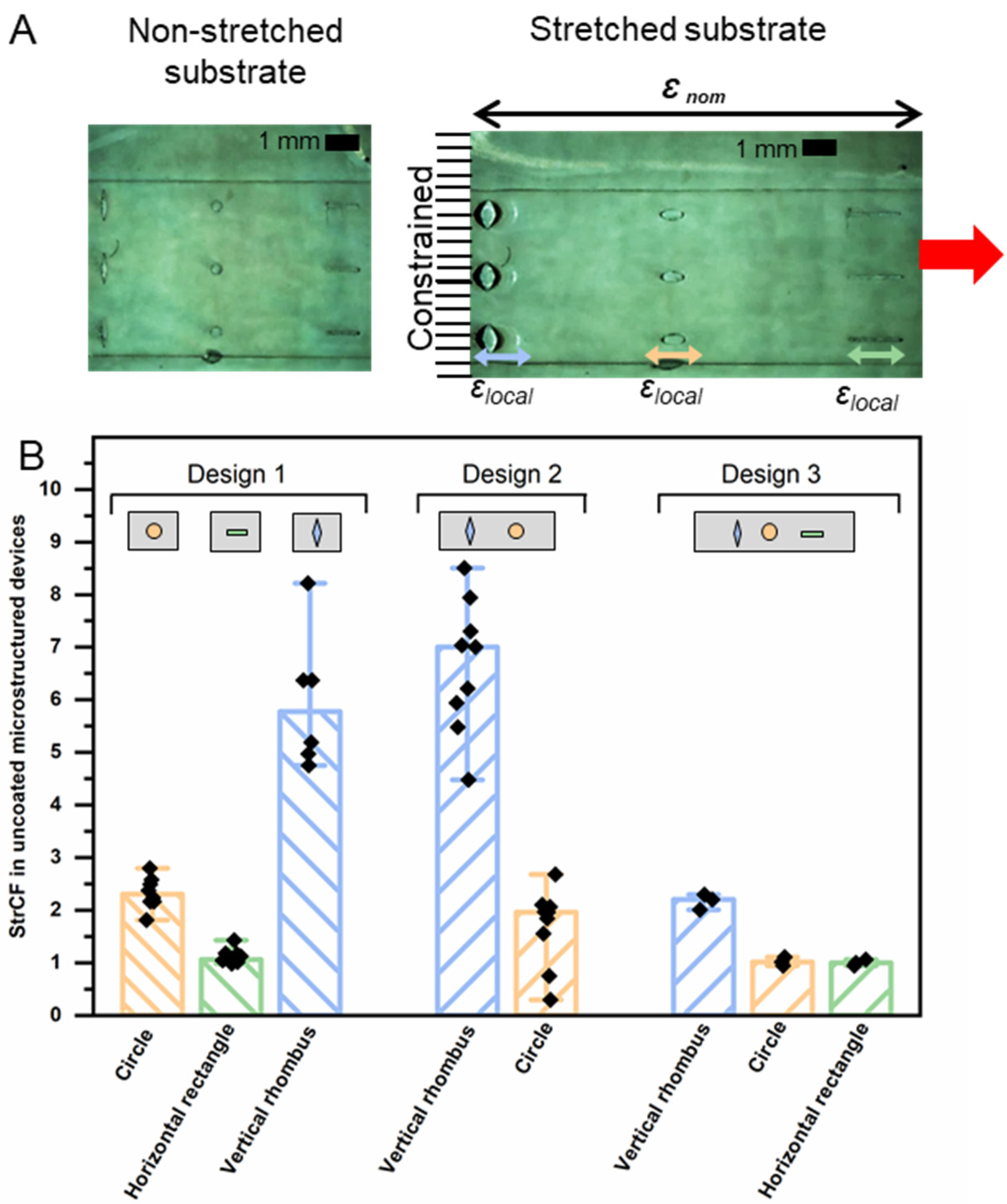
3.3. Functional Evaluation of Sequential Release Capability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calderon-Ospina, C.-A.; Ruiz-Garzon, J.A.; Rojas-Velandia, C.A. Drug therapeutic failures as a cause of admission to an intensive care unit at a university hospital. J. Res. Pharm. Pract. 2019, 8, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Cheng, M.; Goyal, P.; Kim, D.H.; Hummel, S.L.; Dhoble, A.; Deshmukh, A.; Aparasu, R.; Holmes, H.M. Medication Complexity Among Older Adults with HF: How Can We Assess Better? Drugs Aging 2022, 39, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shaidani, S.; Theodossiou, S.K.; Hartzell, E.J.; Kaplan, D.L. Localized, on-demand, sustained drug delivery from biopolymer-based materials. Expert Opin. Drug Deliv. 2022, 19, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Tuncaboylu, D.C.; Wischke, C. Opportunities and Challenges of Switchable Materials for Pharmaceutical Use. Pharmaceutics 2022, 14, 2331. [Google Scholar] [CrossRef] [PubMed]
- Tuncaboylu, D.C.; Friess, F.; Wischke, C.; Lendlein, A. A multifunctional multimaterial system for on-demand protein release. J. Control. Release 2018, 284, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Mazarura, K.R.; Kumar, P.; Choonara, Y.E. Customised 3D printed multi-drug systems: An effective and efficient approach to polypharmacy. Expert Opin. Drug Deliv. 2022, 19, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-H.; Samandari, M.; Li, C.; Li, H.; Song, D.; Zhang, Y.; Tamayol, A.; Wang, X. Multimodal sensing and therapeutic systems for wound healing and management: A review. Sensors Actuators Rep. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef]
- Aschkenasy, C.; Kost, J. On-demand release by ultrasound from osmotically swollen hydrophobic matrices. J. Control. Release 2005, 110, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Abbaspourrad, A.; Chen, D.; Campbell, E.; Zhao, H.; Li, Y.; Li, Q.; Weitz, D.A. Osmotic Pressure Triggered Rapid Release of Encapsulated Enzymes with Enhanced Activity. Adv. Funct. Mater. 2017, 27, 1700975. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical Force-Triggered Drug Delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Kaplan, J.A.; Colson, Y.L.; Grinstaff, M.W. Mechanoresponsive materials for drug delivery: Harnessing forces for controlled release. Adv. Drug Deliv. Rev. 2017, 108, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Lai, X.; Luo, Z.; Chen, Y.; Loh, X.J.; Ye, E.; Li, Z.; Wu, C.; Wu, Y.-L. Recent advances in mechanical force-responsive drug delivery systems. Nanoscale Adv. 2022, 4, 3462–3478. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.; Li, P.-Y.; Saati, S.; Agrawal, R.N.; Humayun, M.S.; Meng, E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 2009, 11, 959–970. [Google Scholar] [CrossRef]
- Kim, B.; Yoo, S.; Kim, Y.-J.; Park, J.; Kang, B.; Haam, S.; Kang, S.-W.; Kang, K.; Jeong, U. A Strain-Regulated, Refillable Elastic Patch for Controlled Release. Adv. Mater. Interfaces 2016, 3, 1500803. [Google Scholar] [CrossRef]
- Di, J.; Yao, S.; Ye, Y.; Cui, Z.; Yu, J.; Ghosh, T.K.; Zhu, Y.; Gu, Z. Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots. ACS Nano 2015, 9, 9407–9415. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Hemmerlé, J.; Mutterer, J.; Ollivier, S.; Voegel, J.-C.; Schaaf, P.; Lavalle, P. Mechanically Responding Nanovalves Based on Polyelectrolyte Multilayers. Nano Lett. 2007, 7, 657–662. [Google Scholar] [CrossRef]
- Mertz, D.; Vogt, C.; Hemmerlé, J.; Mutterer, J.; Ball, V.; Voegel, J.-C.; Schaaf, P.; Lavalle, P. Mechanotransductive surfaces for reversible biocatalysis activation. Nat. Mater. 2009, 8, 731–735. [Google Scholar] [CrossRef]
- Wang, J.; Kaplan, J.A.; Colson, Y.L.; Grinstaff, M.W. Stretch-Induced Drug Delivery from Superhydrophobic Polymer Composites: Use of Crack Propagation Failure Modes for Controlling Release Rates. Angew. Chem. Int. Ed. 2016, 55, 2796–2800. [Google Scholar] [CrossRef] [PubMed]
- Elman, N.M.; Duc, H.L.H.; Cima, M.J. An implantable MEMS drug delivery device for rapid delivery in ambulatory emergency care. Biomed. Microdevices 2009, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Farra, R.; Sheppard, N.F.; McCabe, L.; Neer, R.M.; Anderson, J.M.; Santini, J.T.; Cima, M.J.; Langer, R. First-in-Human Testing of a Wirelessly Controlled Drug Delivery Microchip. Sci. Transl. Med. 2012, 4, 122ra21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, Y. 2—Stress concentration. In Metal Fatigue: Effects of Small Defects and Nonmetallic Inclusions, 2nd ed.; Murakami, Y., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 13–27. [Google Scholar]
- Schijve, J. Fatigue of aircraft materials and structures. Int. J. Fatigue 1994, 16, 21–32. [Google Scholar] [CrossRef]
- Kim, B.C.; Matsuoka, T.; Moraes, C.; Huang, J.; Thouless, M.D.; Takayama, S. Guided fracture of films on soft substrates to create micro/nano-feature arrays with controlled periodicity. Sci. Rep. 2013, 3, srep03027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelung, R.; Aktas, O.C.; Franc, J.; Biswas, A.; Kunz, R.; Elbahri, M.; Kanzow, J.; Schürmann, U.; Faupel, F. Strain-controlled growth of nanowires within thin-film cracks. Nat. Mater. 2004, 3, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ha, D.; Kim, T. Cracking-assisted photolithography for mixed-scale patterning and nanofluidic applications. Nat. Commun. 2015, 6, 6247. [Google Scholar] [CrossRef] [Green Version]
- Fredi, G.; Simon, F.; Sychev, D.; Melnyk, I.; Janke, A.; Scheffler, C.; Zimmerer, C. Bioinspired Polydopamine Coating as an Adhesion Enhancer Between Paraffin Microcapsules and an Epoxy Matrix. ACS Omega 2020, 5, 19639–19653. [Google Scholar] [CrossRef]
- Rezaeepazhand, J.; Jafari, M. Stress concentration in metallic plates with special shaped cutout. Int. J. Mech. Sci. 2010, 52, 96–102. [Google Scholar] [CrossRef]
- Woo, J.-H.; Na, W.-B. Effect of Cutout Orientation on Stress Concentration of Perforated Plates with Various Cutouts and Bluntness. Int. J. Ocean Syst. Eng. 2011, 1, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Tong, W. Cracking and decohesion of a thin Al2O3 film on a ductile Al–5%Mg substrate. Acta Mater. 2005, 53, 477–485. [Google Scholar] [CrossRef]
- Johnston, I.; McCluskey, D.; Tan, C.K.L.; Tracey, M. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromechanics Microengineering 2014, 24, 035017. [Google Scholar] [CrossRef]
- Ding, X.; Liu, J.; Harris, T.A.L. A review of the operating limits in slot die coating processes. AIChE J. 2016, 62, 2508–2524. [Google Scholar] [CrossRef]
- Gleason, K.K. Nanoscale control by chemically vapour-deposited polymers. Nat. Rev. Phys. 2020, 2, 347–364. [Google Scholar] [CrossRef]
- Moritz, P.; Bürger, F.; Wegewitz, L.; Maus-Friedrichs, W. Preparation techniques of thin cyanoacrylate adhesive films for interface analysis. J. Adhes. 2020, 98, 963–978. [Google Scholar] [CrossRef]
- Wischke, C.; Weigel, J.; Neffe, A.T.; Lendlein, A. Understanding Instability and Rupture of Poly(Alkyl-2-Cyanoacrylate) Capsules. Int. J. Artif. Organs 2011, 34, 243–248. [Google Scholar] [CrossRef]
- Spagnoli, A.; Terzano, M.; Brighenti, R.; Artoni, F.; Carpinteri, A. How Soft Polymers Cope with Cracks and Notches. Appl. Sci. 2019, 9, 1086. [Google Scholar] [CrossRef] [Green Version]
- Ebara, M.; Hoffman, J.M.; Hoffman, A.S.; Stayton, P.S. Switchable surface traps for injectable bead-based chromatography in PDMS microfluidic channels. Lab Chip 2006, 6, 843–848. [Google Scholar] [CrossRef]
- Civjan, S.; Margetis, P.M.; Reddick, R.L. Properties of n-butyl-α-cyanoacrylate mixtures. J. Dental. Res. 1969, 48, 536–542. [Google Scholar] [CrossRef]
- Yokoyama, T. Experimental determination of impact tensile properties of adhesive butt joints with the split Hopkinson bar. J. Strain Anal. Eng. Des. 2003, 38, 233–245. [Google Scholar]
- Altabal, O.; Wischke, C. Analyzing the mechanical properties of free-standing PACA thin films using microindentation technique. Polymers 2022, 14, 4863. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altabal, O.; Wischke, C.; Lendlein, A. Design of Reservoirs Enabling Stress-Induced Sequential Release Systems. Pharmaceutics 2022, 14, 2611. https://doi.org/10.3390/pharmaceutics14122611
Altabal O, Wischke C, Lendlein A. Design of Reservoirs Enabling Stress-Induced Sequential Release Systems. Pharmaceutics. 2022; 14(12):2611. https://doi.org/10.3390/pharmaceutics14122611
Chicago/Turabian StyleAltabal, Osamah, Christian Wischke, and Andreas Lendlein. 2022. "Design of Reservoirs Enabling Stress-Induced Sequential Release Systems" Pharmaceutics 14, no. 12: 2611. https://doi.org/10.3390/pharmaceutics14122611






