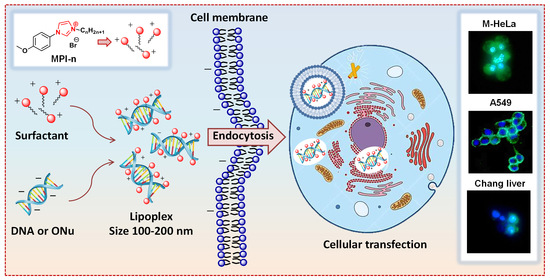
Complexation of Oligo- and Polynucleotides with Methoxyphenyl-Functionalized Imidazolium Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Sample Preparation
2.1.1. Reagents and Probes
2.1.2. Solution Preparation
2.2. Methods
2.2.1. Dynamic and Electrophoretic Light Scattering
2.2.2. Gel Electrophoresis
2.2.3. Fluorescence Spectroscopy
2.2.4. Circular Dichroism Experiments
2.2.5. Transmission Electron Microscopy
2.2.6. Cytotoxicity Assay
2.2.7. Flow Cytometry Assay
2.2.8. Fluorescence Microscopy
2.2.9. DNA Transfection
2.2.10. Hemagglutination Assay
2.2.11. X-ray Crystallography
3. Results and Discussion
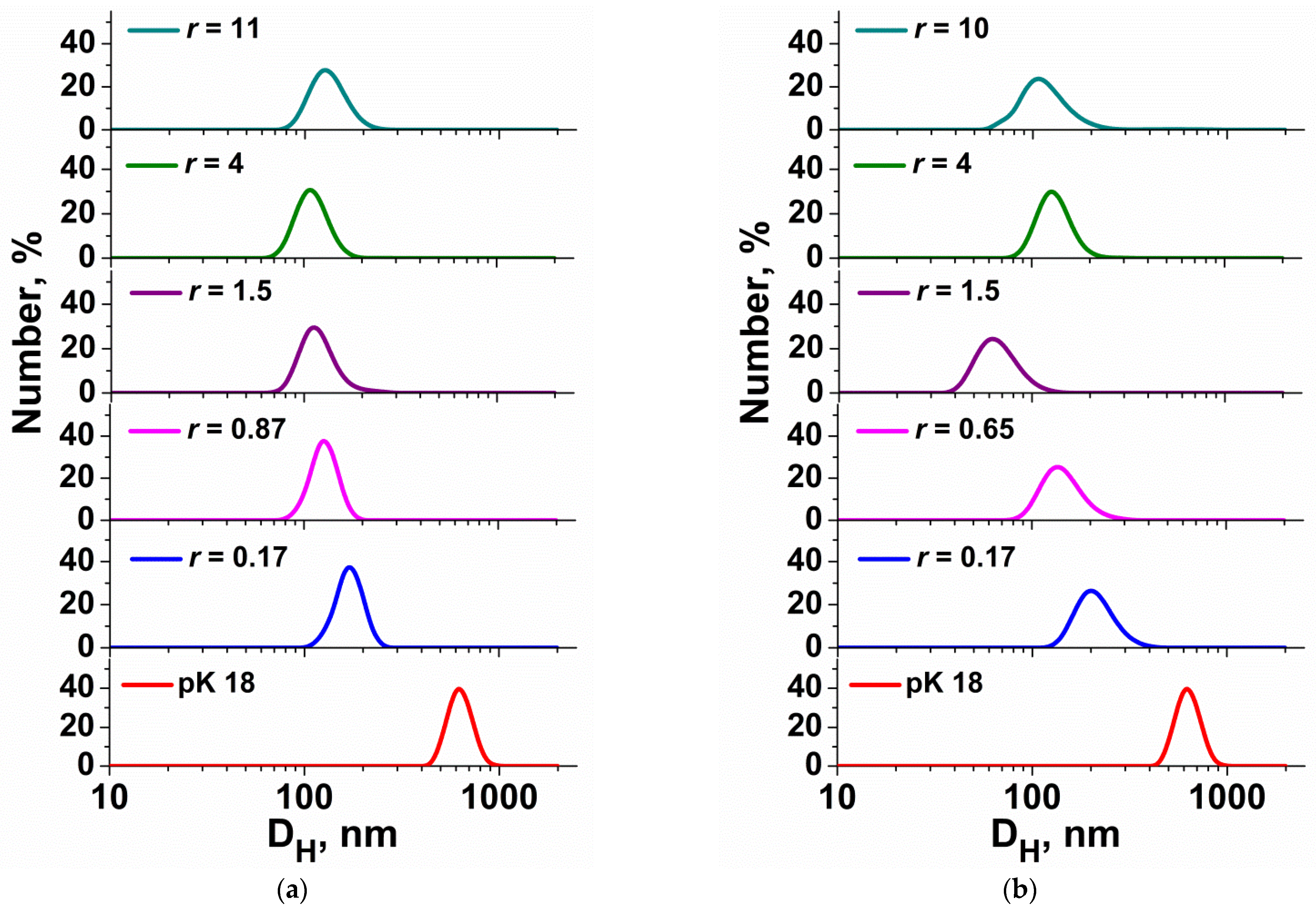
3.1. DLS Measuring for Surfactant/ONu and Surfactant/DNA Complexes
3.2. Zeta Potential for Surfactant/ONu and Surfactant/DNA Complexes
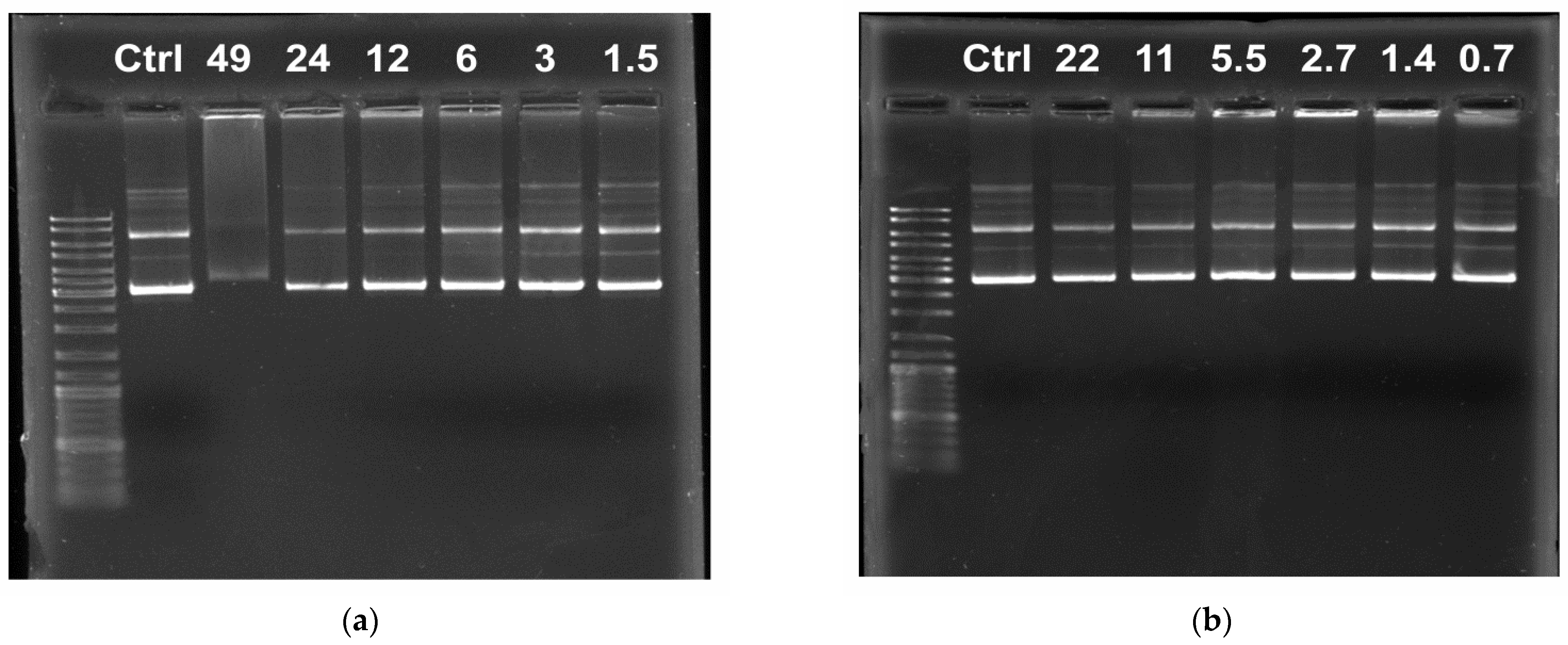
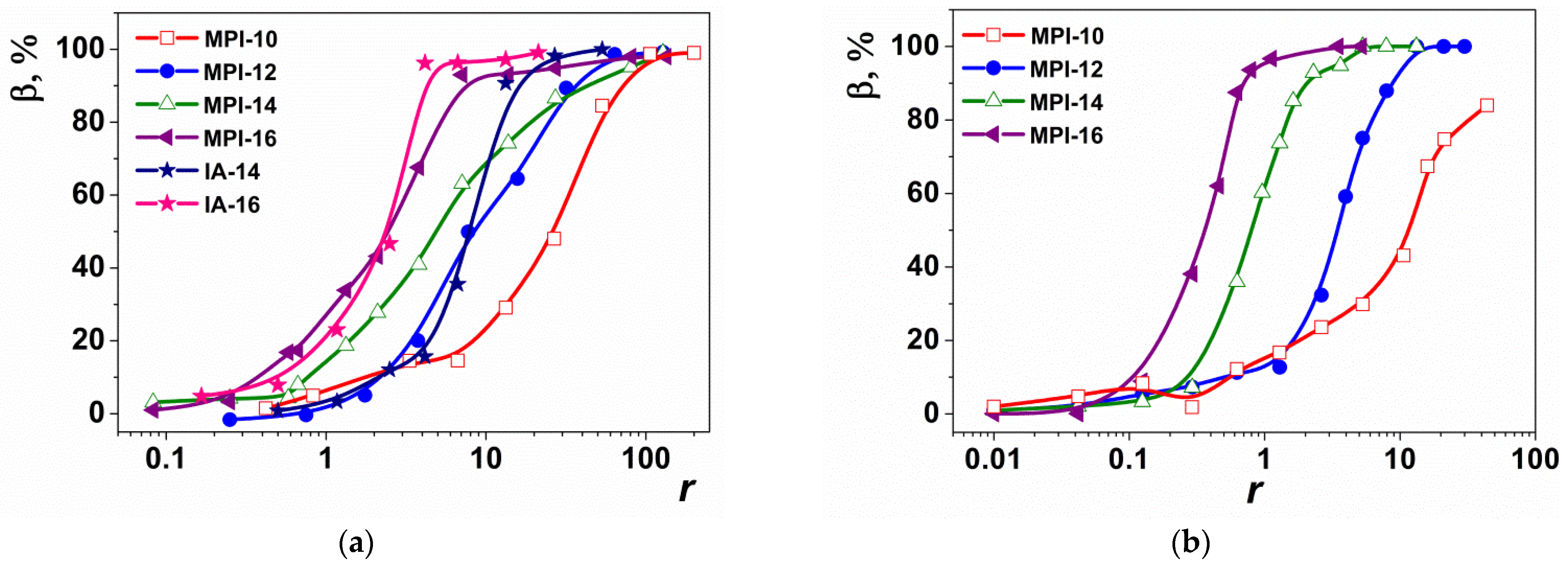
3.3. Gel Electrophoresis
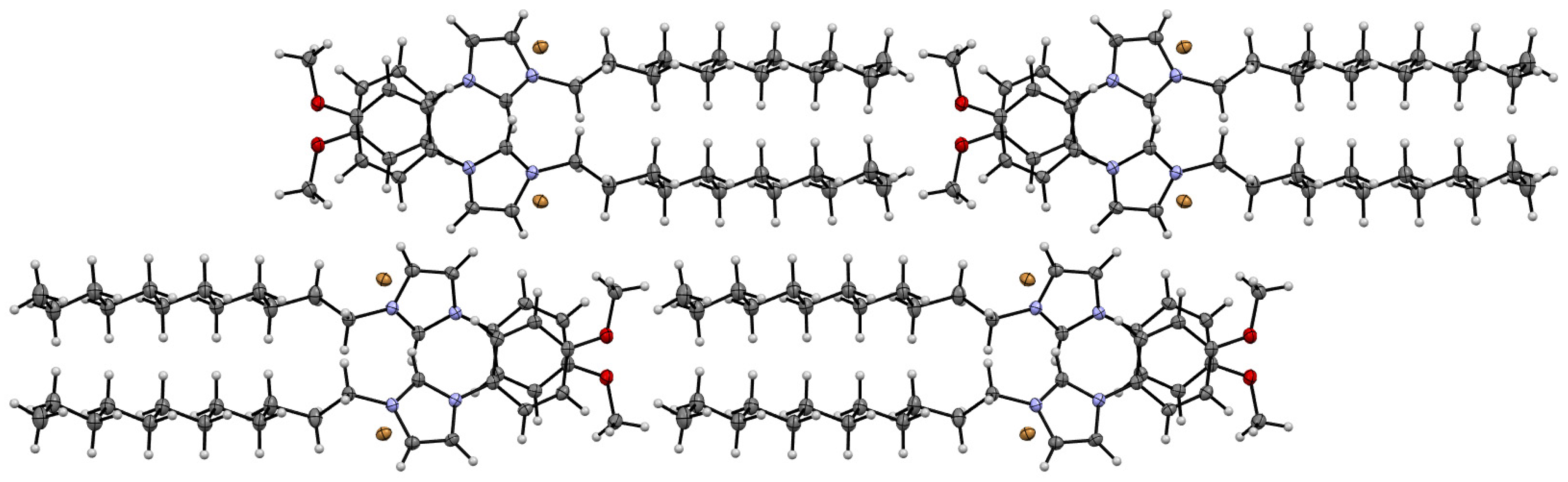
3.4. Single-Crystal X-ray Investigations of the Structure of the MPI-12
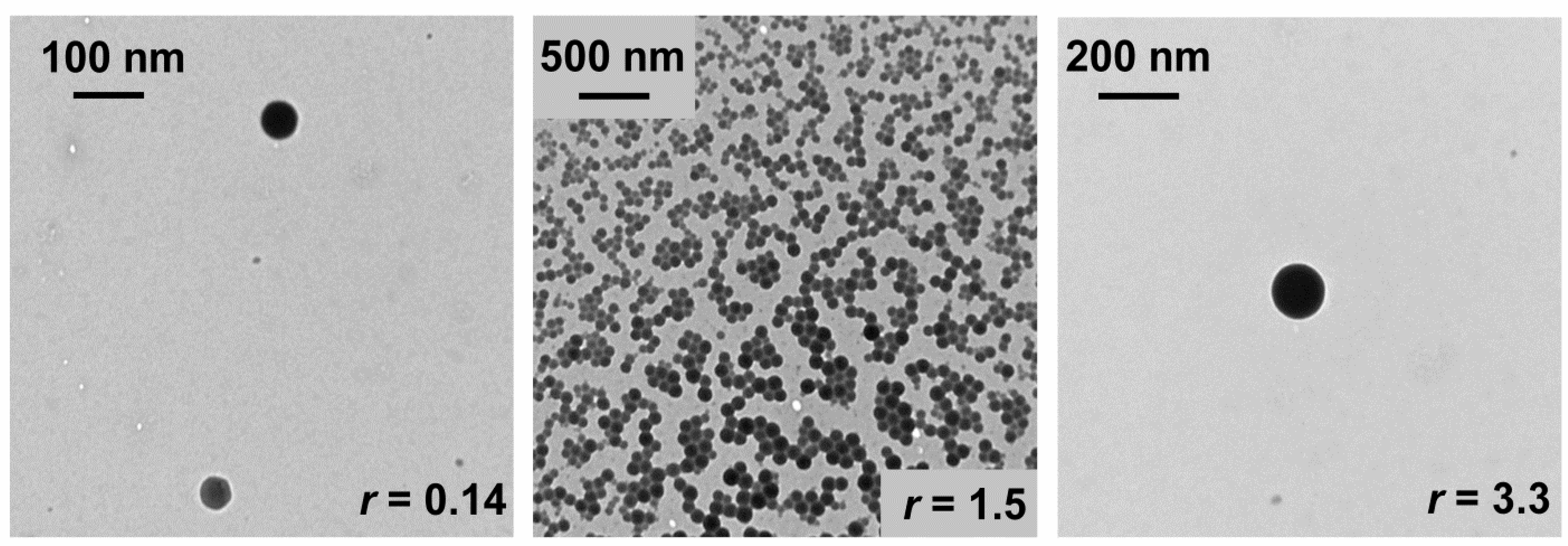
3.5. Transmission Electron Microscopy
3.6. Ethidium Bromide (EB) Exclusion Assay for Surfactant/ONu and Surfactant/DNA Complexes
3.7. Circular Dichroism Studies
3.8. Cytotoxicity Assay of MPI-n/ONu and MPI-n/DNA Complexes In Vitro
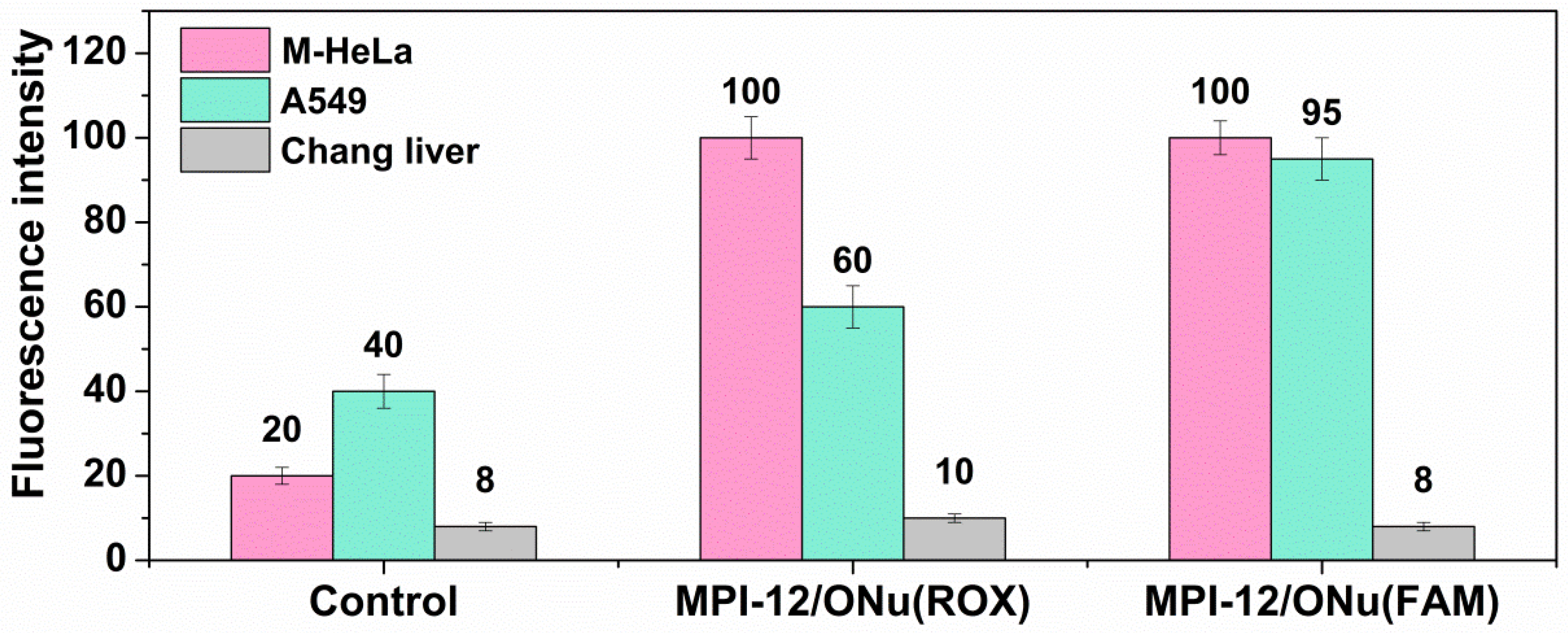
3.9. Flow Cytometry Assay
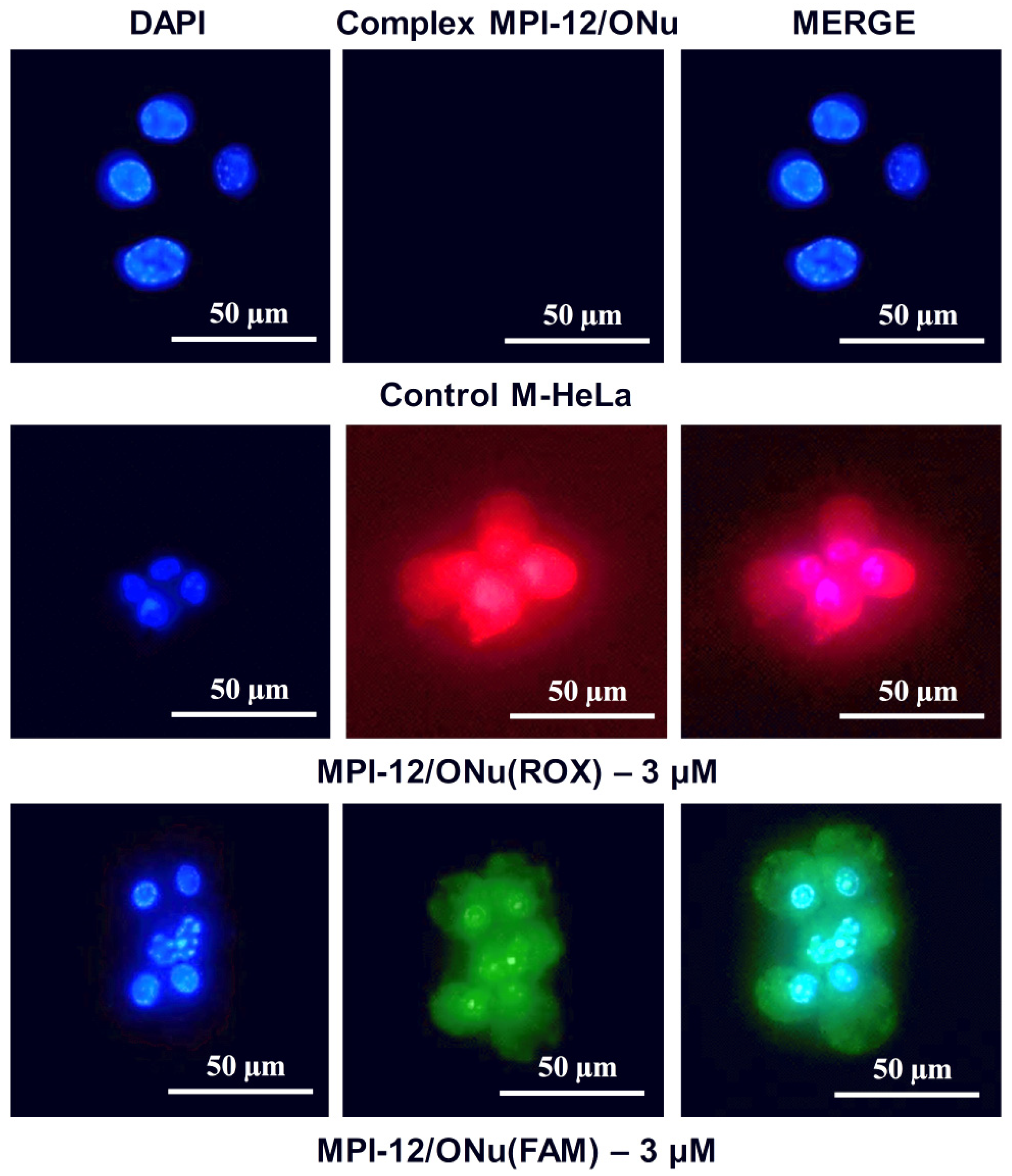
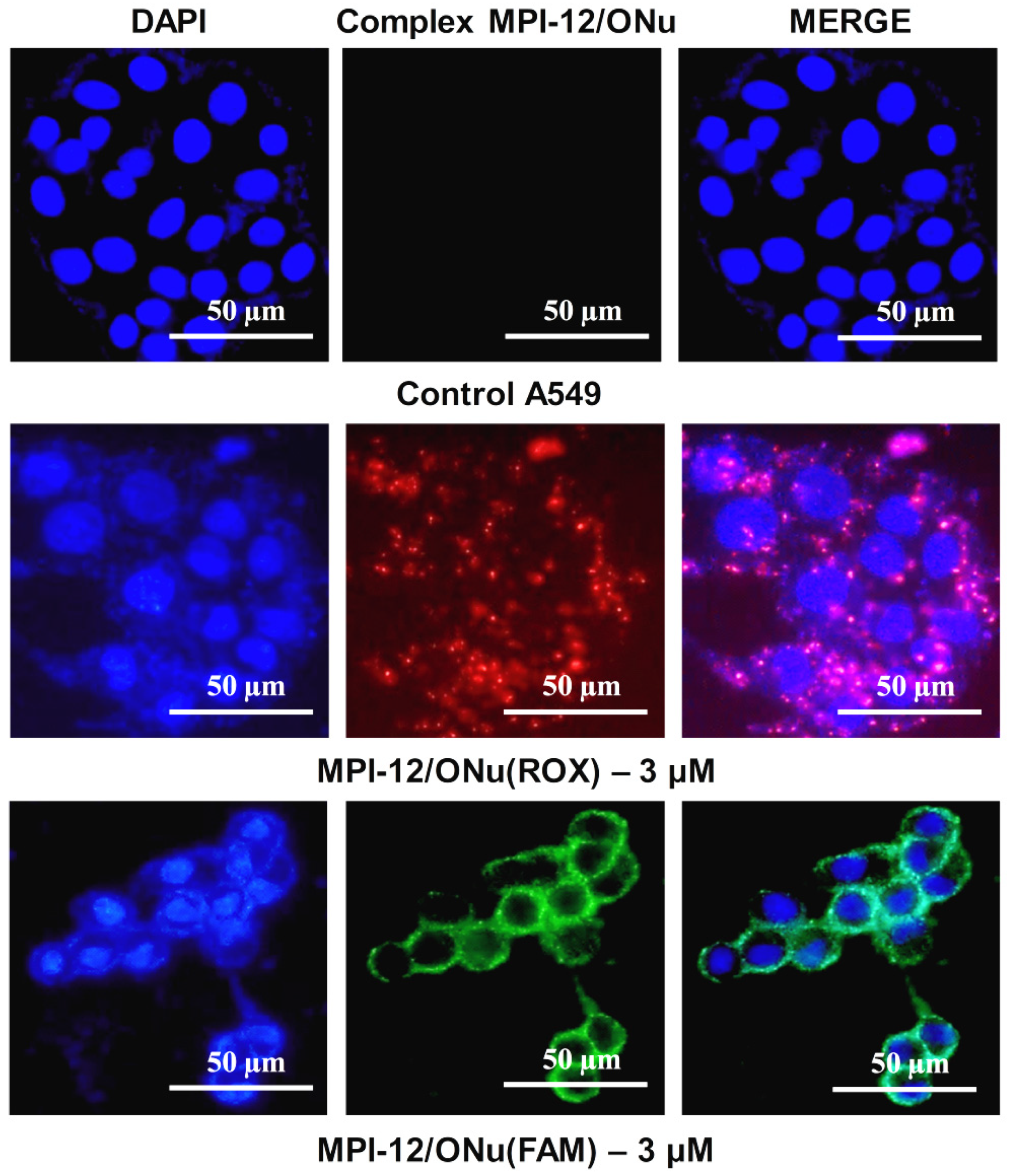
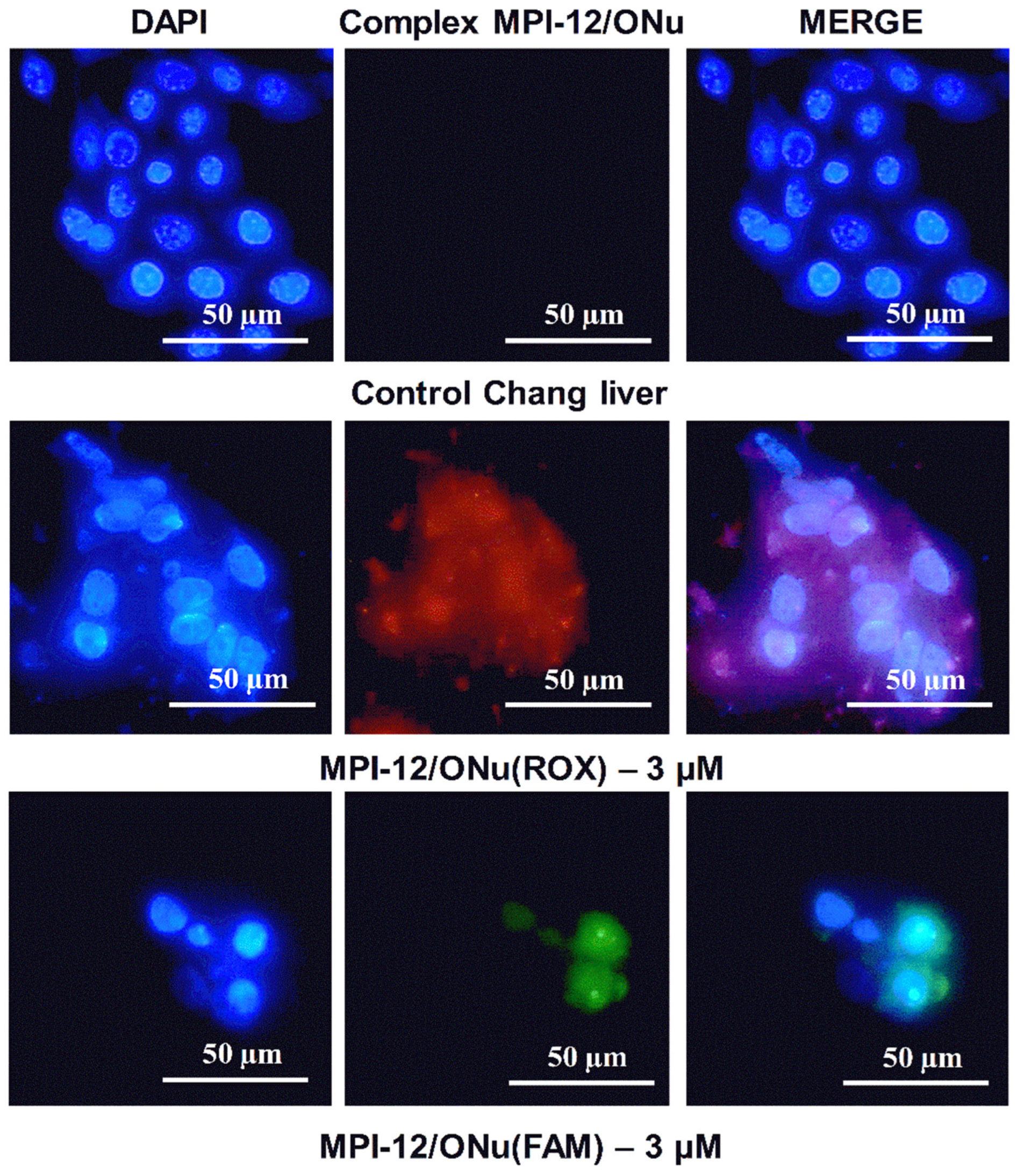
3.10. Fluorescence Microscopy
3.11. In Vitro Transfection Studies into M-HeLa and A549 Cell Lines
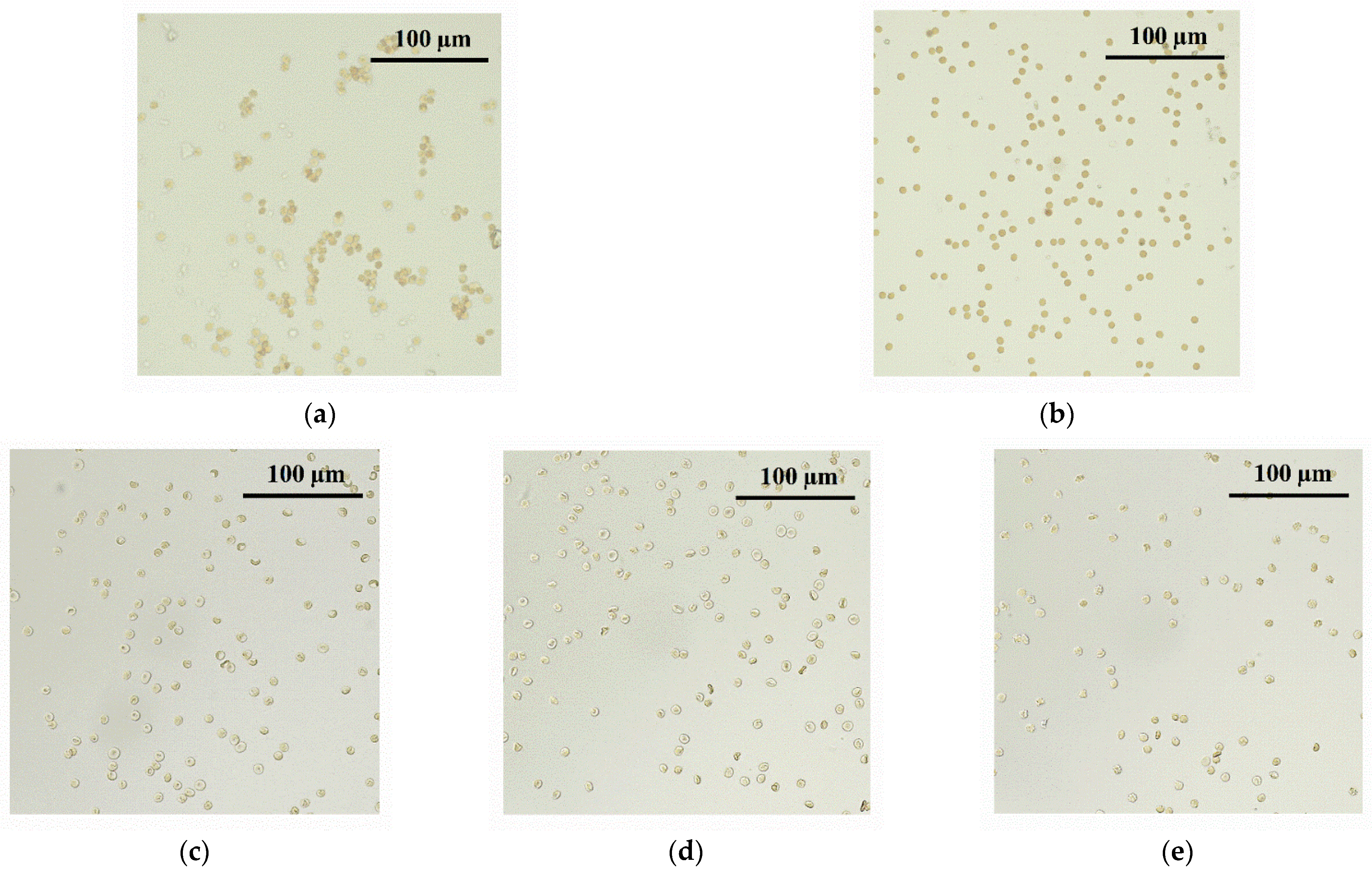
3.12. Hemagglutination Analysis for MPI/ONu Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.; Arora, S.; Singh, J.; Layek, B. A Review of the Tortuous Path of Nonviral Gene Delivery and Recent Progress. Int. J. Biol. Macromol. 2021, 183, 2055–2073. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Dehshahri, A.; Sagar Madamsetty, V.; Zahmatkeshan, M.; Tavakol, S.; Makvandi, P.; Khorsandi, D.; Pardakhty, A.; Ashrafizadeh, M.; Ghasemipour Afshar, E.; et al. In Vivo Gene Delivery Mediated by Non-Viral Vectors for Cancer Therapy. J. Control. Release 2020, 325, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, W.; Wilkowska, M.; Skrzypczak, A.; Kozak, M. Ammonium Gemini Surfactants Form Complexes with Model Oligomers of SiRNA and DsDNA. Int. J. Mol. Sci. 2019, 20, 5546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic Gemini Surfactant Properties, Its Potential as a Promising Bioapplication Candidate, and Strategies for Improving Its Biocompatibility: A Review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef] [PubMed]
- Pal Singh, P.; Vithalapuram, V.; Metre, S.; Kodipyaka, R. Lipoplex-Based Therapeutics for Effective Oligonucleotide Delivery: A Compendious Review. J. Liposome Res. 2020, 30, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, W.; Wilkowska, M.; Peplińska, B.; Skrzypczak, A.; Kozak, M. Structural Characterization of Transfection Nanosystems Based on Tricationic Surfactants and Short Double Stranded Oligonucleotides. Biochem. Biophys. Res. Commun. 2019, 518, 706–711. [Google Scholar] [CrossRef]
- Amer, M.H. Gene Therapy for Cancer: Present Status and Future Perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Huang, L. Lipid Nanoparticles for Gene Delivery. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 88, pp. 13–36. ISBN 978-0-12-800148-6. [Google Scholar]
- Santana-Armas, M.L.; Tros de Ilarduya, C. Strategies for Cancer Gene-Delivery Improvement by Non-Viral Vectors. Int. J. Pharm. 2021, 596, 120291. [Google Scholar] [CrossRef]
- Gigante, A.; Li, M.; Junghänel, S.; Hirschhäuser, C.; Knauer, S.; Schmuck, C. Non-Viral Transfection Vectors: Are Hybrid Materials the Way Forward? MedChemComm 2019, 10, 1692–1718. [Google Scholar] [CrossRef]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic Lipids for Gene Delivery: Many Players, One Goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, J.; Zhang, Z.; Zheng, G. Lipid-Based Nanoparticles in the Systemic Delivery of SiRNA. Nanomed. 2014, 9, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, M.Á.; Morán, C.; Raluy, M.; Pérez, L. Gemini Histidine Based Surfactants: Characterization; Surface Properties and Biological Activity. J. Mol. Liq. 2019, 289, 111156. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Vasilieva, E.A.; Voronin, M.A.; Kuznetsova, D.A.; Valeeva, F.G.; Mirgorodskaya, A.B.; Lukashenko, S.S.; Zakharov, V.M.; Mukhitov, A.R.; Faizullin, D.A.; et al. Soft Nanocontainers Based on Hydroxyethylated Geminis: Role of Spacer in Self-Assembling, Solubilization, and Complexation with Oligonucleotide. J. Phys. Chem. C 2020, 124, 2178–2192. [Google Scholar] [CrossRef]
- He, S.; Fang, J.; Zhong, C.; Ren, F.; Wang, M. Controlled PVEGF Delivery via a Gene-Activated Matrix Comprised of a Peptide-Modified Non-Viral Vector and a Nanofibrous Scaffold for Skin Wound Healing. Acta Biomater. 2022, 140, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Posvyatenko, A.V.; Larin, S.S.; Ananikov, V.P. Ionic Liquids: Prospects for Nucleic Acid Handling and Delivery. Nucleic Acids Res. 2021, 49, 1201–1234. [Google Scholar] [CrossRef] [PubMed]
- Elouahabi, A.; Ruysschaert, J.-M. Formation and Intracellular Trafficking of Lipoplexes and Polyplexes. Mol. Ther. 2005, 11, 336–347. [Google Scholar] [CrossRef]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Zeng, M.; Xu, Q.; Zhou, D.; Sigen, A.; Alshehri, F.; Lara-Sáez, I.; Zheng, Y.; Li, M.; Wang, W. Highly Branched Poly(β-Amino Ester)s for Gene Delivery in Hereditary Skin Diseases. Adv. Drug Deliv. Rev. 2021, 176, 113842. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Cheng, R.; Cai, K.; Wang, X.; Xie, C.; Xu, T.; Yuan, C. Interaction of Calf Thymus DNA and Glucose-Based Gemini Cationic Surfactants with Different Spacer Length: A Spectroscopy and DLS Study. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2022, 267, 120606. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.R.; Kumar, M.; Murumkar, P.R. Further Studies on Cationic Gemini Amphiphiles as Carriers for Gene Delivery─The Effect of Linkers in the Structure and Other Factors Affecting the Transfection Efficacy of These Amphiphiles. ACS Omega 2021, 6, 33370–33388. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, K.; Li, S. Nucleic Acids Based Polyelectrolyte Complexes: Their Complexation Mechanism, Morphology, and Stability. Chem. Mater. 2021, 33, 7923–7943. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Samarkina, D.A.; Semenov, V.E.; Saifina, L.F.; Zakharova, L.Y. Amphiphilic Macrocyclic Derivative of Pyrimidine: Self-Assembly, Solubilization and Interaction with DNA Decamer. Macroheterocycles 2017, 10, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Cortesi, R.; Esposito, E.; Menegatti, E.; Gambari, R.; Nastruzzi, C. Effect of Cationic Liposome Composition on in Vitro Cytotoxicity and Protective Effect on Carried DNA. Int. J. Pharm. 1996, 139, 69–78. [Google Scholar] [CrossRef]
- Li, X.; Sun, D.; Chen, Y.; Wang, K.; He, Q.; Wang, G. Studying Compaction-Decompaction of DNA Molecules Induced by Surfactants. Biochem. Biophys. Res. Commun. 2018, 495, 2559–2565. [Google Scholar] [CrossRef]
- Gawęda, S.; Morán, M.C.; Pais, A.A.C.C.; Dias, R.S.; Schillén, K.; Lindman, B.; Miguel, M.G. Cationic Agents for DNA Compaction. J. Colloid Interface Sci. 2008, 323, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Cai, K.; Cheng, R.; Wang, C.; Xia, Y.; Xu, T.; Gan, C. Interactions with CtDNA of Novel Sugar-Based Gemini Cationic Surfactants. Int. J. Biol. Macromol. 2020, 156, 805–811. [Google Scholar] [CrossRef]
- Mel’nikov, S.M.; Sergeyev, V.G.; Yoshikawa, K. Transition of Double-Stranded DNA Chains between Random Coil and Compact Globule States Induced by Cooperative Binding of Cationic Surfactant. J. Am. Chem. Soc. 1995, 117, 9951–9956. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mandal, S.S. Interaction of Surfactants with DNA. Role of Hydrophobicity and Surface Charge on Intercalation and DNA Melting. Biochim. Biophys. Acta BBA Biomembr. 1997, 1323, 29–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Zhang, Z.; Qiao, H.; Liu, M.; Shen, M.; Yuan, T.; Chen, J.; Dionysiou, D.D. Spectroscopic Study on Interaction between Three Cationic Surfactants with Different Alkyl Chain Lengths and DNA. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015, 151, 237–246. [Google Scholar] [CrossRef] [PubMed]
- López-López, M.; López-Cornejo, P.; Martín, V.I.; Ostos, F.J.; Checa-Rodríguez, C.; Prados-Carvajal, R.; Lebrón, J.A.; Huertas, P.; Moyá, M.L. Importance of Hydrophobic Interactions in the Single-Chained Cationic Surfactant-DNA Complexation. J. Colloid Interface Sci. 2018, 521, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lebrón, J.A.; López-Cornejo, P.; García-Dionisio, E.; Huertas, P.; García-Calderón, M.; Moyá, M.L.; Ostos, F.J.; López-López, M. Cationic Single-Chained Surfactants with a Functional Group at the End of the Hydrophobic Tail DNA Compacting Efficiency. Pharmaceutics 2021, 13, 589. [Google Scholar] [CrossRef]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional Supramolecular Systems: Design and Applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Khamatgalimov, A.R.; Kovalenko, V.I.; Zakharova, L.Y. Cationic Amphiphiles Bearing Imidazole Fragment: From Aggregation Properties to Potential in Biotechnologies. Colloids Surf. Physicochem. Eng. Asp. 2017, 529, 990–997. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Kuznetsov, D.M.; Lukashenko, S.S.; Zakharov, V.M.; Sapunova, A.S.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Salakhieva, D.V.; et al. Polymer–Colloid Complexes Based on Cationic Imidazolium Amphiphile, Polyacrylic Acid and DNA Decamer. Molecules 2021, 26, 2363. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, G.; Ao, M.; Yang, Y.; Wang, C. DNA Compaction to Multi-Molecular DNA Condensation Induced by Cationic Imidazolium Gemini Surfactants. Colloids Surf. Physicochem Eng. Asp. 2012, 414, 33–40. [Google Scholar] [CrossRef]
- Bhadani, A.; Misono, T.; Singh, S.; Sakai, K.; Sakai, H.; Abe, M. Structural Diversity, Physicochemical Properties and Application of Imidazolium Surfactants: Recent Advances. Adv. Colloid Interface Sci. 2016, 231, 36–58. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Kashapov, R.R.; Zakharova, L.Y. Self-Assembled Systems Based on Novel Hydroxyethylated Imidazolium-Containing Amphiphiles: Interaction with DNA Decamer, Protein and Lipid. Chem. Phys. Lipids 2019, 223, 104791. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Ahtamyanova, L.R.; Lukashenko, S.S.; Kusova, A.M.; Zuev, Y.F.; Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Kuznetsov, D.M.; et al. Novel Self-Assembling Systems Based on Imidazolium Amphiphiles with Cleavable Urethane Fragment for Construction of Soft Nanocontainers for Biomedicine Application. J. Mol. Liq. 2020, 298, 111961. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.; Samarkina, D.; Semenov, V.; Syakaev, V.; Giniyatullin, R.; Gogoleva, N.; Reznik, V.; Latypov, S.; Konovalov, A.; Pokrovsky, A.; et al. Novel Dicationic Pyrimidinic Surfactant: Self-Assembly and DNA Complexation. Colloids Surf. Physicochem. Eng. Asp. 2015, 480, 113–121. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Kuznetsov, D.M.; Amerhanova, S.K.; Buzmakova, E.V.; Lyubina, A.P.; Syakaev, V.V.; Nizameev, I.R.; Kadirov, M.K.; Voloshina, A.D.; Zakharova, L.Y. Cationic Imidazolium Amphiphiles Bearing a Methoxyphenyl Fragment: Synthesis, Self-Assembly Behavior, and Antimicrobial Activity. Langmuir 2022, 38, 4921–4934. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Kuznetsov, D.M.; Zakharov, V.M.; Zakharova, L.Y. Interaction of Bovine Serum Albumin with Cationic Imidazolium Surfactants Containing a Methoxyphenyl Fragment. Russ. J. Gen. Chem. 2022, 92, 1262–1270. [Google Scholar] [CrossRef]
- Kusnetsova, D.; Vasilieva, E.; Kuznetsov, D.; Buzmakova, E.; Zakharova, L. Aggregation Behavior of the Mixed Systems Based on Imidazolium Surfactant Containing Carbamate Fragment and Polyacrylic Acid. Rev. Adv. Chem. 2022, 12, 123–125. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs Using Liposomes Containing Cationic Surfactants. ACS Omega 2022, 7, 25741–25750. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasilieva, E.A.; Pavlov, R.V.; Zueva, I.V.; Babaev, V.M.; Kuznetsov, D.M.; Voloshina, A.D.; Petrov, K.A.; Zakharova, L.Y.; et al. Oxime Therapy for Brain AChE Reactivation and Neuroprotection after Organophosphate Poisoning. Pharmaceutics 2022, 14, 1950. [Google Scholar] [CrossRef]
- Kuznetsov, D.M.; Kuznetsova, D.A.; Zakharova, L.Y. Liposomes Modified with Borneol-Containing Surfactants for Transdermal Delivery of Hydrophilic Substrates. Russ. Chem. Bull. 2022, 71, 1887–1896. [Google Scholar] [CrossRef]
- Kuznetsov, D.M.; Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Nikitin, Y.N.; Zakharova, L.Y. Triallyl Ammonium Amphiphiles: Self-Assembly and Complexation with Bovine Serum Albumin. Surf. Innov. 2022, 10, 298–311. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Kuznetsova, D.A.; Saifina, L.F.; Shulaeva, M.M.; Semenov, V.E.; Zakharova, L.Y. Novel Dicationic Pyrimidine-Based Nucleolipid Bearing Piperidine Head Groups: Synthesis, Aggregation Behavior, Solubilization Capacity and Interaction with DNA Decamer. Colloids Surf. Physicochem. Eng. Asp. 2020, 599, 124853. [Google Scholar] [CrossRef]
- Salakhieva, D.; Shevchenko, V.; Németh, C.; Gyarmati, B.; Szilágyi, A.; Abdullin, T. Structure–Biocompatibility and Transfection Activity Relationships of Cationic Polyaspartamides with (Dialkylamino)Alkyl and Alkyl or Hydroxyalkyl Side Groups. Int. J. Pharm. 2017, 517, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Vasilieva, E.A.; Lenina, O.A.; Nizameev, I.R.; Kadirov, M.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Cationic Liposomes Mediated Transdermal Delivery of Meloxicam and Ketoprofen: Optimization of the Composition, in Vitro and in Vivo Assessment of Efficiency. Int. J. Pharm. 2021, 605, 120803. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.Y.; Voloshina, A.D.; Ibatullina, M.R.; Zhiltsova, E.P.; Lukashenko, S.S.; Kuznetsova, D.A.; Kutyreva, M.P.; Sapunova, A.S.; Kufelkina, A.A.; Kulik, N.V.; et al. Self-Assembling Metallocomplexes of the Amphiphilic 1,4-Diazabicyclo[2.2.2]Octane Derivative as a Platform for the Development of Nonplatinum Anticancer Drugs. ACS Omega 2022, 7, 3073–3082. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Pavlov, R.V.; Sapunova, A.S.; Voloshina, A.D.; Sibgatullina, G.V.; Samigullin, D.V.; Petrov, K.A.; Zakharova, L.Y.; et al. Comparative Study of Cationic Liposomes Modified with Triphenylphosphonium and Imidazolium Surfactants for Mitochondrial Delivery. J. Mol. Liq. 2021, 330, 115703. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kuznetsova, D.A.; Kushnazarova, R.A.; Gabdrakhmanov, D.R.; Zhukova, N.A.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Sinyashin, O.G.; Mamedov, V.A.; et al. Soft Nanocarriers for New Poorly Soluble Conjugate of Pteridine and Benzimidazole: Synthesis and Cytotoxic Activity against Tumor Cells. J. Mol. Liq. 2020, 317, 114007. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Gaynanova, G.A.; Vasileva, L.A.; Kuznetsov, D.M.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Nizameev, I.R.; Sibgatullina, G.V.; et al. Novel Biocompatible Liposomal Formulations for Encapsulation of Hydrophilic Drugs—Chloramphenicol and Cisplatin. Colloids Surf. Physicochem. Eng. Asp. 2021, 610, 125673. [Google Scholar] [CrossRef]
- Banerjee, N.; Sengupta, S.; Roy, A.; Ghosh, P.; Das, K.; Das, S. Functional Alteration of a Dimeric Insecticidal Lectin to a Monomeric Antifungal Protein Correlated to Its Oligomeric Status. PLoS ONE 2011, 6, e18593. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Pi-Boleda, B.; Ramisetty, S.; Illa, O.; Branchadell, V.; Dias, R.S.; Ortuño, R.M. Efficient DNA Condensation Induced by Chiral β-Amino Acid-Based Cationic Surfactants. ACS Appl. Bio Mater. 2021, 4, 7034–7043. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Liu, D.; Mori, A.; Huang, L. Role of Liposome Size and RES Blockade in Controlling Biodistribution and Tumor Uptake of GM1-Containing Liposomes. Biochim. Biophys. Acta BBA Biomembr. 1992, 1104, 95–101. [Google Scholar] [CrossRef]
- Elsana, H.; Olusanya, T.O.B.; Carr-wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of Novel Cationic Gene Based Liposomes with Cyclodextrin Prepared by Thin Film Hydration and Microfluidic Systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hu, X.; Zhang, Q.; Guan, P. Crystal Structure and Aggregation Behavior in Water of Ionic Liquid 1-Hexadecyl-3-Methylimidazolium Bromide. Mater. Lett. 2010, 64, 794–797. [Google Scholar] [CrossRef]
- Dasgupta, A.; Das, P.K.; Dias, R.S.; Miguel, M.G.; Lindman, B.; Jadhav, V.M.; Gnanamani, M.; Maiti, S. Effect of Headgroup on DNA−Cationic Surfactant Interactions. J. Phys. Chem. B 2007, 111, 8502–8508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumrudov, V.A.; Zhiryakova, M.V.; Goulko, A.A. Ethidium Bromide as a Promising Probe for Studying DNA Interaction with Cationic Amphiphiles and Stability of the Resulting Complexes. Langmuir 2002, 18, 10348–10356. [Google Scholar] [CrossRef]
- Wani, F.A.; Behera, K.; Padder, R.A.; Husain, M.; Malik, M.A.; Al-Thabaiti, N.S.; Ahmad, R.; Patel, R. Micellization, Anti-Proliferative Activity and Binding Study of Cationic Gemini Surfactants with Calf Thymus DNA. Colloid Interface Sci. Commun. 2020, 34, 100221. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Z.; Song, Y.; Liu, S.; Gao, W.; Qiao, H.; Guo, L.; Wang, J. Investigation on Interaction of DNA and Several Cationic Surfactants with Different Head Groups by Spectroscopy, Gel Electrophoresis and Viscosity Technologies. Chemosphere 2017, 168, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, J.; Nakura, H.; Maeda, S. In Situ Measurement of Circular Dichroism of DNA Adsorbing onto a Solid Surface. J. Biochem. Biophys. Methods 2004, 61, 349–357. [Google Scholar] [CrossRef]
- Santhiya, D.; Dias, R.S.; Shome, A.; Das, P.K.; Miguel, M.G.; Lindman, B.; Maiti, S. Role of Linker Groups between Hydrophilic and Hydrophobic Moieties of Cationic Surfactants on Oligonucleotide−Surfactant Interactions. Langmuir 2009, 25, 13770–13775. [Google Scholar] [CrossRef] [PubMed]
- Uma, V.; Kanthimathi, M.; Weyhermuller, T.; Nair, B.U. Oxidative DNA Cleavage Mediated by a New Copper (II) Terpyridine Complex: Crystal Structure and DNA Binding Studies. J. Inorg. Biochem. 2005, 99, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Ding, Y.; Guo, R. Coil-Globule Structure Transition and Binding Characteristics of DNA Molecules Induced by Isoquinoline-Based Photoactive Ionic Liquid Surfactant. Colloids Surf. Physicochem. Eng. Asp. 2017, 531, 150–163. [Google Scholar] [CrossRef]
- Kong, D.-M.; Wang, J.; Zhu, L.-N.; Jin, Y.-W.; Li, X.-Z.; Shen, H.-X.; Mi, H.-F. Oxidative DNA Cleavage by Schiff Base Tetraazamacrocyclic Oxamido Nickel(II) Complexes. J. Inorg. Biochem. 2008, 102, 824–832. [Google Scholar] [CrossRef]
- Costa, D.; Briscoe, W.H.; Queiroz, J. Polyethylenimine Coated Plasmid DNA–Surfactant Complexes as Potential Gene Delivery Systems. Colloids Surf. B Biointerfaces 2015, 133, 156–163. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex Morphologies and Their Influences on Transfection Efficiency in Gene Delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef]
- Dass, C.R. Lipoplex-Mediated Delivery of Nucleic Acids: Factors Affecting in Vivo Transfection. J. Mol. Med. 2004, 82, 579–591. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Atobe, K.; Barichello, J.M.; Ishida, T.; Kiwada, H. Complex Formation with Plasmid DNA Increases the Cytotoxicity of Cationic Liposomes. Biol. Pharm. Bull. 2007, 30, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Sánchez, C.I.; Ochoa Lara, K.; Martínez-Soto, J.M.; Martínez-Higuera, A.; Iñiguez-Palomares, R.A.; Moreno-Corral, R.; Rodríguez-León, E.; Soto-Guzmán, A.; Rodríguez-Beas, C. Physicochemical Characterization and Viability Assays of a Promising Formulation of Liposomes (DODAB-DOPC) in Complexation with CtDNA. J. Nanomater. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Lawrence, M.J. Surfactant Systems: Their Use in Drug Delivery. Chem. Soc. Rev. 1994, 23, 417. [Google Scholar] [CrossRef]
- Hoekstra, S.A. Dick Cationic Lipid-Mediated Transfection in Vitro and in Vivo. Mol. Membr. Biol. 2001, 18, 129–143. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Engberts, J.B.F.N.; Hoekstra, D. Gene Delivery by Cationic Lipid Vectors: Overcoming Cellular Barriers. Eur. Biophys. J. 2007, 36, 349–362. [Google Scholar] [CrossRef]
- Xiang, S.; Tong, H.; Shi, Q.; Fernandes, J.C.; Jin, T.; Dai, K.; Zhang, X. Uptake Mechanisms of Non-Viral Gene Delivery. J. Control. Release 2012, 158, 371–378. [Google Scholar] [CrossRef]
- Resina, S.; Prevot, P.; Thierry, A.R. Physico-Chemical Characteristics of Lipoplexes Influence Cell Uptake Mechanisms and Transfection Efficacy. PLoS ONE 2009, 4, e6058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durymanov, M.; Reineke, J. Non-Viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018, 9, 971. [Google Scholar] [CrossRef] [Green Version]
- Symens, N.; Soenen, S.J.; Rejman, J.; Braeckmans, K.; De Smedt, S.C.; Remaut, K. Intracellular Partitioning of Cell Organelles and Extraneous Nanoparticles during Mitosis. Adv. Drug Deliv. Rev. 2012, 64, 78–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. In Cancer Nanotechnology; Grobmyer, S.R., Moudgil, B.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 624, pp. 25–37. ISBN 978-1-60761-608-5. [Google Scholar]
- Azzopardi, E.; Nash, R. A Critical Evaluation of Importance–Performance Analysis. Tour. Manag. 2013, 35, 222–233. [Google Scholar] [CrossRef]
- Palchetti, S.; Digiacomo, L.; Giulimondi, F.; Pozzi, D.; Peruzzi, G.; Ferri, G.; Amenitsch, H.; Cardarelli, F.; Mahmoudi, M.; Caracciolo, G. A Mechanistic Explanation of the Inhibitory Role of the Protein Corona on Liposomal Gene Expression. Biochim. Biophys. Acta BBA Biomembr. 2020, 1862, 183159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, F.; Huang, L. Implications of Pharmacokinetic Behavior of Lipoplex for Its Inflammatory Toxicity. Adv. Drug Deliv. Rev. 2005, 57, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Giselbrecht, J.; Wiedemann, S.; Reddy Pinnapireddy, S.; Goergen, N.; Loppnow, H.; Sedding, D.; Erdmann, F.; Bakowsky, U.; Hause, G.; Lúcio, M.; et al. Nucleic Acid Carrier Composed of a Branched Fatty Acid Lysine Conjugate—Interaction Studies with Blood Components. Colloids Surf. B Biointerfaces 2019, 184, 110547. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, H.; Servel, N.; Domb, A.; Barenholz, Y. Lipoplex-Induced Hemagglutination: Potential Involvement in Intravenous Gene Delivery. Gene Ther. 2002, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]






| Compounds | IC50 (µM) | ||
|---|---|---|---|
| Cancer Cell Lines | Normal Cell Line | ||
| a M-HeLa | b A549 | c Chang Liver | |
| MPI-10/ONu | 30 ± 2.3 | 21.0 ± 1.8 | 28.7 ± 2.3 |
| MPI-12/ONu | 4.8 ± 0.4 | 7.1 ± 0.6 | 10.6 ± 0.8 |
| MPI-14/ONu | 6.2 ± 0.5 | 10.7 ± 0.8 | 7.9 ± 0.6 |
| MPI-16/ONu | 5.1 ± 0.4 | 14.8 ± 1.2 | 7.2 ± 0.6 |
| MPI-10/pK18 | 3.2 ± 0.3 | 9.4 ± 0.7 | 8.4 ± 0.7 |
| MPI-12/pK18 | 4.4 ± 0.3 | 5.8 ± 0.5 | 7.0 ± 0.6 |
| MPI-14/pK18 | 3.0 ± 0.2 | 5.8 ± 0.5 | 7.0 ± 0.6 |
| MPI-16/pK18 | 3.1 ± 0.1 | 5.0 ± 0.4 | 8.7 ± 0.7 |
| MPI-10 [47] | 28.3 ± 2.2 | 22.1 ± 1.7 | 26.9 ± 2.1 |
| MPI-12 [47] | 18.0 ± 1.4 | 23.7 ± 1.9 | 13.2 ± 1.1 |
| MPI-14 [47] | 6.3 ± 0.5 | 22.4 ± 1.7 | 10.0 ± 0.9 |
| MPI-16 [47] | 11.5 ± 0.9 | 27.8 ± 2.2 | 13.1 ± 1.2 |
| Compounds | A549 | M-HeLa |
|---|---|---|
| In serum-free medium (α-MEM/0% FBS) | ||
| Lipofectamine 3000 | 0.055 ± 0.010 | 0.233 ± 0.019 |
| MPI-12 | 0.486 ± 0.026 | 0.980 ± 0.003 |
| MPI-16 | 1.650 ± 0.010 | 1.945 ± 0.008 |
| In a medium with serum (α-MEM/10% FBS) | ||
| Lipofectamine 3000 | 0.013 ± 0.002 | 0.420 ± 0.008 |
| MPI-12 | 0.427 ± 0.022 | 0.269 ± 0.001 |
| MPI-16 | 0.643 ± 0.002 | 0.436 ± 0.012 |
| CONu, mM | r = 0.6 | r = 1 | r = 2 | ||||
|---|---|---|---|---|---|---|---|
| CMPI-12, mM | Agglutination +/− | CMPI-12, mM | Agglutination +/− | CMPI-12, mM | Agglutination +/− | ||
| A | 0.002 | 0.001 | − | 0.002 | − | 0.004 | − |
| B | 0.004 | 0.002 | − | 0.004 | − | 0.007 | − |
| C | 0.007 | 0.004 | − | 0.007 | − | 0.014 | − |
| D | 0.014 | 0.008 | − | 0.014 | − | 0.028 | − |
| E | 0.028 | 0.017 | − | 0.028 | − | 0.056 | − |
| F | 0.056 | 0.034 | − | 0.056 | + | 0.113 | + |
| G | 0.113 | 0.068 | + | 0.113 | + | 0.225 | + |
| H | 0.225 | 0.135 | + | 0.225 | + | 0.450 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, D.A.; Kuznetsov, D.M.; Vasileva, L.A.; Amerhanova, S.K.; Valeeva, D.N.; Salakhieva, D.V.; Nikolaeva, V.A.; Nizameev, I.R.; Islamov, D.R.; Usachev, K.S.; et al. Complexation of Oligo- and Polynucleotides with Methoxyphenyl-Functionalized Imidazolium Surfactants. Pharmaceutics 2022, 14, 2685. https://doi.org/10.3390/pharmaceutics14122685
Kuznetsova DA, Kuznetsov DM, Vasileva LA, Amerhanova SK, Valeeva DN, Salakhieva DV, Nikolaeva VA, Nizameev IR, Islamov DR, Usachev KS, et al. Complexation of Oligo- and Polynucleotides with Methoxyphenyl-Functionalized Imidazolium Surfactants. Pharmaceutics. 2022; 14(12):2685. https://doi.org/10.3390/pharmaceutics14122685
Chicago/Turabian StyleKuznetsova, Darya A., Denis M. Kuznetsov, Leysan A. Vasileva, Syumbelya K. Amerhanova, Dilyara N. Valeeva, Diana V. Salakhieva, Viktoriia A. Nikolaeva, Irek R. Nizameev, Daut R. Islamov, Konstantin S. Usachev, and et al. 2022. "Complexation of Oligo- and Polynucleotides with Methoxyphenyl-Functionalized Imidazolium Surfactants" Pharmaceutics 14, no. 12: 2685. https://doi.org/10.3390/pharmaceutics14122685