1. Introduction
CUR is a hydrophobic polyphenol without nutraceutical value, and is used as a food additive [
1]. According to the US Food and Drug Administration (FDA), 95%
w/w curcuminoids (comprising CUR, bisdemethoxycurcumin, and demethoxycurcumin) at doses up to 12 g/day have the status of “Generally Recognized as Safe” (GRAS) [
2]. CUR, on the other hand, has been known from ancient times to act against a variety of pathological conditions; its use against cancer, inflammatory, neurological, cardiovascular, and skin diseases is well documented in current academic settings. The exploitation of such therapeutic potential is hampered, however, by CUR’s structural lability to environmental conditions (photodegradation), even in aqueous media at physiological pH [
3], its poor oral absorption [
4], and its fast degradation in blood (administered by oral route, its inert conjugates are rapidly excreted, and systemically is transformed into hydro derivatives while some evidence indicates its degradation leads to products with useful biological activity [
5]. Today, there is a uniform consensus about the inclusion of CUR into nanoparticles of variable chemical, size, and surface nature, as an efficient strategy to protect CUR’s structure, increase its bioavailability, and modulate its pharmacokinetics [
6]. In other words, CUR-based nanomedicines may enable, in the future, the pharmaceutical use of CUR.
Photosensitization is part of the pleiotropic activity of curcuminoids [
7,
8,
9]. The photodynamic mechanism utilizes a non-toxic chromophore, known as a photosensitizer (PS), which is excited from its ground singlet state (S
0) to an excited singlet state (S
n) with appropriated wavelength (typically from the visible to near infrared spectrum). Afterwards, the PS can lose energy by emitting fluorescence or heat via internal conversion, thus returning to S
0, or it can be transformed to a longer living excited triplet state PS (T
1) by so-called inter-system crossing. From this state, it can return to its S
0 either by phosphorescence emission or by two mechanisms generating ROS, as follows: in type I mechanisms, a charge (i.e., electrons) is transferred to surrounding substrates leading to formation of superoxide radicals (O2
•−) that can undergo dismutation into hydrogen peroxide (H
2O
2), the precursor of the highly reactive free hydroxyl radicals (HO
•−), which are formed via Fenton-like reactions. In contrast, in type II mechanisms, energy (but no charge) is transferred directly to the ground state molecular oxygen (
3O
2), originating energized molecular oxygen, known as singlet oxygen (1O
2) [
7,
10,
11].
However, CUR is not excitable with light from the first therapeutic window at 650–850 nm, capable of penetrating several centimeters within biological tissues [NIR radiation] [
12], since its absorption spectra display an intense peak in the blue zone at 420 nm [
13,
14], coincident with the Soret band at 425 nm from blood porphyrins [
15]. CUR- mediated PDT, therefore, is of limited therapeutic application: ~1% of 450 nm light reaches a depth of around 1.6 mm; 1% of wavelength higher than 650 nm light instead, penetrates about 4.75–5 mm [
16,
17]. Because of these reasons, CUR-mediated PDT is restricted to the treatment of superficial targets, such as skin infections [
18]; and was recently proposed as food preservation technology [
19]. Even superficial tumors on the skin display a three-dimensional morphology and their volumes penetrate several microns and up to millimeters below the epithelia [
20]. In such settings, the fact that blue light is absorbed by the microvascular bed from subcutaneous tissue in the skin and does not efficiently penetrate beyond the stratum corneum reduces the efficiency of PDT to treat skin tumors. CUR-mediated PDT, however, may be used to treat epithelial tumors where light absorption across the skin is not a limiting factor [
21]. Lung epithelial tumors lack stratum corneum, and the tumor cells are relatively exposed to the light of the respiratory tract. PDT is an approved therapy for the ablation of endobronchial tumors [
22]. In such treatments, the photosensitizer is intravenously administered to directly contact the intima (one main effect of PDT is the damage of the vasculature that feeds the tumor) and then diffuse or extravasate into the tumor microenvironment. The design of nebulized formulations of CUR for antitumoral PDT has recently been addressed by Bakowski [
23]. Nebulized formulations of CUR should be able to act on tumor cells, their microenvironment, and be available for vasculature when delivered from the outer side of the vessels.
Our group has previously reported the performance of nATC, nebulized nanovesicles solubilizing ~600 µM CUR, prepared with polar lipids from the archaea
H. tebenquichense, naturally targeted to cells expressing the SRA1. The loading of considerable amounts of CUR (150 μg CUR/15 mg lipids/mL) required the addition of Tween 80 to the lipid bilayers. By SAXS and Raman, it was determined that CUR, surrounded by Tween 80 molecules, experienced a tight immobilization interacting with the terminal side of diether archaeolipid chains of high microviscosity and low lateral mobility. Such interaction was absent in liposomes, which slowly released CUR along storage. nATC resulted not only in being structurally stable in front of nebulization stress, but importantly, also against dilution and storage for months. Nebulized at 5 μg CUR/mL on an inflamed air–liquid interface made of epithelial A549 cells (a cell line used as non-small lung tumor cells (NSLTC) model), nATC increased TEER, normalized the permeation of Lucifer Yellow, and decreased the release of IL-6, TNF-α, and IL-8 [
24]. In other words, nATC magnified the anti-inflammatory activity of CUR and repaired the epithelial damage (this last effect is exclusively owed to the archaeolipid matrix) without inducing cytotoxicity. The effect of PDT on photosensitizers delivered on the surface of vessels and of tumor microenvironment, is currently unknown. To the best of our knowledge, this is the first report exploring the PDT of CUR in archaeosomes made of diether lipids, upon being endocytosed by A549 cells, by THP-1 monocytes derived macrophages and on a superficial vasculature, modeled with a chick embryo CAM.
2. Materials and Methods
2.1. Materials
CUR, cholesterol (chol), 3-(4,5-dimetiltiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), phorbol 12-13-acetate (PMA), 2-mercaptoethanol, trizma base, Tween 80, Tween 20, Triton X-100, sodium dodecyl sulfate (SDS), and ammonium persulfate were from Sigma-Aldrich (St. Louis, MO, USA). 1,2-hydrogenated-L-α-phosphatidylcholine (HSPC) was from Northern Lipids Inc. (Vancouver, BC, Canada). Roswell Park Memorial Institute medium (RPMI), penicillin–streptomycin sulphate, glutamine, sodium pyruvate, and trypsin/ethylenediamine tetraacetic acid were from Thermo Fisher Scientific (Waltham, MA, USA). Acrylamide, bis-acrylamide, and TEMED were obtained from Bio-Rad Laboratories (Hercules, CA, USA). Fetal bovine serum (FBS) was from Internegocios (Buenos Aires, Argentina). Yeast extract, Griess Reagent Solution “A” and Griess Reagent Solution “B” were from Laboratorios Britania S.A. (Buenos Aires, Argentina). The other reagents were of analytic grade from Anedra, Research AG (Buenos Aires, Argentina).
2.2. Archaebacteria Growth, Extraction, and Characterization of Total Polar Archaeolipids (TPA)
H. tebenquichense archaea, isolated from soil samples of Salina Chica, Peninsula de Valdés, Chubut, Argentina were grown in basal medium supplemented with yeast extract and glucose [
25] in a 25-L homemade stainless-steel bioreactor at 40 °C and harvested 72 h after growth. TPA were extracted from biomass using the Bligh and Dyer method modified for extreme halophiles [
26]. Around 700 mg TPA were isolated from each culture batch. The reproducibility of each TPA extract composition was routinely screened by phosphate content [
27] and electrospray–ionization mass spectrometry [
28].
2.3. Preparation of CUR Nanovesicles
Conventional nanovesicles containing Tween 80 (HSPC: chol: Tween 80 1:0.33:0.53
w:
w, nLT), nLT loaded with CUR (HSPC: chol: Tween 80: CUR at 1:0.33:0.53:0.053
w:
w, nLTC), TPA-nanovesicles containing Tween 80 (TPA: Tween 80, 1:0.4
w:
w, nAT) and nAT loaded with CUR (TPA: Tween 80:CUR at 1:0.4:0.04
w:
w, nATC) were prepared by the film hydration method, and were homogenized by sonication and extrusion as described before [
24]. The resulting nanovesicles were sterilized by passage through a 0.22 µm sterile filter and stored at 4 °C protected from light.
2.4. Structural Characterization of CUR Nanovesicles
Phospholipids were quantified by a colorimetric phosphate microassay [
27]. CUR was quantified by absorbance at 425 nm upon complete disruption of one volume of nanovesicles suspension in 150 volumes of methanol [
24]. Size and ζ potential were determined by dynamic light scattering and phase analysis light scattering, respectively, using a nanoZsizer apparatus (Malvern Instruments, Malvern, UK).
2.5. LED Irradiation Device
A homemade LED irradiation device with a wavelength of 420 nm was used at a power of 30 mW/cm
2 (
Figure S1). The cell culture plates or samples were fit into the device at a distance of 10 cm from the LED. Radiation fluence was calculated as a function of radiation time.
2.6. Cell Lines and Culture Conditions
Human epithelial lung cell line A549 (ATCC® CCL-185™) was maintained in RPMI supplemented with 10 % FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine. The human monocyte cell line THP-1 (ATCC TIB-202™) was maintained in RPMI supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol and 1 mM sodium pyruvate. THP-1 monocytes were differentiated into macrophages by treatment with 100 ng/mL PMA for 24 h. All cell lines were grown in a humidified atmosphere of 5% CO2 at 37 °C.
2.7. Fluorescence, Absorbance Spectra, and Induction of ROS and Reactive Nitrogen Species (RNS)
Fluorescence emission measurements λex = 425 nm and absorption measurements of free CUR and CUR nanovesicles at 40 μM in RPMI supplemented with 5% SFB before and after samples irradiation (fluence of 9 J/cm2) were obtained with Cytation™ 5 (BioTek Instruments, Inc., Winooski, VT, USA).
For measuring extracellular ROS under cell-free conditions, 1 mM of the general oxidative stress indicator chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA), (Thermo Fisher Scientific, Waltham, MA, USA) was deacetylated with 0.01 M NaOH at 37 °C for 30 min protected from light [
29]. Free CUR or CUR nanovesicles were diluted to 20 and 40 μM in a 5 μM solution of CM-H2DCF in Tris buffer pH 7.4. After 30 min incubation fluorescence intensity was measured at λex = 490 nm and λem = 530 nm in a Cytation™ 5. A positive control of ROS production was carried out by incubating 400 μM of hydrogen peroxide with the fluorescent probe.
ROS or RNS generation by A549 cells after PDT was measured using CM-H2DCFDA dye and Griess method, respectively. Briefly, A549 cells were seeded in 48-well plates with a density of 2 × 104 cells per well and grown for 24 h. Then, cells were incubated with nATC, nLTC or free CUR at concentrations of 20 and 40 μM in RPMI supplemented with 5% FBS. Cells were incubated at 37 °C to allow nanovesicles uptake, then plates were irradiated with a fluence of 9 J/cm2 or were incubated in the dark (non-irradiated). After 24 h incubation at 37 °C in a 5% CO2 atmosphere, supernatants were collected for RNS quantification and cells were washed with PBS. ROS was quantified in culture cells by adding 4.4 μg/mL CM-H2DCFDA dye in PBS. After 30 min incubation at 37 °C in a 5% CO2 atmosphere, cells were washed. Fluorescence intensity at λex = 492 nm and λem = 517 nm was determined in a Cytation™ 5. A positive control of ROS production was carried out by incubating cells with 400 μM of hydrogen peroxide. RNS was quantified in culture supernatants after precipitating cells and debris, by adding 1 volume of Griess Reactive Solution “A” and 1 volume of Griess Reactive Solution “B”. After 15 min incubation protected from light, the absorbance was measured at 525 nm in a Cytation™ 5 instrument. RNS concentrations were calculated by comparison with absorbance at 525 nm of standard calibration solutions made with sodium nitrite from 2.5 to 30 μM.
2.8. Cytotoxicity of CUR Nanovesicles upon Irradiation
Cell viability upon 24 h of incubation with nanovesicles was measured by the MTT assay. Briefly, A549 cells were seeded in 96-well plates with a density of 1 × 104 cells per well and grown for 24 h. Then, cells were incubated with CUR nanovesicles or free CUR, (prepared from a stock of 13,6 μM CUR in dimethyl sulfoxide (DMSO)), at concentrations of 0.625, 1.25, 2.5, 5, 10, 20, and 40 μM of CUR in RPMI supplemented with 5% FBS. DMSO at 0.3% v/v and empty nanovesicles at total lipid concentrations of 0.25, 0.5, 1, and 3 mg/mL were also tested. Cells were incubated at 37 °C for 1 h to allow nanovesicle cellular uptake and then plates were irradiated for 1, 5, or 10 min, corresponding to a fluence of 1.8, 9, and 18 J/cm2, respectively. For the non-irradiated control, plates were incubated without irradiation. For washing the control, after 1 h incubation with samples, cell supernatants were removed, washed twice with PBS and plates were irradiated with a fluence of 9 J/cm2. After irradiation, cells were incubated for 24 h at 37 °C in a 5% CO2 atmosphere, then the medium was removed, cells were washed with PBS and 100 µL of 5 mg/mL MTT solution were added to each well. After 2 h incubation at 37 °C, MTT solution was removed, the insoluble formazan crystals were dissolved in DMSO, and absorbance was measured at 570 nm using Cytation™ 5. The cell viability was expressed as a percentage of cells untreated and IC50 values were determined for each condition applying a non-linear regression model of dose-response curve, (Inhibitor) vs. normalized response with GraphPad Prism 8.0.1 (Graph Pad Software, Inc., San Diego, CA, USA).
2.9. ΔΨm
The effect of CUR nanovesicles on ΔΨm of A549 was determined using JC-1 dye mitochondria staining kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer guidelines. Briefly, A549 cells were seeded in 48-well plates with a density of 2 × 104 cells per well and grown for 24 h. Then, cells were incubated with nATC, nLTC or free CUR at concentrations of 5, 10, 20, and 40 μM in RPMI supplemented with 5% FBS. Cells were incubated at 37 °C to allow nanovesicles uptake, then plates were irradiated with a fluence of 9 J/cm2 or incubated in the dark (non-irradiated). After 24 h incubation, supernatants were discarded, cells were washed with PBS and incubated with 2.5 μg/mL JC-1 staining mixture for 15 min at 37 °C. Upon supernatant removal and PBS washing, the fluorescence intensity of each well was measured in Cytation™ 5. The fluorescence of JC-1 monomers was determined at λex = 490 nm and λem = 530 nm and JC-1 aggregates at λex = 525 nm and λem = 590 nm. The positive control was carried out by incubating cells with a solution of 100 ng/mL of valinomycin.
2.10. Apoptosis/Necrosis Assays
Induction of apoptosis/necrosis on A549 cells by PDT was determined by staining with Annexin V, propidium iodide (PI) or YO-PRO™-1 dyes (Thermo Fisher Scientific, Waltham, MA, USA). A549 cells were seeded at a density of 3.2 × 105 cells per well in 12-well plates and grown for 24 h. Then, cells were incubated with CUR nanovesicles or free CUR at concentrations of 20 and 40 μM in RPMI supplemented with 5% FBS for 1 h at 37 °C and cells were irradiated with a fluence of 9 J/cm2 and incubated for 1, 6 or 24 h. For Annexin V/PI staining, after 1 and 6 h incubation, cells were trypsinized, washed with PBS, and resuspended in 20 μL of binding buffer. Then, cells were incubated with fluorescein-conjugated Annexin V reagent for 15 min at room temperature in the dark. Thereafter, cells were washed and resuspended on binding buffer with PI for 15 min at 4 °C. A total of 1 × 104 cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San José, CA, USA) and data analysis was performed with the FlowJo 10.8.0 software (Flowjo, LLC, Ashland, OR, USA). Annexin V-positive cells were considered apoptotic, whereas Annexin V and PI-positive cells were considered necrotic. For YO-PRO™-1 and PI stanning, after 24 h incubation, cell supernatants were discarded, washed, and incubated at 0.5 μM YO-PRO™-1 and incubated for 15 min at room temperature. After that, PI was added to each well and incubated for 15 min at 4 °C and washed with PBS. Fluorescence microscopies were made with Cytation™ 5. YO-PRO™-1 positive cells were considered apoptotic, whereas YO-PRO™-1 and PI-positive cells were considered necrotic.
2.11. Intracellular ATP Content
The intracellular ATP content of A549 cells after PDT was determined with CellTiter-Glo
® Luminescent Cell Viability Assay (Promega, Madison, WI, USA) according to manufacturer guidelines. Briefly, A549 cells were seeded and incubated with nATC, nLTC, or free CUR as stated in
Section 2.9. After 24 h incubation at 37 °C in a 5% CO
2 atmosphere, supernatants were discarded, and cells were washed with PBS and incubated with fresh medium for 30 min at room temperature. Then, one volume of CellTiter-Glo
® reactive was added to cell media in each well, stirred for 2 min in an orbital shaker, and incubated for 10 min at room temperature until signal stabilization. The luminescence of each well was measured in a Cytation™ 5.
2.12. Lactate Dehydrogenase (LDH) Leakage
The LDH release on A549 cells supernatant after PDT was determined with CytoTox 96
® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA), according to manufacturer guidelines. Briefly, A549 cells were seeded and incubated with nATC, nLTC, or free CUR as stated in
Section 2.8. Cells were incubated at 37 °C to allow nanovesicles uptake, then plates were irradiated with a fluence of 9 J/cm
2 or incubated in the dark (non-irradiated). After 24 h incubation at 37 °C in a 5% CO
2 atmosphere an aliquot of 35 μL supernatants was transferred to fresh 96-well plates, 35 μL of the CytoTox 96
® Reagent were added to each well and incubated for 30 min at room temperature. The reaction was terminated by adding 35 μL of Stop solution. The absorbance was measured at λ 490 nm in Cytation™ 5.
2.13. CUR Cellular Uptake and Lysosomal Damage
CUR cellular uptake was measured by fluorescence confocal microscopy. LysoTracker™ Red DND-99 (LR), (Thermo Fisher Scientific, Waltham, MA, USA), a fluorescent probe that accumulates in acidic compartments such as lysosomes, was used as an indicator of lysosomal health, as well as a marker to study CUR colocalization in lysosomes. A549 cells were seeded at a density of 1.2 × 10
5 cells per well in 12-well plates with rounded cover slips on the bottom and allowed to attach overnight. Then, cells were incubated with CUR nanovesicles or free CUR at concentrations of 40 μM in RPMI supplemented with 5% FBS for 1 h or 6 h at 37 °C. For LR assays samples were incubated for 1 h, irradiated with a fluence of 9 J/cm
2, and incubated for an additional 5 h. Then supernatants were discarded, cells were washed with PBS, and incubated with 3.8 µM LR for 30 min at 37 °C for endosomal–lysosomal staining. Afterward cells were washed and fixed for 5 min with formaldehyde 3.75% (
w/v) in PBS. For cellular uptake determinations, samples were incubated for 1 h at 37 °C, washed with PBS and cells were fixed. Finally, preparations were washed three times in PBS and mounted in a 90% (
w/v) glycerol solution. Fluorescence microscopies were made with a Leica laser-scanning spectral confocal microscope TCS SP8; (Leica Microsystem, Wetzlar, Germany). Pearson’s correlation coefficient (Rr), which describes the correlation of intensity distribution between channels, was calculated using Fiji Software [
30]. The degree of colocalization was determined from Rr values between −0.2–0.09 (weak colocalization), 0.1–0.48 (moderate colocalization), and 0.49–0.84 (strong colocalization) [
31].
2.14. Tumor-Associated Macrophages (TAM) Modulation
TAM-like macrophages were generated by cultivating THP-1 macrophages with A549 cell culture supernatant [
32]. THP-1 cells were seeded in 24-well plates with a density of 6 × 10
4 cells per well in RPMI supplemented with 10% FBS, pyruvate sodium 1% (
w/v), 100 ng/mL of PMA, and incubated for 24 h to allow macrophage transformation. A549 cells were seeded at a density of 2 × 10
5 cells per well in 48-well plates and grown for 24 h. Then, A549 cells were incubated with CUR nanovesicles or free CUR at concentrations of 5 μM and 40 μM for 1 h at 37 °C and were irradiated with a fluence of 9 J/cm
2 or left in the dark (non-irradiated), and incubated for additional 24 h. Then 250 μL of A549 supernatants were transferred to each THP-1 well and 50 μL of fresh RPMI medium was added. After 24 h, the supernatants were stored at −20 °C for cytokine determination. THP-1 cells were washed with PBS, trypsinized, washed again, and labeled with the CD204-APC antibody (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min, except for the control cells. Cells were then washed with PBS and fixed with formaldehyde 3.75% (
w/v) in PBS for 10 min at 4 °C. A total of 2 × 10
4 cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San José, CA, USA), and data analysis was performed with FlowJo software. Human IL-8 and IL-6 concentrations were measured by enzyme-linked immunosorbent assay BD OptEIA™ (BD Biosciences, San Jose, CA, USA) following the manufacturer’s instructions. Absorbance measurements were carried out at 450 nm on a microplate reader in a microplate reader.
2.15. Migration Assay
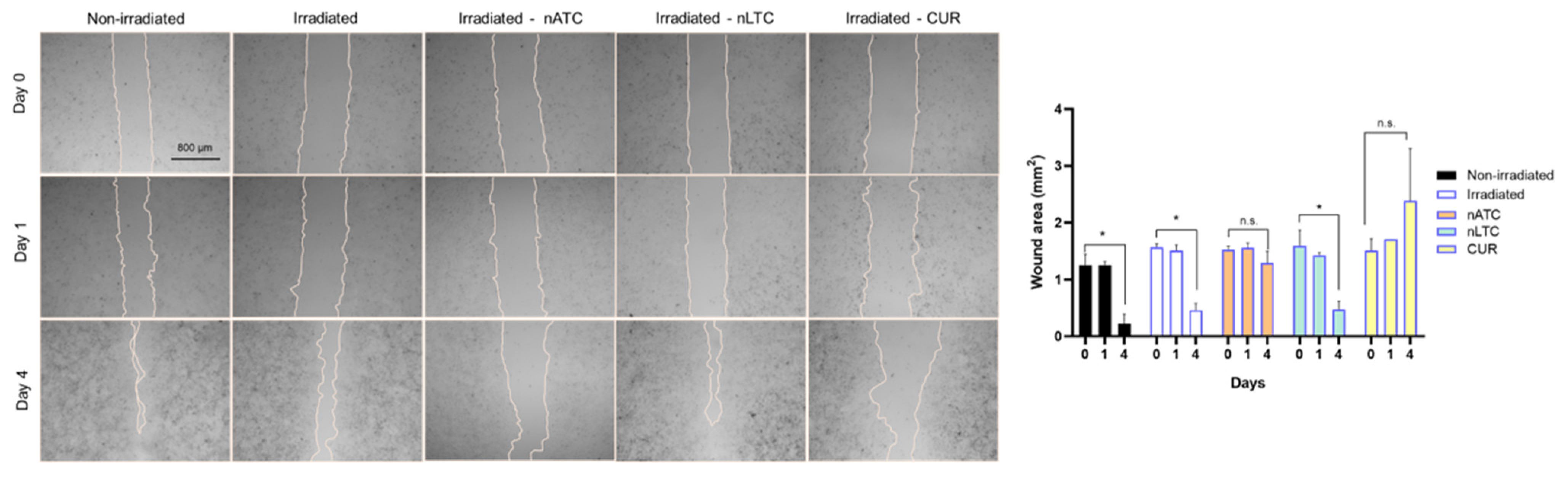
A549 cells were seeded at a density of 3.2 × 105 cells per well in 12-well plates and grown for 48 h to complete confluence in RPMI supplemented with 10% FBS. Scratch wounds were created in confluent monolayers using a sterile p200 pipette tip. Following washing, cells were cultured in RPMI without SFB and incubated with CUR nanovesicles or free CUR at concentrations of 5 μM. After that, cells were irradiated with a fluence of 9 J/cm2 and incubated at 37 °C. After 24 h incubation, cells were washed with PBS and incubated with RPMI without SFB for an additional 72 h. Repopulation of the wounded areas was observed under phase-contrast microscopy with Cytation™ 5. The size of the scratch wound area was determined at 0, 24, and 96 h using Fiji Software.
2.16. MMP Activity
The effect of nanovesicles on MMP activity was determined using the MMP-substrate fluorogenic peptide FS-6 (Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2) (Sigma-Aldrich, St. Louis, MO, USA) according to a method previously described [
33]. Briefly, A549 cells were seeded and incubated with nanovesicles as stated in
Section 2.9. After 24 h incubation, 72 μL of cell supernatant was mixed with 8 μL of FS-6 (50 μM) in a 96-well black plate. Fluorescence was followed every 30 min for 6 h at λex = 320 nm and λem = 405 nm in a Cytation™ 5. MMP activity was expressed as the relative fluorescence units (RFU) emitted per hour.
The gelatinolytic activity of MMP-2 and MMP-9 was analyzed by gelatin zymography [
34]. Briefly, A549 cells were seeded and incubated with CUR nanovesicles and free CUR as stated in
Section 2.9. After 24 h incubation, cell supernatants were collected, concentrated three times by Speed Vac System AES 1010 (Savant, GMI, Inc., Ramsey, MN, USA), and stored at −80 °C until use. Gels were prepared with 10% (
w/v) polyacrylamide containing 0.1% (
m/v) SDS and 0.2 mg/mL gelatin from porcine skin type A (Sigma-Aldrich, St. Louis, MO, USA). On each electrophoretic lane, the same amount of total protein supernatants (1000 μg each) was electrophoresed. Electrophoresis was performed using running buffer (0.025 M Tris/0.192 glycine pH 8.3 containing 0.1%
w/v SDS) at a constant voltage of 120 V. The gels were washed for 1 h in washing buffer (50 mM Tris-HCl, pH 7.4; 5 mM CaCl
2; 1 μM ZnCl
2 and 2.5%
w/v Triton-X 100) to remove SDS. Then, gels were incubated for 48 h at 37 °C in incubation buffer (50 mM Tris-HCl, pH 7.4, 5 mM CaCl
2, 1 μM ZnCl
2, 1%
w/v Triton-X100). After that, gels were stained for 48 h with 0.5% (
w/v) Coomassie Brilliant Blue R250 to visualize bands of proteolytic activity. Gels were washed out in 5% acetic acid and 10% methanol. Sites of gelatinolytic activity were detectable as clear bands against a background of uniform staining. The intensity of the gelatinolytic bands was measured with Fiji Software. The bands of pro- and active MMP-2 were detected at 72 kDa and 62 kDa and those of pro and active MMP-9 at 92 kDa and 82 kDa, respectively.
2.17. Vascular Damage Assay on the CAM
The chicken CAM was used as a model to determine vascular damage produced by photodynamic therapy. Fertilized, specific pathogen-free White Leghorn eggs were supplied by Instituto Rosenbusch S.A. (Buenos Aires, Argentina) on incubation day 7. Undamaged eggs were incubated at 38.0 ± 0.1 °C in a relative humidity of 65 ± 2% and under automatic rotation in an egg incubator (A.Dami Incubadoras, Buenos Aires, Argentina). Between three and six eggs weighing 45–75 g were used to study each condition.
On days 8–9, the air chamber of the eggs was marked and a perforation of 15 mm was made on the shell, then, the internal white membrane was exposed, moistened with 0.9% (w/v) NaCl, and removed. A picture of the CAM (time 0) was obtained with a Leica S8APO stereoscopic magnifying glass and recorded with a Leica DMC2900 camera (Leica Microsystem, Wetzlar, Germany). Then, 30 μL of 2 mg/mL solution of mucins from porcine stomach type II (Sigma-Aldrich, St. Louis, MO, USA) was applied on the CAM, immediately after 300 μL of nATC, nLTC, nAT, or nLT at CUR 40 μM and 1 mg/mL of total lipids or PBS were applied over the mucin layer and irradiated with a fluence of 9 J/cm2 or kept in the dark (non-irradiated). Control eggs were incubated with PBS and left in the dark or irradiated with a fluence of 9 J/cm2 for an irradiation control.
Pictures were obtained at 2, 4, 6, and 8 h after sample incubation and irradiation. The effects on the membrane blood vessels analyzed were lysis and hemorrhage. Lysis was evidenced by the disappearance of small blood vessels, and hemorrhage as bleeding of the vessels on the CAM. Vascular damage parameter results from the sum of the lysis and hemorrhage scores were observed at different times and corrected by a time factor that considers the vascular damage onset.
2.18. Statistical Analysis
Statistical analyses were performed by one-way analysis of variance followed by Dunnet’s test using Prisma 8.0.1 Software. The p-value of < 0.05 was considered statistically significant. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; n.s. represents non-significant (p > 0.05). Statistical analyses for vascular damage assays were performed by the Kruskal–Wallis non-parametric test followed by Dunn’s multiple comparisons. Differences were considered statistically significant at a p-value of < 0.05.
4. Discussion
CUR is a poorly hydrosoluble and poorly bioavailable drug, and its particulate formulations are mostly designed to increase its oral bioavailability, or, less frequently, to act as a controlled release depot after parenteral administration [
6,
47]. The design of targeted formulations of CUR aiming to modify its pharmacodynamics has been much less frequently addressed. Targeted formulations often reduce the structural simplicity of nanomedicines, and simplicity is not only a plus but an essential requirement of any scalable nanoparticulate formulation [
48]. In this work, we explored the performance of nATC, a structurally stable and naturally targeted nanovesicular formulation of CUR, as a photodynamic agent on A549 cells. Different from liposomal formulations, nATC retains CUR upon dilution and displays a relatively simple targeted structure, a fact that may simplify its scaling up and characterization.
Our results must be analyzed in terms of feasible in vivo future uses, this is the nebulization suitability of each formulation, and the ability to retain CUR over time. Since free CUR cannot be nebulized, and nLTC releases CUR crystals during storage, the present comparison between nATC and nLTC and free CUR was performed only to assess the role of the archaeolipid matrix and to compare the effects of nanovesicles vs. those of free CUR after cell uptake. In such context, it was found that the archaeolipid matrix of nATC did not exert any additional cyto or phototoxicity compared to the liposomal matrix; after 24 h of irradiated, free CUR, nLTC, and nATC caused the same lysosomal damage, and a decrease in ΔΨm, induced the same extent of ATP consumption, whereas the extra and intracellular generation of ROS induced by 40 or 20 μM of CUR nanovesicles, was practically the same. The cytotoxicity of non-irradiated formulations followed the trend: CUR> nATC> nLTC; upon irradiation at 9 J/cm
2, the order of damage was modified and the IC
50 values were substantially reduced by 30-fold for nATC and nLTC, resulting in the nanovesicles being more cytotoxic than free CUR. This signified that nanovesicles, independently of their chemical nature, would favor the exertion of intracellular phototoxicity. This may be related to the higher ability of CUR nanovesicles to produce ROS after irradiation and RNS in the dark. The only effect that could be ascribed solely to the archaeolipids matrix was the fact that non-irradiated, 40 μM CUR nATC impaired the production of MMP-2 and MMP-9 by A549 cells. This chemical effect is in line with the previously reported ability of nATC in repairing epithelia [
49].
Apart from A549 phototoxicity, two remarkable effects of nATC were caused. One was that upon internalization of 5 uM nATC and irradiation, their migration was impaired. The other was that conditioned media from A549 cells treated with 5 μM nATC and irradiated reduced the expression of the CD204 marker on macrophages derived from THP-1 monocytes. Used as models for TAM, the reduced expression of CD204 suggests a reduction of their pro-tumoral M2 phenotype. This is important since the tumoral microenvironment of epithelial tumors such as NSCLC predominantly contains M2 phenotypic TAM [
50], which is known to express high SRA1 levels, a receptor responsible for the extensive internalization of nATC [
51]. Hence, besides direct antitumoral activity, the irradiation of these tumors may reprogram their TAM to an antitumor phenotype, as judged by the increased production of pro-inflammatory cytokines that followed the treatment with nATC.
CAM has been previously used to model vessels submitted to CUR-PDT. The CAM surface received a high CUR concentration (100 μM CUR in saline solution 0.1% ethanol) and was irradiated at high fluence (30 J/cm
2), which first induced shrinkage and ended in a complete collapse of small vessels after 30 min [
52].
In our approach, the CAM was used to model vessels under a mucin layer covering the CAM surface, which was additionally covered with a mucin layer ~0.06 mm thick (comparable thickness to the 5–50 μm thick mucus layer covering the epithelia of lung bronchia) [
53]. After 8 h and at 40 μM, irradiated CUR nanovesicles induced the shrinking and further collapse of the vessels. These results suggest therefore that in vivo, the mucin layer on the lung airways would not impair the access of nebulized vesicles to the subjacent epithelia. This also showed that upon topical administration of CUR formulations and blue light irradiation, the generation of phototoxicity at low depth (capillary plexus is located immediately below the chorionic epithelium, the outer cell layer of the CAM [
54]) from a surface was possible. Remarkably, different from nLTC, non-irradiated nATC was harmless, even after 8 h. Therefore, while the vascular damage caused by nATC was limited to irradiated zones, that of nLTC would escape from the light control.
In addition, recently, the group of Bakowski explored the performance of nebulized CUR nanovesicles to perform antitumoral PDT, consisting of bilayers lacking Tween 80 and containing 90% weight DPPC (synthetic hydrogenated ordinary phospholipid) and 10% TEL (tetraether from the archaea
Sulfolobus acidocaldarius) [
23].
S. acidocaldarius is a thermoacidophile microorganism thriving in extreme conditions of temperature (80 °C) and acidity (pH < 2); its lipids are highly thermostable caldarchaeols containing pentacycles, that form monolayers [
55]. In there, the use of CAM was performed classically, without mucins and determining the damage induced 5 min after nebulization.
Because of their simpler growth conditions and lower costs, halophilic archaea are of the outmost biotechnological potential as sources of polar and non-polar archaeolipids [
56]. Importantly, the biotechnological use of archaea products is in its beginning stage. The polar archaeolipids possess a biotechnological readiness level 3 [
57]. This signifies that archaeolipids are products whose demand is satisfied by tiny amounts commercialized at high prices. In such context, archaeosomes from halophilic archaea constitute novel structural platforms for drug delivery, alternative to liposomes. Besides strongly trapping poorly hydrosoluble drugs, these archaeosomes are less refractory to oxidation and hydrolytic attacks than those made of tetraethers, but strong enough to maintain their colloidal structure against realistic aggressive environments. As proof of the versatility of such structural platform, recently void archaeosomes made of halophilic dieters were revealed to be bioactive, displaying an intense anti-inflammatory activity on vascular endothelial cells [
58]. Remarkably, the reduced viability and migration of A549 cells, the vessel lysis, the reduced expression of CD204 by THP1-derived macrophages (as TAM models), or the chemical reduction of MMP release and pro-inflammatory cytokines previously reported [
22] could be exerted by nATC between a short range of concentrations of 5–40 μM CUR. Our results suggest therefore that the activity of nATC occurred at multiple levels, that can be triggered and controlled by a blue light of low fluency according to the specific need.