Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy
Abstract
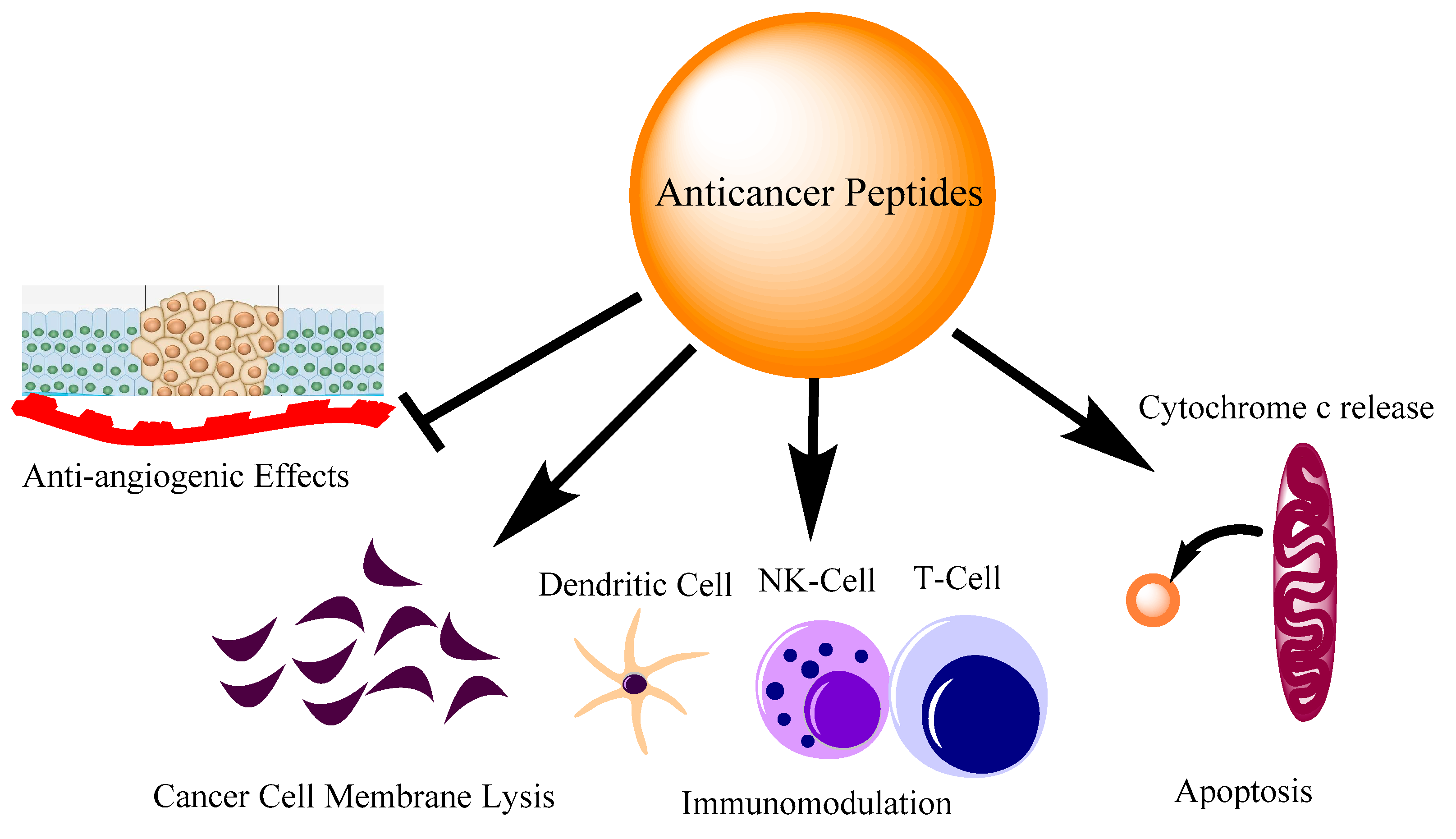
:1. Introduction
2. Where Anti-Cancer Peptides Stand
3. Naturally Occurring AMPs/ACPs with Immunomodulatory Activities
3.1. Amino Acid Arrangement and Their Biophysical Parameters Determine Anti-Cancer and Immunomodulatory Properties
3.2. Knowledge of Structural Determinants of AMPs/ACPs Are Same as for Immunomodulatory Peptides
3.2.1. Size
3.2.2. Amino Acid Prevalence
3.2.3. Charge
3.2.4. Conformation
3.2.5. Hydrophobicity, Amphipathicity and Hydrophobic Moment
3.2.6. Polar Angle
3.2.7. Peptide Self Assembly
3.2.8. Chemical Modifications
3.3. Role of Non-Natural Amino Acids in Improving the Anti-Cancer Activity
4. Cell Selectivity
5. Mechanism of Membrane Targeting and Entry to the Cell
5.1. Barrel-Stave Model
5.2. The Carpet Model
5.3. Toroidal Pore Model
5.4. Cell-Penetrating Mechanism
6. Design of Anti-Cancer Peptides as Vaccines to Influence the Immune System
7. Limitations of Anti-Cancer Peptides with Immunomodulatory Activity and Plausible Resolution
8. Summary and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byun, J.S.; Gardner, K. Wounds that will not heal: Pervasive cellular reprogramming in cancer. Am. J. Pathol. 2013, 182, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchison, T.J. The proliferation rate paradox in antimitotic chemotherapy. Mol. Biol. Cell 2012, 23, 1–6. [Google Scholar] [CrossRef]
- Liu, B.; Ezeogu, L.; Zellmer, L.; Yu, B.; Xu, N.; Joshua Liao, D. Protecting the normal in order to better kill the cancer. Cancer Med. 2015, 4, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Wirsdorfer, F.; de Leve, S.; Jendrossek, V. Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity? Int. J. Mol. Sci. 2018, 20, 24. [Google Scholar] [CrossRef] [Green Version]
- Lara, O.D.; Krishnan, S.; Wang, Z.; Corvigno, S.; Zhong, Y.; Lyons, Y.; Dood, R.; Hu, W.; Qi, L.; Liu, J.; et al. Tumor core biopsies adequately represent immune microenvironment of high-grade serous carcinoma. Sci. Rep. 2019, 9, 17589. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, H.C.; Bloom, S.R.; Murphy, K.G. Peptides and their potential role in the treatment of diabetes and obesity. Rev. Diabet. Stud. 2011, 8, 355–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieco, P.; Gomez-Monterrey, I. Natural and synthetic peptides in the cardiovascular diseases: An update on diagnostic and therapeutic potentials. Arch. Biochem. Biophys. 2019, 662, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, R.; Bahmani, A.; Afshar, S. Investigating the role of peptides in effective therapies against cancer. Cancer Cell Int. 2022, 22, 139. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Recio, C.; Maione, F.; Iqbal, A.J.; Mascolo, N.; De Feo, V. The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front. Pharm. 2016, 7, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharm 2022, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.F.; Wang, J.D.; Uen, W.C. Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockshott, I.D. Clinical pharmacokinetics of goserelin. Clin. Pharm. 2000, 39, 27–48. [Google Scholar] [CrossRef]
- Swayzer, D.V.; Gerriets, V. Leuprolide; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Guzman-Soto, I.; Salinas, E.; Quintanar, J.L. Leuprolide Acetate Inhibits Spinal Cord Inflammatory Response in Experimental Autoimmune Encephalomyelitis by Suppressing NF-kappaB Activation. Neuroimmunomodulation 2016, 23, 33–40. [Google Scholar] [CrossRef]
- Katai, M.; Sakurai, A.; Inaba, H.; Ikeo, Y.; Yamauchi, K.; Hashizume, K. Octreotide as a rapid and effective painkiller for metastatic carcinoid tumor. Endocr. J. 2005, 52, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Theodoropoulou, M.; Zhang, J.; Laupheimer, S.; Paez-Pereda, M.; Erneux, C.; Florio, T.; Pagotto, U.; Stalla, G.K. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006, 66, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.B.; He, L.Y.; Jiang, H.Y.; Chen, Y.X. Role of helicity on the anticancer mechanism of action of cationic-helical peptides. Int. J. Mol. Sci. 2012, 13, 6849–6862. [Google Scholar] [CrossRef]
- Kumari, T.; Verma, D.P.; Kuldeep, J.; Dhanabal, V.B.; Verma, N.K.; Sahai, R.; Tripathi, A.K.; Saroj, J.; Ali, M.; Mitra, K.; et al. 10-Residue MyD88-Peptide Adopts beta-Sheet Structure, Self-Assembles, Binds to Lipopolysaccharides, and Rescues Mice from Endotoxin-Mediated Lung-Infection and Death. ACS Chem. Biol. 2022, 75, 2431–2446. [Google Scholar] [CrossRef]
- Tandon, A.; Harioudh, M.K.; Ishrat, N.; Tripathi, A.K.; Srivastava, S.; Ghosh, J.K. An MD2-derived peptide promotes LPS aggregation, facilitates its internalization in THP-1 cells, and inhibits LPS-induced pro-inflammatory responses. Cell. Mol. Life Sci. 2018, 75, 2431–2446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, X.; Qi, G.; Tang, Y.; Guo, Y.; Si, J.; Liang, L. Roles and Mechanisms of Human Cathelicidin LL-37 in Cancer. Cell Physiol. Biochem. 2018, 47, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Wan, L.; Cai, H.W.; Li, S.F.; Li, Y.P.; Cheng, J.Q.; Lu, X.F. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharm. Sin. 2011, 32, 79–88. [Google Scholar] [CrossRef]
- Ceremuga, M.; Stela, M.; Janik, E.; Gorniak, L.; Synowiec, E.; Sliwinski, T.; Sitarek, P.; Saluk-Bijak, J.; Bijak, M. Melittin-A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 2020, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Bae, H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef] [Green Version]
- Tipgomut, C.; Wongprommoon, A.; Takeo, E.; Ittiudomrak, T.; Puthong, S.; Chanchao, C. Melittin Induced G1 Cell Cycle Arrest and Apoptosis in Chago-K1 Human Bronchogenic Carcinoma Cells and Inhibited the Differentiation of THP-1 Cells into Tumour- Associated Macrophages. Asian Pac. J. Cancer Prev. 2018, 19, 3427–3434. [Google Scholar] [CrossRef] [Green Version]
- Kong, G.M.; Tao, W.H.; Diao, Y.L.; Fang, P.H.; Wang, J.J.; Bo, P.; Qian, F. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J. Gastroenterol. 2016, 22, 3186–3195. [Google Scholar] [CrossRef] [Green Version]
- Gajski, G.; Domijan, A.M.; Zegura, B.; Stern, A.; Geric, M.; Novak Jovanovic, I.; Vrhovac, I.; Madunic, J.; Breljak, D.; Filipic, M.; et al. Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon 2016, 110, 56–67. [Google Scholar] [CrossRef]
- Asthana, N.; Yadav, S.P.; Ghosh, J.K. Dissection of antibacterial and toxic activity of melittin: A leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J. Biol. Chem. 2004, 279, 55042–55050. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Srivastava, S.; Singh, M.; Bajpai, V.K.; Ghosh, J.K. Consequences of alteration in leucine zipper sequence of melittin in its neutralization of lipopolysaccharide-induced proinflammatory response in macrophage cells and interaction with lipopolysaccharide. J. Biol. Chem. 2012, 287, 1980–1995. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, H.; Liu, L.; Wang, B.; Sun, G. Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy. J. Transl. Med. 2016, 14, 155. [Google Scholar] [CrossRef] [Green Version]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Lee, M.K.; Kim, K.L.; Hahm, K.S. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 1997, 50, 279–285. [Google Scholar] [CrossRef]
- Ryu, S.; Choi, S.Y.; Acharya, S.; Chun, Y.J.; Gurley, C.; Park, Y.; Armstrong, C.A.; Song, P.I.; Kim, B.J. Antimicrobial and anti-inflammatory effects of Cecropin A(1-8)-Magainin2(1-12) hybrid peptide analog p5 against Malassezia furfur infection in human keratinocytes. J. Investig. Derm. 2011, 131, 1677–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Shao, X.; Sun, B.; Huang, W.; Qiu, F.; Chen, Y.; Shi, Y.K.; Zhang, E.Y.; Wang, C.; Zhao, X. Anticancer mechanism of peptide P18 in human leukemia K562 cells. Org. Biomol. Chem. 2010, 8, 984–987. [Google Scholar] [CrossRef]
- Nan, Y.H.; Jeon, Y.J.; Park, I.S.; Shin, S.Y. Antimicrobial peptide P18 inhibits inflammatory responses by LPS- but not by IFN-gamma-stimulated macrophages. Biotechnol. Lett. 2008, 30, 1183–1187. [Google Scholar] [CrossRef]
- Almeida, J.R.; Mendes, B.; Lancellotti, M.; Franchi, G.C., Jr.; Passos, O.; Ramos, M.J.; Fernandes, P.A.; Alves, C.; Vale, N.; Gomes, P.; et al. Lessons from a Single Amino Acid Substitution: Anticancer and Antibacterial Properties of Two Phospholipase A2-Derived Peptides. Curr. Issues Mol. Biol. 2021, 44, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Haney, E.F.; Hilchie, A.L.; Corcoran, J.A.; Hyndman, M.E.; Hancock, R.E.W.; Vogel, H.J. Selective anticancer activity of synthetic peptides derived from the host defence peptide tritrpticin. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183228. [Google Scholar] [CrossRef]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef]
- Ghiselli, R.; Giacometti, A.; Cirioni, O.; Mocchegiani, F.; Orlando, F.; Silvestri, C.; Di Matteo, F.; Abbruzzetti, A.; Scalise, G.; Saba, V. Efficacy of the bovine antimicrobial peptide indolicidin combined with piperacillin/tazobactam in experimental rat models of polymicrobial peritonitis. Crit. Care Med. 2008, 36, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.T.; Liu, Z.D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.F. Recent Advances in Small Peptides of Marine Origin in Cancer Therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Therapeutic Properties and Biological Benefits of Marine-Derived Anticancer Peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef] [Green Version]
- Iijima, N.; Tanimoto, N.; Emoto, Y.; Morita, Y.; Uematsu, K.; Murakami, T.; Nakai, T. Purification and characterization of three isoforms of chrysophsin, a novel antimicrobial peptide in the gills of the red sea bream, Chrysophrys major. Eur. J. Biochem. 2003, 270, 675–686. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.; Chen, J.Y. Characteristics of the antitumor activities in tumor cells and modulation of the inflammatory response in RAW264.7 cells of a novel antimicrobial peptide, chrysophsin-1, from the red sea bream (Chrysophrys major). Peptides 2011, 32, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Kumari, T.; Harioudh, M.K.; Yadav, P.K.; Kathuria, M.; Shukla, P.K.; Mitra, K.; Ghosh, J.K. Identification of GXXXXG motif in Chrysophsin-1 and its implication in the design of analogs with cell-selective antimicrobial and anti-endotoxin activities. Sci. Rep. 2017, 7, 3384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Swithenbank, L.; Cox, P.; Harris, L.G.; Dudley, E.; Sinclair, K.; Lewis, P.; Cappiello, F.; Morgan, C. Temporin A and Bombinin H2 Antimicrobial Peptides Exhibit Selective Cytotoxicity to Lung Cancer Cells. Science 2020, 2020, 3526286. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, A.; Tripathi, A.K.; Tandon, A.; Ghosh, J.K. Modulation of anti-endotoxin property of Temporin L by minor amino acid substitution in identified phenylalanine zipper sequence. Biochem. J. 2016, 473, 4045–4062. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Mocchegiani, F.; Orlando, F.; Silvestri, C.; Bozzi, A.; Di Giulio, A.; Luzi, C.; Mangoni, M.L.; et al. Interaction of antimicrobial peptide temporin L with lipopolysaccharide in vitro and in experimental rat models of septic shock caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 2478–2486. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Kumari, T.; Tandon, A.; Sayeed, M.; Afshan, T.; Kathuria, M.; Shukla, P.K.; Mitra, K.; Ghosh, J.K. Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat. Acta. Biomater. 2017, 57, 170–186. [Google Scholar] [CrossRef]
- Alvarez, C.A.; Santana, P.A.; Salinas-Parra, N.; Beltran, D.; Guzman, F.; Vega, B.; Acosta, F.; Mercado, L. Immune Modulation Ability of Hepcidin from Teleost Fish. Animals 2022, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.M.; Hilchie, A.L.; McMillan, K.A.M.; Liwski, R.S.; Hoskin, D.W.; Power Coombs, M.R. The Acute Phase Protein Hepcidin Is Cytotoxic to Human and Mouse Myeloma Cells. Anticancer Res. 2021, 41, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Niemirowicz, K.; Wnorowska, U.; Watek, M.; Wollny, T.; Gluszek, K.; Gozdz, S.; Levental, I.; Bucki, R. The Role of Cathelicidin LL-37 in Cancer Development. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.X.; Cheng, A.S.; To, K.F.; Tong, J.H.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef] [Green Version]
- Porter, R.J.; Murray, G.I.; Alnabulsi, A.; Humphries, M.P.; James, J.A.; Salto-Tellez, M.; Craig, S.G.; Wang, J.M.; Yoshimura, T.; McLean, M.H. Colonic epithelial cathelicidin (LL-37) expression intensity is associated with progression of colorectal cancer and presence of CD8(+) T cell infiltrate. J. Pathol. Clin. Res. 2021, 7, 495–506. [Google Scholar] [CrossRef]
- Tuomela, J.M.; Sandholm, J.A.; Kaakinen, M.; Hayden, K.L.; Haapasaari, K.M.; Jukkola-Vuorinen, A.; Kauppila, J.H.; Lehenkari, P.P.; Harris, K.W.; Graves, D.E.; et al. Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro. Breast. Cancer Res. Treat. 2016, 155, 261–271. [Google Scholar] [CrossRef]
- Ren, S.X.; Shen, J.; Cheng, A.S.; Lu, L.; Chan, R.L.; Li, Z.J.; Wang, X.J.; Wong, C.C.; Zhang, L.; Ng, S.S.; et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [Green Version]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Wodlej, C.; Riedl, S.; Rinner, B.; Leber, R.; Drechsler, C.; Voelker, D.R.; Choi, J.Y.; Lohner, K.; Zweytick, D. Interaction of two antitumor peptides with membrane lipids—Influence of phosphatidylserine and cholesterol on specificity for melanoma cells. PLoS ONE 2019, 14, e0211187. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.H.; Pan, C.Y.; Chen, N.F.; Yang, S.N.; Hsieh, S.; Wen, Z.H.; Chen, W.F.; Wang, J.W.; Lu, W.H.; Kuo, H.M. Piscidin-1 Induces Apoptosis via Mitochondrial Reactive Oxygen Species-Regulated Mitochondrial Dysfunction in Human Osteosarcoma Cells. Sci. Rep. 2020, 10, 5045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Tripathi, A.K.; Kathuria, M.; Shree, S.; Tripathi, J.K.; Purshottam, R.K.; Ramachandran, R.; Mitra, K.; Ghosh, J.K. Single Amino Acid Substitutions at Specific Positions of the Heptad Repeat Sequence of Piscidin-1 Yielded Novel Analogs That Show Low Cytotoxicity and In Vitro and In Vivo Antiendotoxin Activity. Antimicrob. Agents Chemother. 2016, 60, 3687–3699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, P.G.; Lamoureux, L.; Swingle, K.L.; Mukundan, H.; Montano, G.A. Lipopolysaccharide-induced dynamic lipid membrane reorganization: Tubules, perforations, and stacks. Biophys. J. 2014, 106, 2395–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Yamamoto, K.; Sato, Y.; Inoue, S.; Morinaga, T.; Hirano, E. Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed. Res. 2016, 37, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Liscano, Y.; Onate-Garzon, J.; Delgado, J.P. Peptides with Dual Antimicrobial-Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
- Bai, D.; Yu, S.; Zhong, S.; Zhao, B.; Qiu, S.; Chen, J.; Lunagariya, J.; Liao, X.; Xu, S. d-Amino Acid Position Influences the Anticancer Activity of Galaxamide Analogs: An Apoptotic Mechanism Study. Int. J. Mol. Sci. 2017, 18, 544. [Google Scholar] [CrossRef] [Green Version]
- Barras, D.; Chevalier, N.; Zoete, V.; Dempsey, R.; Lapouge, K.; Olayioye, M.A.; Michielin, O.; Widmann, C. A WXW motif is required for the anticancer activity of the TAT-RasGAP317-326 peptide. J. Biol. Chem. 2014, 289, 23701–23711. [Google Scholar] [CrossRef] [Green Version]
- Walrant, A.; Bauza, A.; Girardet, C.; Alves, I.D.; Lecomte, S.; Illien, F.; Cardon, S.; Chaianantakul, N.; Pallerla, M.; Burlina, F.; et al. Ionpair-pi interactions favor cell penetration of arginine/tryptophan-rich cell-penetrating peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183098. [Google Scholar] [CrossRef]
- Jobin, M.L.; Blanchet, M.; Henry, S.; Chaignepain, S.; Manigand, C.; Castano, S.; Lecomte, S.; Burlina, F.; Sagan, S.; Alves, I.D. The role of tryptophans on the cellular uptake and membrane interaction of arginine-rich cell penetrating peptides. Biochim. Biophys. Acta 2015, 1848, 593–602. [Google Scholar] [CrossRef]
- Tyagi, A.; Kapoor, P.; Kumar, R.; Chaudhary, K.; Gautam, A.; Raghava, G.P. In silico models for designing and discovering novel anticancer peptides. Sci. Rep. 2013, 3, 2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.B.; Wang, X.F.; Wang, H.Y.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.Z.; Wang, C.; Lang, L.; Zhou, Y.; Wang, H.; Shang, D.J. Design of potent, non-toxic anticancer peptides based on the structure of the antimicrobial peptide, temporin-1CEa. Arch. Pharm. Res. 2013, 36, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Piyadasa, H.; Hemshekhar, M.; Osawa, N.; Lloyd, D.; Altieri, A.; Basu, S.; Krokhin, O.V.; Halayko, A.J.; Mookherjee, N. Disrupting Tryptophan in the Central Hydrophobic Region Selectively Mitigates Immunomodulatory Activities of the Innate Defence Regulator Peptide IDR-1002. J. Med. Chem. 2021, 64, 6696–6705. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Faiyaz, S.; Choi, K.G.; Krokhin, O.V.; Mookherjee, N. Immunomodulatory Functions of the Human Cathelicidin LL-37 (aa 13-31)-Derived Peptides are Associated with Predicted alpha-Helical Propensity and Hydrophobic Index. Biomolecules 2019, 9, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, S.; Fei, W.; Feng, Y.; Shen, L.; Yang, X.; Wang, M.; Wu, M. Prediction of Anticancer Peptides with High Efficacy and Low Toxicity by Hybrid Model Based on 3D Structure of Peptides. Int. J. Mol. Sci. 2021, 22, 5630. [Google Scholar] [CrossRef]
- Kozlowski, M.R.; Kozlowski, R.E. A novel, small peptide with activity against human pancreatic cancer. Am. J. Cancer Res. 2020, 10, 1356–1365. [Google Scholar]
- Ohtake, T.; Fujimoto, Y.; Ikuta, K.; Saito, H.; Ohhira, M.; Ono, M.; Kohgo, Y. Proline-rich antimicrobial peptide, PR-39 gene transduction altered invasive activity and actin structure in human hepatocellular carcinoma cells. Br. J. Cancer 1999, 81, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Bian, D.; Li, W.; Xie, Y.; Li, X.; Lv, J.; Tang, R. Host defense peptide LL-37 is involved in the regulation of cell proliferation and production of pro-inflammatory cytokines in hepatocellular carcinoma cells. Amino. Acids. 2021, 53, 471–484. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhagat, D.; Mahalwal, M.; Sharma, N.; Raghava, G.P.S. AntiCP 2.0: An updated model for predicting anticancer peptides. Brief. Bioinform. 2021, 22, bbaa153. [Google Scholar] [CrossRef] [PubMed]
- Maraming, P.; Klaynongsruang, S.; Boonsiri, P.; Peng, S.F.; Daduang, S.; Rungsa, P.; Tavichakorntrakool, R.; Chung, J.G.; Daduang, J. Anti-metastatic Effects of Cationic KT2 Peptide (a Lysine/Tryptophan-rich Peptide) on Human Melanoma A375.S2 Cells. In Vivo 2021, 35, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Lind, D.S. Arginine and cancer. J. Nutr. 2004, 134, 2837S–2841S, discussion 2853S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdukiewicz, M.; Sidorczuk, K.; Rafacz, D.; Pietluch, F.; Bakala, M.; Slowik, J.; Gagat, P. CancerGram: An Effective Classifier for Differentiating Anticancer from Antimicrobial Peptides. Pharmaceutics 2020, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, S.; Zhang, L.; Zhao, Y.; Huang, L.; Gong, X.; Wang, H.; Shang, D. Cell surface binding, uptaking and anticancer activity of L-K6, a lysine/leucine-rich peptide, on human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 8293. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.L.; Yip, B.S.; Chen, K.H.; Yu, H.Y.; Chih, Y.H.; Cheng, H.T.; Chou, Y.T.; Cheng, J.W. Novel antimicrobial peptides with high anticancer activity and selectivity. PLoS ONE 2015, 10, e0126390. [Google Scholar] [CrossRef] [Green Version]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Rationally designed short cationic alpha-helical peptides with selective anticancer activity. J. Colloid. Interface Sci. 2022, 607, 488–501. [Google Scholar] [CrossRef]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Correlation between the secondary structure and surface activity of beta-sheet forming cationic amphiphilic peptides and their anticancer activity. Colloids Surf. B Biointerfaces 2022, 209, 112165. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Ding, Y.; Lv, S.; You, R.; Hadianamrei, R.; Tomeh, M.A.; Zhao, X. Anticancer effect of rationally designed alpha-helical amphiphilic peptides. Colloids Surf. B Biointerfaces 2022, 220, 112841. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, Q.; Yan, Q.; Hao, X.; Chen, Y. Alpha-helical cationic anticancer peptides: A promising candidate for novel anticancer drugs. Mini Rev. Med. Chem. 2015, 15, 73–81. [Google Scholar] [CrossRef]
- Bae, S.; Oh, K.; Kim, H.; Kim, Y.; Kim, H.R.; Hwang, Y.I.; Lee, D.S.; Kang, J.S.; Lee, W.J. The effect of alloferon on the enhancement of NK cell cytotoxicity against cancer via the up-regulation of perforin/granzyme B secretion. Immunobiology 2013, 218, 1026–1033. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Y.; Dong, W.; Guan, Y.; Shang, D. Effect of hydrophobicity on distinct anticancer mechanism of antimicrobial peptide chensinin-1b and its lipoanalog PA-C1b in breast cancer cells. Int. J. Biochem. Cell. Biol. 2022, 143, 106156. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open. Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Pedron, C.N.; Andrade, G.P.; Sato, R.H.; Torres, M.T.; Cerchiaro, G.; Ribeiro, A.O.; Oliveira, V.X., Jr. Anticancer activity of VmCT1 analogs against MCF-7 cells. Chem. Biol. Drug Des. 2018, 91, 588–596. [Google Scholar] [CrossRef]
- Uematsu, N.; Matsuzaki, K. Polar angle as a determinant of amphipathic alpha-helix-lipid interactions: A model peptide study. Biophys. J. 2000, 79, 2075–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef] [Green Version]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pentlavalli, S.; Coulter, S.; Laverty, G. Peptide Nanomaterials for Drug Delivery Applications. Curr. Protein Pept. Sci. 2020, 21, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Yang, Z.; Zhao, X. Controlled release of paclitaxel from a self-assembling peptide hydrogel formed in situ and antitumor study in vitro. Int. J. Nanomed. 2011, 6, 2143–2153. [Google Scholar] [CrossRef]
- Han, Y.Y.; Liu, H.Y.; Han, D.J.; Zong, X.C.; Zhang, S.Q.; Chen, Y.Q. Role of glycosylation in the anticancer activity of antibacterial peptides against breast cancer cells. Biochem. Pharm. 2013, 86, 1254–1262. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Morita, K.; Nishimura, K.; Yamamoto, S.; Shimizu, N.; Yashiro, T.; Kawabata, R.; Aoi, T.; Tamura, A.; Maruyama, T. In Situ Synthesis of an Anticancer Peptide Amphiphile Using Tyrosine Kinase Overexpressed in Cancer Cells. JACS Au 2022, 2, 2023–2028. [Google Scholar] [CrossRef]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Park, E.J.; Min, G.; Choi, J.; Na, D.H.; Bae, J.S. Dual Functioned Pegylated Phospholipid Micelles Containing Cationic Antimicrobial Decapeptide for Treating Sepsis. Theranostics 2017, 7, 3759–3767. [Google Scholar] [CrossRef]
- Jaber, S.; Iliev, I.; Angelova, T.; Nemska, V.; Sulikovska, I.; Naydenova, E.; Georgieva, N.; Givechev, I.; Grabchev, I.; Danalev, D. Synthesis, Antitumor and Antibacterial Studies of New Shortened Analogues of (KLAKLAK)2-NH2 and Their Conjugates Containing Unnatural Amino Acids. Molecules 2021, 26, 898. [Google Scholar] [CrossRef]
- Torfoss, V.; Isaksson, J.; Ausbacher, D.; Brandsdal, B.O.; Flaten, G.E.; Anderssen, T.; Cavalcanti-Jacobsen Cde, A.; Havelkova, M.; Nguyen, L.T.; Vogel, H.J.; et al. Improved anticancer potency by head-to-tail cyclization of short cationic anticancer peptides containing a lipophilic beta(2,2) -amino acid. J. Pept. Sci. 2012, 18, 609–619. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta. Biochim. Biophys. Sin. (Shanghai) 2017, 49, 916–925. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, H.; Xu, W. Applications and modifications of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) in peptides and peptidomimetics design and discovery. Curr. Protein Pept. Sci. 2010, 11, 752–758. [Google Scholar] [CrossRef]
- Sayago, F.J.; Calaza, M.I.; Jimenez, A.I.; Cativiela, C. Versatile methodology for the synthesis and alpha-functionalization of (2R,3aS,7aS)-octahydroindole-2-carboxylic acid. Tetrahedron 2008, 64, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Misawa, T.; Doi, M.; Demizu, Y. Extent of Helical Induction Caused by Introducing alpha-Aminoisobutyric Acid into an Oligovaline Sequence. ACS Omega 2018, 3, 6395–6399. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.P. Antibacterial and anticancer activity of a series of novel peptides incorporating cyclic tetra-substituted C(alpha) amino acids. Bioorg. Med. Chem. 2016, 24, 4056–4065. [Google Scholar] [CrossRef]
- Burgess, A.W. Designing amino acids to determine the local conformations of peptides. Proc. Natl. Acad. Sci. USA 1994, 91, 2649–2653. [Google Scholar] [CrossRef] [Green Version]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to generate highly stable D-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB. Proc. Natl. Acad. Sci. USA 2018, 115, 1505–1510. [Google Scholar] [CrossRef] [Green Version]
- Desai, T.J.; Toombs, J.E.; Minna, J.D.; Brekken, R.A.; Udugamasooriya, D.G. Identification of lipid-phosphatidylserine (PS) as the target of unbiasedly selected cancer specific peptide-peptoid hybrid PPS1. Oncotarget 2016, 7, 30678–30690. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Kanwar, S.S. Phosphatidylserine: A cancer cell targeting biomarker. Semin. Cancer Biol. 2018, 52, 17–25. [Google Scholar] [CrossRef]
- Memmel, S.; Sukhorukov, V.L.; Horing, M.; Westerling, K.; Fiedler, V.; Katzer, A.; Krohne, G.; Flentje, M.; Djuzenova, C.S. Cell surface area and membrane folding in glioblastoma cell lines differing in PTEN and p53 status. PLoS ONE 2014, 9, e87052. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Han, X.; Gu, J.; Li, J.; Lou, W.; Jin, C.; Saiyin, H. The physiological characteristics of the basal microvilli microvessels in pancreatic cancers. Cancer Med. 2020, 9, 5535–5545. [Google Scholar] [CrossRef]
- Herrera-Leon, C.; Ramos-Martin, F.; Antonietti, V.; Sonnet, P.; D’Amelio, N. The impact of phosphatidylserine exposure on cancer cell membranes on the activity of the anticancer peptide HB43. FEBS J. 2022, 289, 1984–2003. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Papo, N.; Shai, Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J. Biol. Chem. 2006, 281, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.; Srivastava, S.; Mishra, N.N.; Tripathi, J.K.; Shukla, P.K.; Ghosh, J.K. Characterization of antimicrobial, cytotoxic, and antiendotoxin properties of short peptides with different hydrophobic amino acids at “a” and “d” positions of a heptad repeat sequence. J. Med. Chem. 2013, 56, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.J.; Sut, T.N.; Tan, S.W.; Yoon, B.K.; Jackman, J.A. Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge. Int. J. Mol. Sci. 2022, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Camilio, K.A.; Wang, M.Y.; Mauseth, B.; Waagene, S.; Kvalheim, G.; Rekdal, O.; Sveinbjornsson, B.; Maelandsmo, G.M. Combining the oncolytic peptide LTX-315 with doxorubicin demonstrates therapeutic potential in a triple-negative breast cancer model. Breast Cancer Res. 2019, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsson, B.; Camilio, K.A.; Haug, B.E.; Rekdal, O. LTX-315: A first-in-class oncolytic peptide that reprograms the tumor microenvironment. Future Med. Chem. 2017, 9, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Ehrenstein, G.; Lecar, H. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys. 1977, 10, 1–34. [Google Scholar] [CrossRef]
- Pouny, Y.; Rapaport, D.; Mor, A.; Nicolas, P.; Shai, Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 1992, 31, 12416–12423. [Google Scholar] [CrossRef]
- Majewska, M.; Zamlynny, V.; Pieta, I.S.; Nowakowski, R.; Pieta, P. Interaction of LL-37 human cathelicidin peptide with a model microbial-like lipid membrane. Bioelectrochemistry 2021, 141, 107842. [Google Scholar] [CrossRef]
- Li, M.; Xi, X.; Ma, C.; Chen, X.; Zhou, M.; Burrows, J.F.; Chen, T.; Wang, L. A Novel Dermaseptin Isolated from the Skin Secretion of Phyllomedusa tarsius and Its Cationicity-Enhanced Analogue Exhibiting Effective Antimicrobial and Anti-Proliferative Activities. Biomolecules 2019, 9, 628. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Leontiadou, H.; Mark, A.E.; Marrink, S.J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta 2008, 1778, 2308–2317. [Google Scholar] [CrossRef] [Green Version]
- Raucher, D.; Ryu, J.S. Cell-penetrating peptides: Strategies for anticancer treatment. Trends. Mol. Med. 2015, 21, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Bitler, B.G.; Schroeder, J.A. Anti-cancer therapies that utilize cell penetrating peptides. Recent Pat. Anticancer Drug Discov. 2010, 5, 99–108. [Google Scholar] [CrossRef]
- Nasiri, F.; Atanaki, F.F.; Behrouzi, S.; Kavousi, K.; Bagheri, M. CpACpP: In Silico Cell-Penetrating Anticancer Peptide Prediction Using a Novel Bioinformatics Framework. ACS Omega 2021, 6, 19846–19859. [Google Scholar] [CrossRef]
- Buteau, C.; Markovic, S.N.; Celis, E. Challenges in the development of effective peptide vaccines for cancer. Mayo. Clin. Proc. 2002, 77, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Magnan, C.N.; Zeller, M.; Kayala, M.A.; Vigil, A.; Randall, A.; Felgner, P.L.; Baldi, P. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics 2010, 26, 2936–2943. [Google Scholar] [CrossRef] [Green Version]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef] [Green Version]
- Larsen, M.V.; Lundegaard, C.; Lamberth, K.; Buus, S.; Lund, O.; Nielsen, M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinform. 2007, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Tang, Y.; Chen, Q.; Liu, Y. The Screening of Therapeutic Peptides for Anti-Inflammation through Phage Display Technology. Int. J. Mol. Sci. 2022, 23, 8554. [Google Scholar] [CrossRef]
- Karami Fath, M.; Babakhaniyan, K.; Zokaei, M.; Yaghoubian, A.; Akbari, S.; Khorsandi, M.; Soofi, A.; Nabi-Afjadi, M.; Zalpoor, H.; Jalalifar, F.; et al. Anti-cancer peptide-based therapeutic strategies in solid tumors. Cell Mol. Biol. Lett. 2022, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, A.; Nistico, N.; Mimmi, S.; Maisano, D.; Vecchio, E.; Fiume, G.; Iaccino, E.; Quinto, I. Phage-Displayed Peptides for Targeting Tyrosine Kinase Membrane Receptors in Cancer Therapy. Viruses 2021, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharm. Toxicol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; La Manna, S.; De Benedictis, I.; Brandi, P.; Marasco, D. Perspectives in Peptide-Based Vaccination Strategies for Syndrome Coronavirus 2 Pandemic. Front. Pharm. 2020, 11, 578382. [Google Scholar] [CrossRef]
- Yang, H.; Cao, J.; Lin, X.; Yue, J.; Zieneldien, T.; Kim, J.; Wang, L.; Fang, J.; Huang, R.P.; Bai, Y.; et al. Developing an Effective Peptide-Based Vaccine for COVID-19: Preliminary Studies in Mice Models. Viruses 2022, 14, 449. [Google Scholar] [CrossRef]



| Sl No | Peptide Name | Sequence |
|---|---|---|
| 1 | LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| 2 | Magainin II | GIGKFLHSAKKFGKAFVGEIMNS |
| 3 | Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ |
| 4 | P18 | KWKLFKKIPKFLHLAKKF |
| 5 | Tritrpticin | VRRFPWWWPFLRR |
| 6 | Indolicidin | ILPWKWPWWPWRR |
| 7 | PuroA | FPVTWKWWKWWKG |
| 8 | Chrysophsin-1 | FFGWLIKGAIHAGKAIHGLIHRRRH |
| 9 | Chrysophsin-2 | FFGWLIRGAIHAGKAIHGLIHRRRH |
| 10 | Temporin L | FVQWFSKFLGRIL |
| 11 | Temporin A | FLPLIGRVLSGIL |
| 12 | Bombinin H2 | LIGPVLGLVGSALGGLLKKI |
| 13 | Hepcidin | ICIFCCGCCHRSKCGMCCKT |
| 14 | KSL-W | KKVVFWVKFK |
| 15 | HB43 | FAKLLAKLAKKLL |
| 16 | LTX-315 * | KKWWKKW-DipK |
| 16 | KTH-222 | LKGQLRCI |
| 17 | K4R2-Nal2-S1 ** | KKKKRR-Nal-Nal-KKWRKWLAKK |
| 18 | PR-39 | RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP |
| 19 | L-K6 | IKKILSKIKKLLK |
| 20 | IK-13 | CIIKKIIKKIIKK |
| 21 | LK-13 | CLLKKLLKKLLKK |
| 22 | Alloferon | HGVSGHGQHGVHG |
| 23 | LactoferricinB (LfcinB) | FKCRRWQWRMKKLGAPSITCVRRAF |
| 24 | RADA16 | RADARADARADARADA |
| 25 | E3PA | AAAAGGGEEE |
| 26 | FLAK50 | FAKLLAKLAKKLL |
| 27 | VmCT1 | FLGALWNVAKSVF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, A.K.; Vishwanatha, J.K. Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy. Pharmaceutics 2022, 14, 2686. https://doi.org/10.3390/pharmaceutics14122686
Tripathi AK, Vishwanatha JK. Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy. Pharmaceutics. 2022; 14(12):2686. https://doi.org/10.3390/pharmaceutics14122686
Chicago/Turabian StyleTripathi, Amit Kumar, and Jamboor K. Vishwanatha. 2022. "Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy" Pharmaceutics 14, no. 12: 2686. https://doi.org/10.3390/pharmaceutics14122686
APA StyleTripathi, A. K., & Vishwanatha, J. K. (2022). Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy. Pharmaceutics, 14(12), 2686. https://doi.org/10.3390/pharmaceutics14122686








