Polymeric Nanoparticles for Drug Delivery in Osteoarthritis
Abstract
:1. Introduction
1.1. Osteoarthritis
1.2. Nanoscale-Delivery Systems and Polymeric Nanoparticles
2. Methods
3. Natural Polymers
3.1. Chitosan
3.2. Hyaluronic Acid
3.3. Dextran Sulfate
3.4. Elastin
3.5. Polyphenols
3.6. Silk Fibroin
4. Synthetic Polymers
4.1. Poly(lactic-co-glycolic Acid) (PLGA)
4.2. Polylactic Acid (PLA)
4.3. Polycaprolactone (PCL)
4.4. Poly(hydroxyethyl) methacrylate (pHEMA)
4.5. Poly(N-isopropylacrylamide) (pNIPAM)
4.6. Poly(amidoamine) (PAA)
4.7. Poly[2-(N,N-dimethylamino)ethyl methacrylate] (PDMAEMA)
4.8. Poly(aspartic Acid) (PAsp)
4.9. Poly(organophosphazene)
4.10. Poly(propylene sulfide) (PPS)
4.11. Polyurethane
4.12. Terpolymers
5. Physicochemical Properties and Tissue Targeting
6. Discussion and Future Challenges
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Hügle, T.; Geurts, J.; Nüesch, C.; Müller-Gerbl, M.; Valderrabano, V. Aging and Osteoarthritis: An Inevitable Encounter? J. Aging Res. 2012, 2012, 950192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EclinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sport. Health 2009, 1, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis pathogenesis. Bone 2012, 51, 249. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular cartilage: Structure and regeneration. Tissue Eng. Part B Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Luyten, F.P.; Facchini, A. Biological aspects of early osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 20, 407–422. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J. Bone Jt. Surg. 2003, 85, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, Y.; Tao, Y.; Lin, W.; Wang, P. Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics 2021, 13, 2166. [Google Scholar] [CrossRef]
- Kammermann, J.R.; Kincaid, S.A.; Rumph, P.F.; Baird, D.K.; Visco, D.M. Tumor necrosis factor-α (TNF-α) in ca-nine osteoarthritis: Immunolocalization of TNF-α, stromelysin and TNF receptors in canine osteoarthritis cartilage. Osteoarthr. Cartil. 1996, 4, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, S.; Satoh, T.; Chiba, J.; Ju, C.; Inoue, K.; Kagawa, J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell. Mol. Ther. 2000, 6, 71–79. [Google Scholar] [CrossRef]
- Na, H.S.; Park, J.S.; Cho, K.H.; Kwon, J.Y.; Choi, J.W.; Jhun, J.; Kim, S.J.; Park, S.H.; Cho, M.L. Interleukin-1-Interleukin-17 Signaling Axis Induces Cartilage Destruction and Promotes Experimental Osteoarthritis. Front. Immunol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Schieker, M.; Conaghan, P.G.; Mindeholm, L.; Praestgaard, J.; Solomon, D.H.; Scotti, C.; Gram, H.; Thuren, T.; Roubenoff, R.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement: Exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2020, 173, 509. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in osteoarthritis: Time for a critical review of the literature. F1000Research 2019, 8, 934. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. J. Am. Med. Assoc. 2001, 286, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Grayson, C.W.; Decker, R.C. Total Joint Arthroplasty for Persons with Osteoarthritis. PM R 2012, 4, S97–S103. [Google Scholar] [CrossRef]
- Hunter, D.J.; Pike, M.C.; Jonas, B.L.; Kissin, E.; Krop, J.; McAlindon, T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2010, 11, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohmander, L.S.; Hellot, S.; Dreher, D.; Krantz, E.F.W.; Kruger, D.S.; Guermazi, A.; Eckstein, F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014, 66, 1820–1831. [Google Scholar] [CrossRef]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res. 2009, 61, 344–352. [Google Scholar] [CrossRef]
- Cohen, S.B.; Proudman, S.; Kivitz, A.J.; Burch, F.X.; Donohue, J.P.; Burstein, D.; Sun, Y.N.; Banfield, C.; Vincent, M.S.; Ni, L.; et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 2011, 13, R125. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, V.; O’Green, A.L.; Bossard, C.; Seo, T.; Lamangan, L.; Ibanez, M.; Ghias, A.; Lai, C.; Do, L.; Cho, S.; et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthr. Cartil. 2019, 27, 1347–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazici, Y.; McAlindon, T.E.; Gibofsky, A.; Lane, N.E.; Lattermann, C.; Skrepnik, N.; Swearingen, C.J.; Simsek, I.; Ghandehari, H.; DiFrancesco, A.; et al. A Phase 2b randomized trial of lorecivivint, a novel intra-articular CLK2/DYRK1A inhibitor and Wnt pathway modulator for knee osteoarthritis. Osteoarthr. Cartil. 2021, 29, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Gouze, J.N.; Gouze, E.; Robbins, P.D.; Ghivizzani, S.C. Osteoarthritis gene therapy. Gene Ther. 2004, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, S.N.; Lindsay, M.A.; Jones, S.W. Oligonucleotide Therapies in the Treatment of Arthritis: A Narrative Review. Biomedicines 2021, 9, 902. [Google Scholar] [CrossRef]
- Colella, F.; Garcia, J.P.; Sorbona, M.; Lolli, A.; Antunes, B.; D’Atri, D.; Barré, F.P.Y.; Oieni, J.; Vainieri, M.L.; Zerrillo, L.; et al. Drug delivery in intervertebral disc degeneration and osteoarthritis: Selecting the optimal platform for the delivery of disease-modifying agents. J. Control. Release 2020, 328, 985–999. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2013, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Tryfonidou, M.A.; de Vries, G.; Hennink, W.E.; Creemers, L.B. “Old Drugs, New Tricks”—Local controlled drug release systems for treatment of degenerative joint disease. Adv. Drug Deliv. Rev. 2020, 160, 170–185. [Google Scholar] [CrossRef]
- Cheng, O.T.; Souzdalnitski, D.; Vrooman, B.; Cheng, J. Evidence-Based Knee Injections for the Management of Arthritis. Pain Med. 2012, 13, 740–753. [Google Scholar] [CrossRef] [Green Version]
- Conaghan, P.G.; Hunter, D.J.; Cohen, S.B.; Kraus, V.B.; Berenbaum, F.; Lieberman, J.R.; Jones, D.G.; Spitzer, A.I.; Jevsevar, D.S.; Nathaniel, P.; et al. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain: A Double-Blinded, Randomized, Placebo-Controlled, Multinational Study. J. Bone Jt. Surg. Am. 2018, 100, 666. [Google Scholar] [CrossRef] [Green Version]
- Owen, S.; Francis, H.; Roberts, M. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br. J. Clin. Pharmacol. 1994, 38, 349. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Kumar, S.; Sharma, B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019, 93, 239–257. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Jin, G.Z. Current Nanoparticle-Based Technologies for Osteoarthritis Therapy. Nanomaterials 2020, 10, 2368. [Google Scholar] [CrossRef]
- Uzieliene, I.; Kalvaityte, U.; Bernotiene, E.; Mobasheri, A. Non-viral Gene Therapy for Osteoarthritis. Front. Bioeng. Biotechnol. 2021, 1, 618399. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Adler-Moore, J.; Proffitt, R.T. AmBisome: Liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 2002, 49, 21–30. [Google Scholar] [CrossRef]
- Corciulo, C.; Castro, C.M.; Coughlin, T.; Jacob, S.; Li, Z.; Fenyö, D.; Rifkin, D.B.; Kennedy, O.D.; Cronstein, B.N. Intraarticular injection of liposomal adenosine reduces cartilage damage in established murine and rat models of osteoarthritis. Sci. Rep. 2020, 10, 13477. [Google Scholar] [CrossRef]
- Bousnaki, M.; Bakopoulou, A.; Kritis, A.; Koidis, P. The Efficacy of Stem Cells Secretome Application in Osteoarthritis: A Systematic Review of In Vivo Studies. Stem Cell Rev. Rep. 2020, 16, 1222–1241. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, J.K.; Jeong, J.; Choy, J.H. Toxicity evaluation of inorganic nanoparticles: Considerations and challenges. Mol. Cell. Toxicol. 2013, 9, 205–210. [Google Scholar] [CrossRef]
- Rahimi, M.; Charmi, G.; Matyjaszewski, K.; Banquy, X.; Pietrasik, J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021, 123, 31–50. [Google Scholar] [CrossRef]
- Tabujew, I.; Peneva, K. CHAPTER 1: Functionalization of Cationic Polymers for Drug Delivery Applications, in Cationic Polymers in Regenerative Medicine. In RSC Polymers Chemistry; Royal Society of Chemistry: London, UK, 2014; pp. 1–29. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Duncan, R. Polymer therapeutics: Top 10 selling pharmaceuticals—What next? J. Control. Release 2014, 190, 371–380. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [Green Version]
- Yoneki, N.; Takami, T.; Ito, T.; Anzai, R.; Fukuda, R.; Kinoshita, K.; Sonotaki, S.; Murakami, Y. One-pot facile preparation of PEG-modified PLGA nanoparticles: Effects of PEG and PLGA on release properties of the particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 66–72. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.A.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Sig. Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Sarvari, R.; Nouri, M.; Agbolaghi, S.; Roshangar, L.; Sadrhaghighi, A.; Seifalian, A.M.; Keyhanvar, P. A summary on non-viral systems for gene delivery based on natural and synthetic polymers. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 246–265. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; XuShi; Zhang, C.; Tang, T.; Dai, K. Direct chitosan-mediated gene delivery to the rabbit knee joints in vitro and in vivo. Biochem. Biophys. Res. Commun. 2006, 341, 202–208. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, X.; Zhang, Q.; Sun, F.; Li, X.; Xiong, C.; Zhang, C.; Fan, H. Chitosan-plasmid DNA nanoparticles encoding small hairpin RNA targeting MMP-3 and -13 to inhibit the expression of dedifferentiation related genes in expanded chondrocytes. J. Biomed. Mater. Res. A 2014, 102, 373–380. [Google Scholar] [CrossRef]
- Li, T.; Yang, J.; Weng, C.; Liu, P.; Huang, Y.; Meng, S.; Li, R.; Yang, L.; Chen, C.; Gong, X. Intra-articular injection of anti-inflammatory peptide-loaded glycol chitosan/fucoidan nanogels to inhibit inflammation and attenuate osteoarthritis progression. Int. J. Biol. Macromol. 2021, 170, 469–478. [Google Scholar] [CrossRef]
- Zhou, P.H.; Qiu, B.; Deng, R.H.; Li, H.J.; Xu, X.F.; Shang, X.F. Chondroprotective effects of hyaluronic acid-chitosan nanoparticles containing plasmid DNA encoding cytokine response modifier A in a rat knee osteoarthritis model. Cell. Physiol. Biochem. 2018, 47, 1207–1216. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Cao, Y.; Huang, T.; Song, D.X.; Tao, H.R. Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes. Int. J. Mol. Med. 2018, 42, 2604–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, M.; Ma, L.; Zhang, X.; Xie, D.; Kang, Y.; Xiao, B. Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer-targeted chemotherapy. Colloids Surf. B Biointerfaces 2019, 177, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, N.A.; Bagheri, F.; Eslaminejad, M.B. Chondroitin sulfate modified chitosan nanoparticles as an efficient and targeted gene delivery vehicle to chondrocytes. Colloids Surf. B Biointerfaces 2022, 219, 112786. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, X.; Zhang, J.; Ma, S.; Jiang, L.; Wei, Q.; Cai, M.; Zhou, F. Synthesis of charged chitosan nanoparticles as functional biolubricant. Colloids Surf. B Biointerfaces 2021, 206, 111973. [Google Scholar] [CrossRef]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef] [PubMed]
- Cullier, A.; Cassé, F.; Manivong, S.; Contentin, R.; Legendre, F.; Garcia-Ac, A.; Sirois, P.; Roullin, G.; Banquy, X.; Moldovan, F.; et al. Functionalized Nanogels with Endothelin-1 and Bradykinin Receptor Antagonist Peptides Decrease Inflammatory and Cartilage Degradation Markers of Osteoarthritis in a Horse Organoid Model of Cartilage. Int. J. Mol. Sci. 2022, 23, 8949. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Amorim, D.; Laranjeira, I.; Almeida, A.; Reis, R.L.; Ferreira, H.; Pinto-Ribeiro, F.; Neves, N.M. Modulating inflammation through the neutralization of Interleukin-6 and tumor necrosis factor-α by biofunctionalized nanoparticles. J. Control. Release 2021, 331, 491–502. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Y.; Zhang, G.; Tang, F.; Zhang, J.; Cao, J.; Liu, C. Targeted elimination of intracellular reactive oxygen species using nanoparticle-like chitosan- superoxide dismutase conjugate for treatment of monoiodoacetate-induced osteoarthritis. Int. J. Pharm. 2020, 590, 119947. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.Q.; Peng, H.; Yu, L.; He, B.; Zhao, Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int. Immunopharmacol. 2015, 28, 34–43. [Google Scholar] [CrossRef]
- Concoff, A.; Sancheti, P.; Niazi, F.; Shaw, P.; Rosen, J. The efficacy of multiple versus single hyaluronic acid injections: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 542. [Google Scholar] [CrossRef]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue integrity signals communicated by high molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 2014, 58, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.J.; Yoon, J.; Rho, J.G.; Han, H.S.; Lee, S.; Oh, Y.S.; Kim, H.; Kim, E.; Kim, S.J.; Lim, Y.T.; et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials 2021, 275, 120967. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Khattab, M.A.; Abd-Allah, H. Intra-articular multifunctional celecoxib loaded hyaluronan nanocapsules for the suppression of inflammation in an osteoarthritic rat model. Int. J. Pharm. 2020, 583, 119378. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Bian, S.; Cheng, Y.; Dong, S.; Liu, J.; Liu, W.; Xiao, C. Dextran sulfate-triamcinolone acetonide conjugate nanoparticles for targeted treatment of osteoarthritis. Int. J. Biol. Macromol. 2020, 158, 1082–1089. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; David, M.A.; Dunshee, L.C.; Scott, R.A.; Urello, M.A.; Price, C.; Kiick, K.L. Thermoresponsive Elastin-b-Collagen-Like Peptide Bioconjugate Nanovesicles for Targeted Drug Delivery to Collagen-Containing Matrices. Biomacromolecules 2017, 18, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Liu, Q.; Yan, J.; Pan, D.; Wang, L.; Xu, Y.; Wang, F.; Liu, Y.; Li, X.; et al. ROS-Responsive Boronate-Stabilized Polyphenol–Poloxamer 188 Assembled Dexamethasone Nanodrug for Macrophage Repolarization in Osteoarthritis Treatment. Adv. Healthc. Mater. 2021, 10, 2100883. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Faragò, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Corciulo, C.; Arabagian, S.; Ulman, A.; Cronstein, B.N. Adenosine-Functionalized Biodegradable PLA-b-PEG Nanoparticles Ameliorate Osteoarthritis in Rats. Sci. Rep. 2019, 9, 7430. [Google Scholar] [CrossRef] [Green Version]
- Sturm, L.; Schwemberger, B.; Menzel, U.; Häckel, S.; Albers, C.E.; Plank, C.; Rip, J.; Alini, M.; Traweger, A.; Grad, S.; et al. In vitro evaluation of a nanoparticle-based mRNA delivery system for cells in the joint. Biomedicines 2021, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Pape, E.; Parent, M.; Pinzano, A.; Sapin-Minet, A.; Henrionnet, C.; Gillet, P.; Scala-Bertola, J.; Gambier, N. Rapamycin-loaded Poly(lactic-co-glycolic) acid nanoparticles: Preparation, characterization, and in vitro toxicity study for potential intra-articular injection. Int. J. Pharm. 2021, 609, 121198. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, S.E.; Kim, H.J.; Park, K.; Song, G.G.; Choi, S.J. A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int. J. Pharm. 2020, 581, 119249. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Ho, M.J.; Kim, S.H.; Cho, H.R.; Kim, H.S.; Choi, Y.S.; Choi, Y.W.; Kang, M.J. Increased localized delivery of piroxicam by cationic nanoparticles after intra-articular injection. Drug Des. Devel. Ther. 2016, 10, 3779–3787. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Gu, L.; Ren, L.; Chen, J.; Li, T.; Wang, X.; Jang, J.; Chen, C.; Sun, L. Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression. Am. J. Transl. Res. 2019, 11, 6775–6789. [Google Scholar]
- Gómez-Gaete, C.; Ferreira, F.; Bustos, P.; Mennickent, S.; Castillo, D.; Chávez, C.; Novoa, P.; Godoy, R. Optimization of rhein-loaded polymeric nanoparticles using a factorial design and evaluation of the cytotoxic and anti-inflammatory effects. Drug Dev. Ind. Pharm. 2018, 44, 1285–1294. [Google Scholar] [CrossRef]
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.N.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J. Control. Release 2019, 309, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, F.; Li, C.; Zhang, B.; An, M.; Lu, M.; Liu, Y.; Liu, Y. Rhein laden pH-responsive polymeric nanoparticles for treatment of osteoarthritis. AMB Express 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Duan, Y.; Zhang, Q.; Sun, D.; Fang, R.H.; Liu-Bryan, R.; Gao, W.; Zhang, L. Cartilage-targeting ultrasmall lipid-polymer hybrid nanoparticles for the prevention of cartilage degradation. Bioeng. Transl. Med. 2021, 6, e10187. [Google Scholar] [CrossRef] [PubMed]
- Zerrillo, L.; Gigliobianco, M.R.; D’atri, D.; Garcia, J.P.; Baldazzi, F.; Ridwan, Y.; Fuentes, G.; Chan, A.; Creemers, L.B.; Censi, R.; et al. PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy. Nanomaterials 2022, 12, 2248. [Google Scholar] [CrossRef] [PubMed]
- Riffault, M.; Six, J.L.; Netter, P.; Gillet, P.; Grossin, L. PLGA-Based Nanoparticles: A Safe and Suitable Delivery Platform for Osteoarticular Pathologies. Pharm. Res. 2015, 32, 3886–3898. [Google Scholar] [CrossRef]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Kim, S.I.; Kim, S.R.; Kim, S.; Joo, Y.; et al. P47phox siRNA-loaded PLGA nanoparticles suppress ROS/oxidative stress-induced chondrocyte damage in osteoarthritis. Polymers 2020, 12, 443. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Park, H.; Shin, N.; Shin, J.; Gwon, D.H.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Hong, J.; Heo, J.Y.; et al. P66shc siRNA nanoparticles ameliorate chondrocytic mitochondrial dysfunction in osteoarthritis. Int. J. Nanomed. 2020, 15, 2379–2390. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, Y.; Wen, Y.; Yu, Q.; Liu, J.; Zhao, Y.; Liu, J.; Ye, G. A photothermal-triggered nitric oxide nanogenerator combined with siRNA for precise therapy of osteoarthritis by suppressing macrophage inflammation. Nanoscale 2019, 11, 6693–6709. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G.A. Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Corciulo, C.; Lendhey, M.; Wilder, T.; Schoen, H.; Cornelissen, A.S.; Chang, G.; Kennedy, O.D.; Cronstein, B.N. Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat. Commun. 2017, 8, 15019. [Google Scholar] [CrossRef] [Green Version]
- Pest, M.A.; Russell, B.A.; Zhang, Y.W.; Jeong, J.W.; Beier, F. Disturbed cartilage and joint homeostasis resulting from a loss of mitogen-inducible gene 6 in a mouse model of joint dysfunction. Arthritis Rheumatol. 2014, 66, 2816–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Agarwal, R.; Diaz-Ruiz, C.A.; Willett, N.J.; Wang, P.; Lee, L.A.; Wang, Q.; Guldberg, R.E.; García, A.J. Nanoengineered particles for enhanced intra-articular retention and delivery of proteins. Adv. Healthc. Mater. 2014, 3, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Volkmer, T.M.; Wang, P.; Lee, L.A.; Wang, Q.; García, A.J. Synthesis of self-assembled IL-1Ra-presenting nanoparticles for the treatment of osteoarthritis. J. Biomed. Mater. Res. A 2016, 104, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Deloney, M.; Smart, K.; Christiansen, B.A.; Panitch, A. Thermoresponsive, hollow, degradable core-shell nanoparticles for intra-articular delivery of anti-inflammatory peptide. J. Control. Release 2020, 323, 47–58. [Google Scholar] [CrossRef]
- McMasters, J.; Poh, S.; Lin, J.B.; Panitch, A. Delivery of anti-inflammatory peptides from hollow PEGylated poly(NIPAM) nanoparticles reduces inflammation in an ex vivo osteoarthritis model. J. Control. Release 2017, 258, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhong, Z.; Lok, M.C.; Jiang, X.; Hennink, W.E.; Feijen, J.; Engbersen, F.J. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjug. Chem. 2007, 18, 138–145. [Google Scholar] [CrossRef]
- Lin, C.; Engbersen, J.F.J. PEGylated bioreducible poly(amido amine)s for non-viral gene delivery. Mater. Sci. Eng. C 2011, 31, 1330–1337. [Google Scholar] [CrossRef]
- Elzes, M.R.; Akeroyd, N.; Engbersen, J.F.J.; Paulusse, J.M.J. Disulfide-functional poly(amido amine)s with tunable degradability for gene delivery. J. Control. Release 2016, 244, 357–365. [Google Scholar] [CrossRef]
- Bedingfield, S.K.; Colazo, J.M.; Yu, F.; Liu, D.D.; Jackson, M.A.; Himmel, L.E.; Cho, H.; Crofford, L.J.; Hasty, K.A.; Duvall, C.L. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 2021, 5, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Bedingfield, S.K.; Colazo, J.M.; Di Francesco, M.; Yu, F.; Liu, D.D.; Di Francesco, V.; Himmel, L.E.; Gupta, M.K.; Cho, H.; Hasty, K.A.; et al. Top-Down Fabricated microPlates for Prolonged, Intra-articular Matrix Metalloproteinase 13 siRNA Nanocarrier Delivery to Reduce Post-traumatic Osteoarthritis. ACS Nano 2021, 15, 14475–14491. [Google Scholar] [CrossRef] [PubMed]
- Aini, H.; Itaka, K.; Fujisawa, A.; Uchida, H.; Uchida, S.; Fukushima, S.; Kataoka, K.; Saito, T.; Chung, U.; Ohba, S. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci. Rep. 2016, 6, 18743. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.E.; Song, H.R.; Kim, Y.M.; Song, S.C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2021, 7, 14–25. [Google Scholar] [CrossRef]
- O’Grady, K.P.; Kavanaugh, T.E.; Cho, H.; Ye, H.; Gupta, M.K.; Madonna, M.C.; Lee, J.; O’Brien, C.M.; Skala, M.C.; Hasty, K.A.; et al. Drug-Free ROS Sponge Polymeric Microspheres Reduce Tissue Damage from Ischemic and Mechanical Injury. ACS Biomater. Sci. Eng. 2018, 4, 1251–1264. [Google Scholar] [CrossRef]
- Rothenfluh, D.A.; Bermudez, H.; O’Neil, C.P.; Hubbell, J.A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat. Mater. 2008, 7, 248–254. [Google Scholar] [CrossRef]
- Fan, W.; Li, J.; Yuan, L.; Chen, J.; Wang, Z.; Wang, Y.; Guo, C.; Mo, X.; Yan, Z. Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 2018, 25, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Pontes-Quero, G.M.; Benito-Garzón, L.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Modulation of Inflammatory Mediators by Polymeric Nanoparticles Loaded with Anti-Inflammatory Drugs. Pharmaceutics 2021, 13, 290. [Google Scholar] [CrossRef]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic Understanding of Nanoparticles’ Interactions with Extracellular Matrix: The Cell and Immune System. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioria, S.; Caputo, F.; Urbán, P.; Maguire, C.M.; Bremer-Hoffmann, S.; Prina-Mello, A.; Calzolai, L.; Mehn, D. Are existing standard methods suitable for the evaluation of nanomedicines: Some case studies. Nanomedicine 2018, 13, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, W.; Lao, F.; Liu, Y.; Wang, L.; Bai, R.; Zhao, Y.; Chen, C. Intracellular dynamics of cationic and anionic polystyrene nanoparticles without direct interaction with mitotic spindle and chromosomes. Biomaterials 2011, 32, 8291–8303. [Google Scholar] [CrossRef] [PubMed]
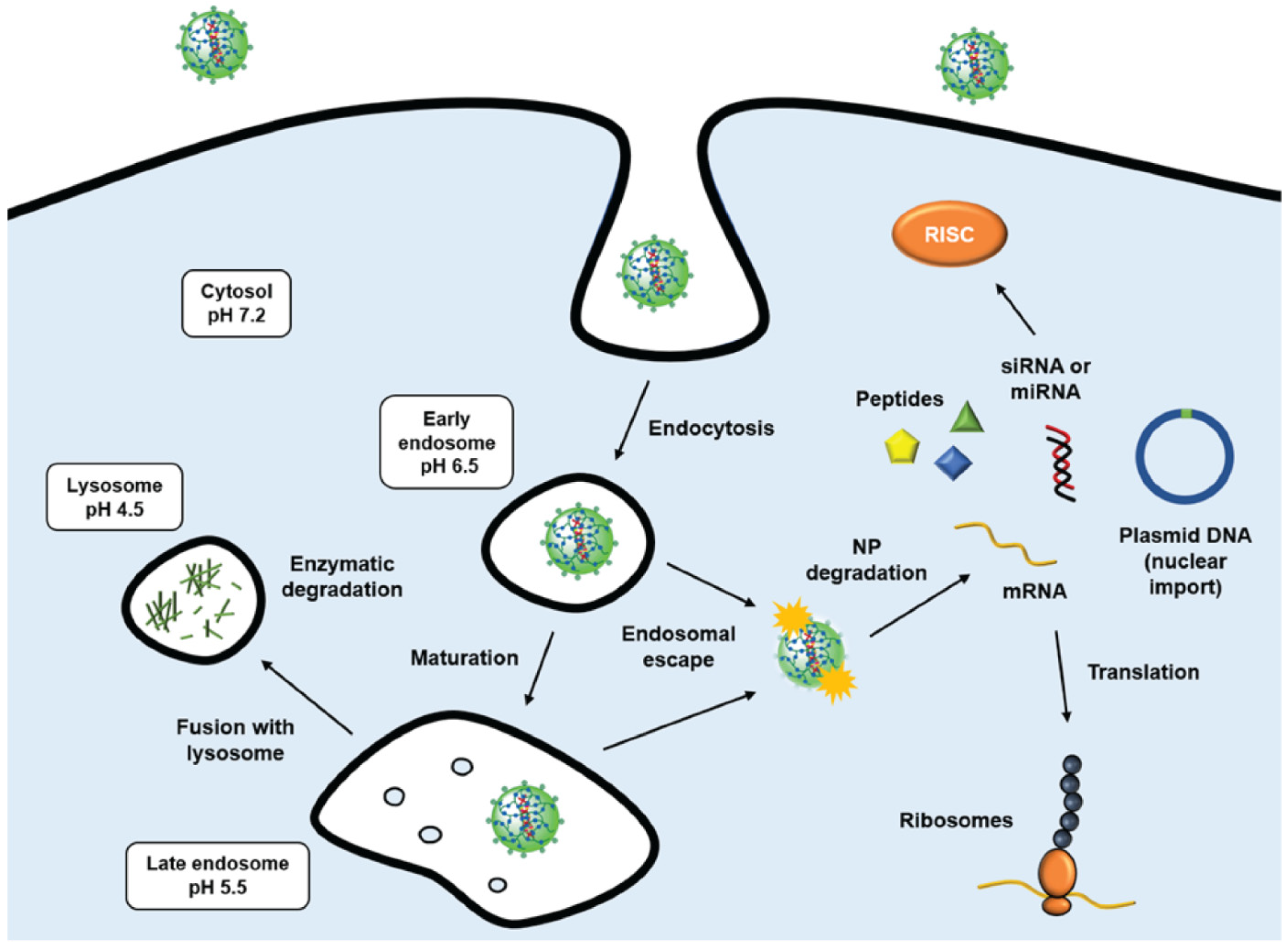
- Freeman, E.C.; Weiland, L.M.; Meng, W.S. Modeling the proton sponge hypothesis: Examining proton sponge effectiveness for enhancing intracellular gene delivery through multiscale modeling. J. Biomater. Sci. Polym. Ed. 2013, 24, 398–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.B.; Dammer, E.B.; Ren, R.J.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Crucho, C.I.C. Stimuli-responsive polymeric nanoparticles for nanomedicine. ChemMedChem 2015, 10, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Manickam, D.S.; Oupický, D. Polyplex gene delivery modulated by redox potential gradients. J. Drug Target. 2006, 14, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Torzilli, P.A.; Arduino, J.M.; Gregory, J.D.; Bansal, M. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 1997, 30, 895–902. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Grodzinsky, A.J. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat. Rev. Rheumatol. 2017, 13, 183–193. [Google Scholar] [CrossRef]
- Brown, S.; Pistiner, J.; Adjei, I.M.; Sharma, B. Nanoparticle Properties for Delivery to Cartilage: The Implications of Disease State, Synovial Fluid, and Off-Target Uptake. Mol. Pharm. 2019, 16, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Labhasetwar, V. Effect of Molecular Structure of Cationic Surfactants on Biophysical Interactions of the Surfactant-modified Nanoparticles with a Model Membrane and Cellular Uptake. Langmuir 2009, 25, 2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahy, N.; de Vries-van Melle, M.L.; Lehmann, J.; Wei, W.; Grotenhuis, N.; Farrell, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J.V.M. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr. Cartil. 2014, 22, 1167–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef]
- Roser, M.; Fischer, D.; Kissel, T. Surface-modified biodegradable albumin nano- and microspheres. II: Effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur. J. Pharm. Biopharm. 1998, 46, 255–263. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.E.; George, G.; Brody, A.R. Sialic acid mediates the initial binding of positively charged inorganic particles to alveolar macrophage membranes. Am. Rev. Respir. Dis. 1987, 135, 1345–1352. [Google Scholar] [CrossRef]
- Zhu, S.; Makosa, D.; Miller, B.F.; Griffin, T.M. Glutathione as a Mediator of Cartilage Oxidative Stress Resistance and Resilience During Aging and Osteoarthritis. Connect. Tissue Res. 2020, 61, 34–47. [Google Scholar] [CrossRef]


| Strengths |
|
| Limitations |
|
| Natural Polymers | |||
| Polymer Name | Surface Chemistry (Charge/Targeting) | Functional Groups/Benefits | Biodegradability |
| Chitosan | Positive charge (cationic) Active targeting possible:
|
| Enzymatic degradation by lysozymes |
| Hyaluronic acid (HA) | Negative charge (anionic) Active targeting possible:
| - | Hydrolysis of β-1,4-glycosidic bonds by hyaluronidases |
| Synthetic Polymers | |||
| Poly(lactic-co-glycolic acid) (PLGA) | Neutral charge Active targeting possible:
|
| Hydrolysis in aqueous media, degradation rate depends on the lactide/glycolide ratio and their molecular weights |
| Polylactic acid (PLA) | Neutral charge |
| Hydrolysis of the ester bonds, degradation rate depends on molecular weight |
| Polycaprolactone (PCL) | Neutral charge |
| Hydrolysis of the ester bonds, slower degradation at physiological pH |
| Poly(hydroxyethyl) methacrylate (pHEMA) | Neutral charge |
| pH and thermosensitive release: ↑ solubility (drug leakage) at cloud point changes from 28 °C to 39 °C (pH = 6.5) |
| Poly(N-isopropylacrylamide) (pNIPAM) | Neutral charge | - | Thermosensitive release: phase transition from a water-soluble to insoluble state at temperatures higher than the LCST (>32 °C) |
| Poly(amidoamine) (PAA) | Positive charge (cationic) | - | Bioreducible NPs, cleavage of disulfide bonds by the intracellular GSH |
| Applicant | Title/Reference | Embodiment for OA Treatment | Experimental Evidence for OA |
|---|---|---|---|
| University of Pennsylvania | Targeting cartilage EGFR pathway for osteoarthritis treatment (WO2022040006A1) | Therapeutic composition comprising a polymeric nanoparticle, a ligand selected to activate an EGFR receptor (e.g., TGFα), and a linker associating the NP and the ligand. | Intra-articular delivery effectively attenuated surgery-induced OA cartilage degeneration, subchondral bone plate sclerosis, and joint pain [82]. |
| New York University | Biodegradable polymeric nanoparticle conjugates and use thereof (WO2017083659A1) | Poly(lactic acid) (PLA) nanoparticle conjugated with adenosine using a polyethylene glycol (PEG) linker. | Intra-articular delivery prevented the development of OA in a rat model of PTOA [83]. |
| 20Med Therapeutics B.V. | Nanogels (WO2012165953A1) | Poly(amidoamine) nanoparticles containing disulfide linkages and a biologically active component such as siRNA, miRNA, DNA, (oligo)peptide or proteins. | Transfection of primary chondrocytes and 3D constructs rich in extracellular matrix (bCH pellets and tendon-like constructs) [84]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontes, A.P.; Welting, T.J.M.; Rip, J.; Creemers, L.B. Polymeric Nanoparticles for Drug Delivery in Osteoarthritis. Pharmaceutics 2022, 14, 2639. https://doi.org/10.3390/pharmaceutics14122639
Pontes AP, Welting TJM, Rip J, Creemers LB. Polymeric Nanoparticles for Drug Delivery in Osteoarthritis. Pharmaceutics. 2022; 14(12):2639. https://doi.org/10.3390/pharmaceutics14122639
Chicago/Turabian StylePontes, Adriano P., Tim J. M. Welting, Jaap Rip, and Laura B. Creemers. 2022. "Polymeric Nanoparticles for Drug Delivery in Osteoarthritis" Pharmaceutics 14, no. 12: 2639. https://doi.org/10.3390/pharmaceutics14122639
APA StylePontes, A. P., Welting, T. J. M., Rip, J., & Creemers, L. B. (2022). Polymeric Nanoparticles for Drug Delivery in Osteoarthritis. Pharmaceutics, 14(12), 2639. https://doi.org/10.3390/pharmaceutics14122639







