Tailoring Lipid-Based Drug Delivery Nanosystems by Synchrotron Small Angle X-ray Scattering
Abstract
1. Introduction
2. Phase Transition of Lipid Nanoparticles for Controlled Drug Delivery
2.1. External Stimuli Responsive Lipid Nanoparticles
2.2. Hybrid Lipid-Based Nanoparticles
3. Functionalization with Polyethylene Glycol (PEG)
4. Coated Nanoparticles
4.1. Biomolecular Corona
4.2. Others
5. Encapsulation Strategies for Gene Delivery
6. Outlook and Future Perspectives
- The design of compounds able to improve the targeted release of drugs exploiting the properties of the molecular corona. The aim is to promote its interaction with specific cell receptors;
- The tailoring of lipid nanoparticles capable of reaching the disease sites and produce therapeutic activity selectively at the target organ;
- The achievement of an effective triggered drug release via the exposure to light, pH variation and temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreuter, J. Nanoparticles—A Historical Perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Filipa Carreiró, A.M.; Oliveira, A.N.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; Santini, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Aqil, M. Lyotropic Liquid Crystalline Nanoparticles: Scaffolds for Delivery of Myriad Therapeutics and Diagnostics. J. Mol. Liq. 2021, 338, 116919. [Google Scholar] [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of Drug Delivery Systems: From 1950 to 2020 and Beyond. J. Control Release 2022, 342, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. PH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.K.; Parikh, N.H.; Patel, J.K.; Pathak, Y.V. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles for Diagnostics. In Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems. In Pharmacodynamics of Nanoparticulate Drug Delivery Systems; Springer: Cham, Switzerland, 2022; pp. 315–331. [Google Scholar]
- Fonseca-Gomes, J.; Loureiro, J.A.; Tanqueiro, S.R.; Mouro, F.M.; Ruivo, P.; Carvalho, T.; Sebastião, A.M.; Diógenes, M.J.; Pereira, M.C. In Vivo Bio-Distribution and Toxicity Evaluation of Polymeric and Lipid-Based Nanoparticles: A Potential Approach for Chronic Diseases Treatment. Int. J. Nanomed. 2020, 15, 8609–8621. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of Biopolymers for Drugs and Probiotics Delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Schneible, J.D.; Daniele, M.A.; Menegatti, S. Natural and Synthetic Biopolymers in Drug Delivery and Tissue Engineering. Biopolym. Biomed. Biotechnol. Appl. 2021, 1, 265–356. [Google Scholar] [CrossRef]
- Byun, M.J.; Lim, J.; Na, S.; Dae, K.; Park, H.; Hyung, T.; Wooram, K.; Park, C.G.; Park, W. Advances in Nanoparticles for Effective Delivery of RNA Therapeutics. BioChip J. 2022, 16, 128–145. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thuy, V.N.; Van, T.V.; Dao, A.H.; Lee, B.J. Nanostructured Lipid Carriers and Their Potential Applications for Versatile Drug Delivery via Oral Administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- Salentinig, S. Supramolecular Structures in Lipid Digestion and Implications for Functional Food Delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 190–201. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.A.; Mumuni, A.-M.; Builders, P.F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; Ali Demir, S., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Cubosomes and Hexosomes as Versatile Platforms for Drug Delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef]
- Umar, H.; Wahab, H.A.; Gazzali, A.M.; Tahir, H.; Ahmad, W. Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers 2022, 14, 3118. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Tian, X.; Chong, Y.; Ge, C. Understanding the Nano–Bio Interactions and the Corresponding Biological Responses. Front. Chem. 2020, 8, 446. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Glatter, O.; Kratky, O. Small Angle X-ray Scattering; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Narayanan, T.; Konovalov, O. Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research. Materials 2020, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Franke, D.; Svergun, D.I. Synchrotron Small-Angle X-Ray Scattering on Biological Macromolecules in Solution. In Synchrotron Light Sources and Free-Electron Lasers: Accelerator Physics, Instrumentation and Science Applications; Jaeschke, E.J., Khan, S., Schneider, J.R., Hastings, J.B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1645–1672. [Google Scholar] [CrossRef]
- Thakral, S.; Kim, K. Small-Angle Scattering for Characterization of Pharmaceutical Materials. TrAC Trends Anal. Chem. 2021, 134, 116144. [Google Scholar] [CrossRef]
- Konstantinidi, A.; Naziris, N.; Chountoulesi, M.; Kiriakidi, S.; Sartori, B.; Kolokouris, D.; Amentisch, H.; Mali, G.; Ntountaniotis, D.; Demetzos, C.; et al. Comparative Perturbation Effects Exerted by the Influenza A M2 WT Protein Inhibitors Amantadine and the Spiro [Pyrrolidine-2,2′-Adamantane] Variant AK13 to Membrane Bilayers Studied Using Biophysical Experiments and Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 9877–9895. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, E.; Grillo, I.; Ristori, S. Small Angle X-Ray and Neutron Scattering: Powerful Tools for Studying the Structure of Drug-Loaded Liposomes. Pharmaceutics 2016, 8, 10. [Google Scholar] [CrossRef]
- Semeraro, E.F.; Marx, L.; Frewein, M.P.K.; Pabst, G. Increasing Complexity in Small-Angle X-Ray and Neutron Scattering Experiments: From Biological Membrane Mimics to Live Cells. Soft Matter 2021, 17, 222–232. [Google Scholar] [CrossRef]
- Kučerka, N.; Nagle, J.F.; Feller, S.E.; Balgavý, P. Models to Analyze Small-Angle Neutron Scattering from Unilamellar Lipid Vesicles. Phys. Rev. E 2004, 69, 9. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of MRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef]
- Arteta, M.Y.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through MRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. PH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea Javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
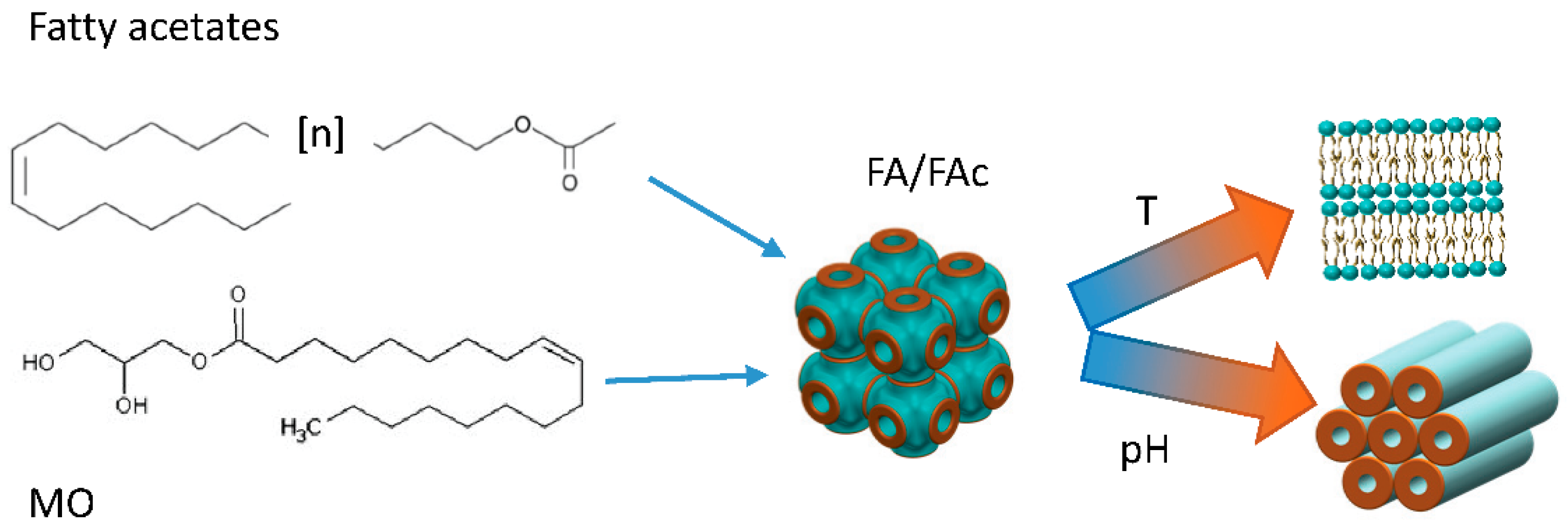
- Zhai, J.; Yap, S.L.; Drummond, C.J.; Tran, N. Controlling the PH Dependent Transition between Monoolein Fd3m Micellar Cubosomes and Hexosomes Using Fatty Acetate and Fatty Acid Additive Mixtures. J. Colloid Interface Sci. 2022, 607, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Zhai, J.; Drummond, C.J.; Tran, N. Synthetic Ionizable Aminolipids Induce a PH Dependent Inverse Hexagonal to Bicontinuous Cubic Lyotropic Liquid Crystalline Phase Transition in Monoolein Nanoparticles. J. Colloid Interface Sci. 2021, 589, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.D.; Mertins, O.; Angelov, B.; Angelova, A. Cubosomal Lipid Nanoassemblies with PH-Sensitive Shells Created by Biopolymer Complexes: A Synchrotron SAXS Study. J. Colloid Interface Sci. 2022, 607, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Seibert, E.; Tetyczka, C.; Kuehnelt, D.; Vidakovic, I.; Kornmueller, K.; Absenger-Novak, M.; Radatović, B.; Vrček, I.V.; Leitinger, G.; et al. Thiolated Chitosan Conjugated Liposomes for Oral Delivery of Selenium Nanoparticles. Pharmaceutics 2022, 14, 803. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Siqueira, S.D.V.S.; Amenitsch, H.; Rades, T.; Müllertz, A. Monoacyl Phosphatidylcholine Inhibits the Formation of Lipid Multilamellar Structures during in Vitro Lipolysis of Self-Emulsifying Drug Delivery Systems. Eur. J. Pharm. Sci. 2017, 108, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi Zenezini, R.; Laganà, A.; Amenitsch, H.; Masuelli, L.; et al. Interplay of Protein Corona and Immune Cells Controls Blood Residency of Liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Hawley, A.; Tan, A.; Boyd, B.J. Coupling in Vitro Cell Culture with Synchrotron SAXS to Understand the Bio-Interaction of Lipid-Based Liquid Crystalline Nanoparticles with Vascular Endothelial Cells. Drug Deliv. Transl. Res. 2020, 10, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Caselli, L.; Mendozza, M.; Muzzi, B.; Toti, A.; Montis, C.; Mello, T.; Di Cesare Mannelli, L.; Ghelardini, C.; Sangregorio, C.; Berti, D. Lipid Cubic Mesophases Combined with Superparamagnetic Iron Oxide Nanoparticles: A Hybrid Multifunctional Platform with Tunable Magnetic Properties for Nanomedical Applications. Int. J. Mol. Sci. 2021, 22, 9268. [Google Scholar] [CrossRef]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; De Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of PEG Lenght on the Intaraction with Biological Fluids.Pdf. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef]
- Caracciolo, G.; Palchetti, S.; Digiacomo, L.; Chiozzi Zenezini, R.; Capriotti, A.L.; Amenitsch, H.; Tentori, P.M.; Palmieri, V.; Papi, M.; Cardarelli, F.; et al. Human Biomolecular Corona of Liposomal Doxorubicin: The Overlooked Factor in Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 22951–22962. [Google Scholar] [CrossRef]
- Siewert, C.D.; Haas, H.; Cornet, V.; Nogueira, S.S.; Nawroth, T.; Uebbing, L.; Ziller, A.; Al-gousous, J.; Radulescu, A.; Schroer, M.A.; et al. Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for MRNA. Cells 2020, 9, 2034. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.S.; Schlegel, A.; Maxeiner, K.; Weber, B.; Barz, M.; Schroer, M.A.; Blanchet, C.E.; Svergun, D.I.; Ramishetti, S.; Peer, D.; et al. Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic MRNA Delivery. ACS Appl. Nano Mater. 2020, 3, 10634–10645. [Google Scholar] [CrossRef]
- Caracciolo, G.; Amenitsch, H. Cationic Liposome/DNA Complexes: From Structure to Interactions with Cellular Membranes. Eur. Biophys. J. 2012, 41, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, E.; Renzi, S.; Digiacomo, L.; Giulimondi, F.; Sartori, B.; Amenitsch, H.; Tassinari, V.; Masuelli, L.; Bei, R.; Cui, L.; et al. Microfluidic Formulation of Dna-Loaded Multicomponent Lipid Nanoparticles for Gene Delivery. Pharmaceutics 2021, 13, 1292. [Google Scholar] [CrossRef]
- Conte, G.; Costabile, G.; Baldassi, D.; Rondelli, V.; Bassi, R.; Colombo, D.; Linardos, G.; Fiscarelli, E.V.; Sorrentino, R.; Miro, A.; et al. Hybrid Lipid/Polymer Nanoparticles to Tackle the Cystic Fibrosis Mucus Barrier in SiRNA Delivery to the Lungs: Does PEGylation Make the Difference? ACS Appl. Mater. Interfaces 2022, 14, 7565–7578. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Mohammad, Y.; Prentice, R.N.; Boyd, B.J.; Rizwan, S.B. Comparison of Cubosomes and Hexosomes for the Delivery of Phenytoin to the Brain. J. Colloid Interface Sci. 2022, 605, 146–154. [Google Scholar] [CrossRef]
- Date, T.; Paul, K.; Singh, N.; Jain, S. Drug–Lipid Conjugates for Enhanced Oral Drug Delivery. AAPS PharmSciTech 2019, 20, 41. [Google Scholar] [CrossRef]
- Khan, N.F.; Salim, M.; Binte Abu Bakar, S.Y.; Ristroph, K.; Prud’homme, R.K.; Hawley, A.; Boyd, B.J.; Clulow, A.J. Small-Volume in Vitro Lipid Digestion Measurements for Assessing Drug Dissolution in Lipid-Based Formulations Using SAXS. Int. J. Pharm. X 2022, 4, 100113. [Google Scholar] [CrossRef]
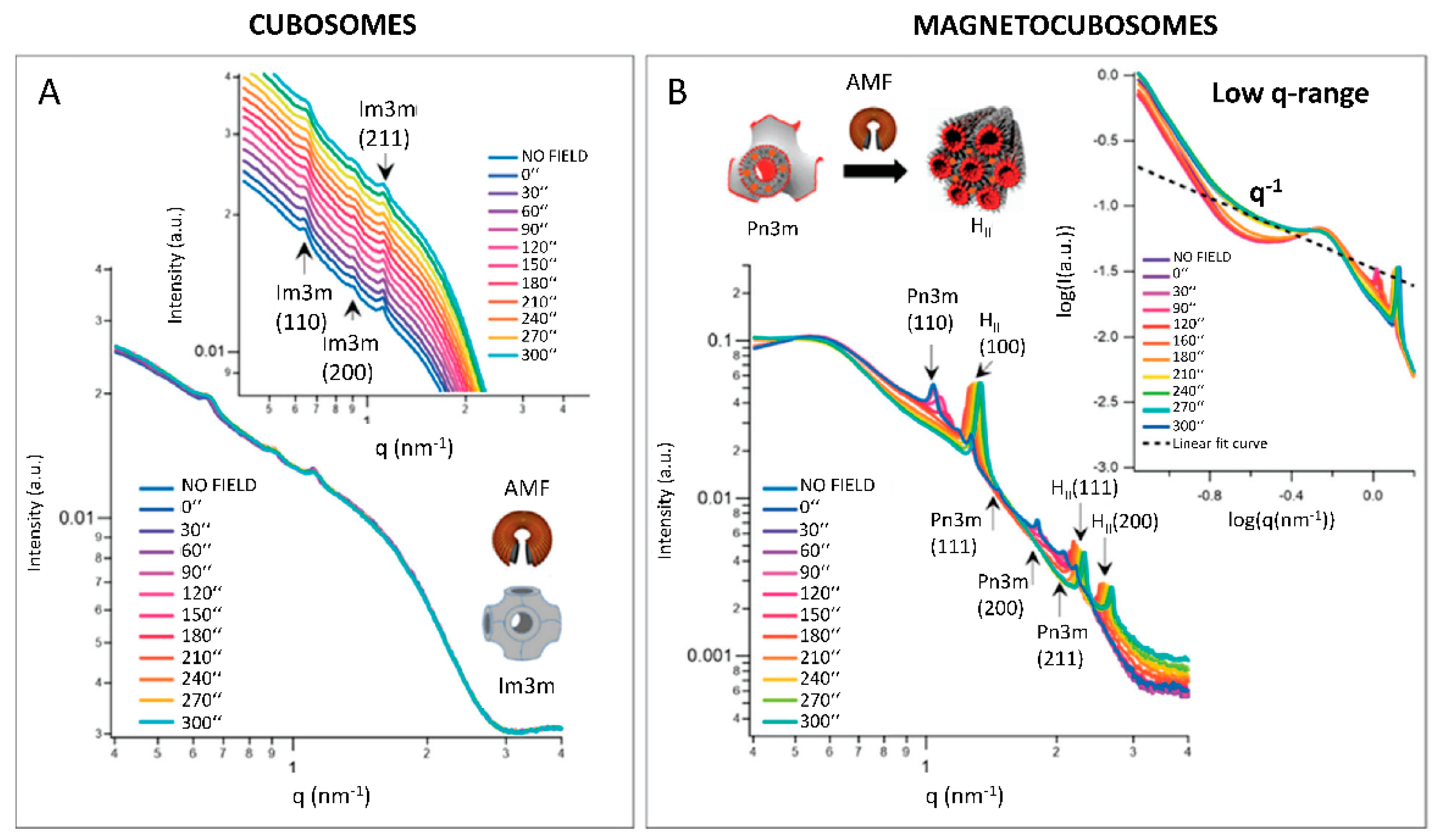
- Mendozza, M.; Montis, C.; Caselli, L.; Wolf, M.; Baglioni, P.; Berti, D. On the Thermotropic and Magnetotropic Phasebehavior of Lipid Liquid Crystals Containingmagnetic Nanoparticles. Nanoscale 2018, 10, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale R. Soc. Chem. 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Ruscigno, S.; Trapani, A.; Di Gioia, S.; Milano, F.; Mandracchia, D.; Comparelli, R.; Castellani, S.; Agostiano, A.; Trapani, G.; et al. Preparation of Drug-Loaded Small Unilamellar Liposomes and Evaluation of Their Potential for the Treatment of Chronic Respiratory Diseases. Int. J. Pharm. 2018, 545, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.R.; Molley, T.G.; Kim, H.; Bharath, P.A.; Kilian, K.A.; Leal, C. The Structural Fate of Lipid Nanoparticles in the Extracellular Matrix †. Mater. Horiz. 2020, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Sarode, A.; Fan, Y.; Byrnes, A.E.; Hammel, M.; Hura, G.L.; Fu, Y.; Kou, P.; Hu, C.; Hinz, F.I.; Roberts, J.; et al. Predictive High-Throughput Screening of PEGylated Lipids in Oligonucleotide-Loaded Lipid Nanoparticles for Neuronal Gene Silencing. Nanoscale Adv. 2022, 4, 2107–2123. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, Z.S.; Hadjidemetriou, M.; Gubbins, J.; Kostarelos, K. Formation of Protein Corona in Vivo Affects Drug Release from Temperature-Sensitive Liposomes. J. Control Release 2018, 27, 157–167. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nature 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Szebeni, J.; Muggia, F.; Gabizon, A.; Barenholz, Y. Activation of Complement by Therapeutic Liposomes and Other Lipid Excipient-Based Therapeutic Products: Prediction and Prevention. Adv. Drug Deliv. Rev. 2011, 63, 1020–1030. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef] [PubMed]


| Application | System | In Situ/Ex Situ SAXS | Investigation on | Ref. | Outcome |
|---|---|---|---|---|---|
| pH-dependent drug release | Cubosomes (MO + Oleic acid + natural derived oil) | Ex situ formulation design | Stability and pH responsivity | [34] | Optimized formulation |
| cubosomes(MO + fatty acetates) | In situ pH responsivity | pH and temperature responsivity | [35] | Improved formulation for pH-dependent phase change | |
| Cubosomes (MO + F127 + aminolipids) | In situ Investigate structural changes at different pH | pH responsivity | [36] | Improved formulation–pH-dependent phase change | |
| Oral drug delivery | cubosomes (Mo + F127 + polysaccharides) | Ex situ Formulation design | pH responsivity | [37] | Improved formulation–pH-dependent phase change |
| Liposomes (chitosan-N-acetylcysteine) | Ex situ Effect of coating on NPs stability | Improvement on the interaction with intestine cells | [38] | Lipid bilayer integrity confirmed after polymer coating | |
| Self-emulsifying drug delivery systems | In situ structural changes during digestion | Poorly soluble drug cargo stability during lipolysis | [39] | Selection of excipient to improve SEDDS stability | |
| Enable prolonged circulation in vivo | Liposomes/protein corona | Ex situ “Tailor-made” protein coating of NPs | Stability during circulation | [40] | Plasma protein coating induce structural changes in liposomes |
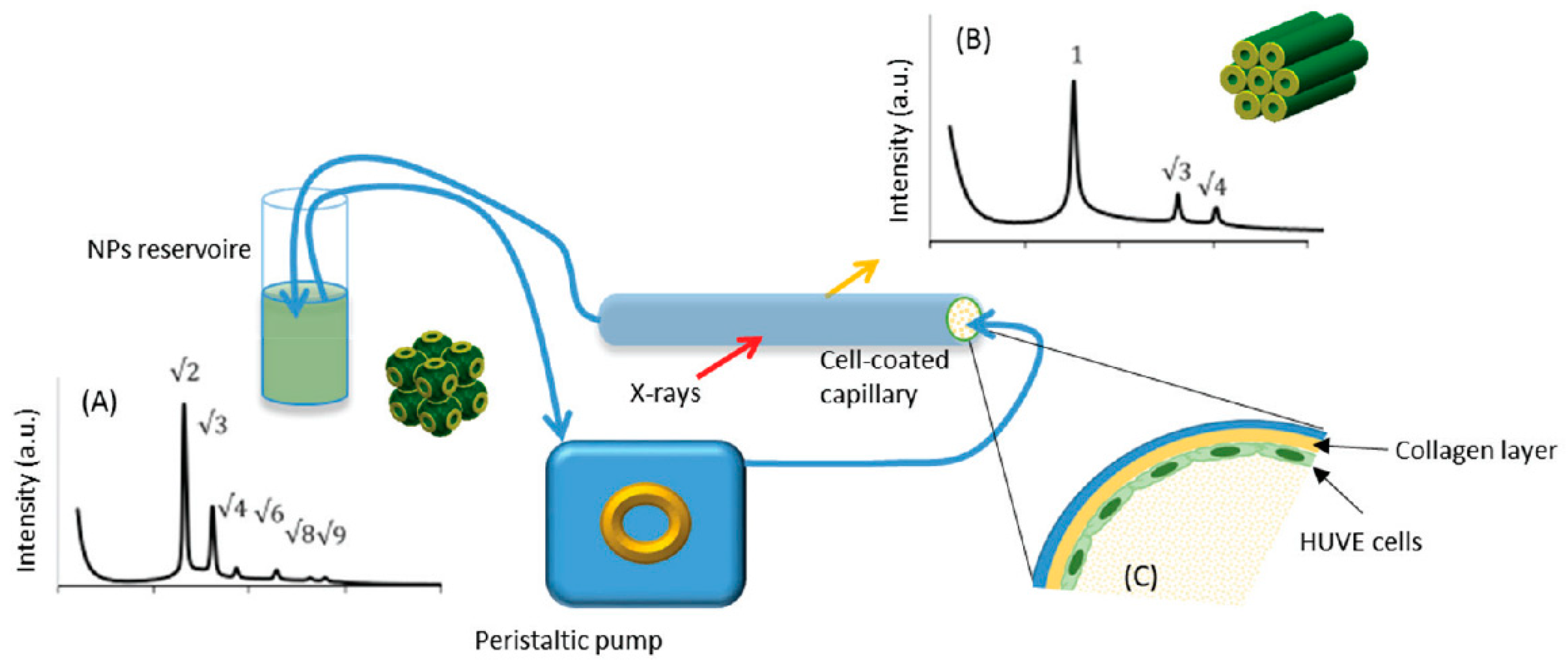
| Interaction betw.lipid-based NPs and live cells | cubosomes (Phytantriol–F127) | In situ Phase transition in contact with HUVE cells | Interaction between structured NPs and cells | [41] | Understanding the dynamics of vascular endothelial cells–lipid NPs interactions |
| Thermally triggered drug/nutrients delivery | cubosomes (MO + superparamagnetic iron oxide) | In situ Investigate phase transition | Temperature responsivity and magnetic trigger | [42] | Formulation of responsive magnetocubosomes |
| Active prostatic cancer cell targeting | PEG-ylated liposomes | Ex situ Effect of PEG chain length on protein corona | Stability, reduction in immune response | [43] | Formulation improvement of PEGylated cationic lipid NPs |
| Delivery of Doxorubicine for cancer treatment | Liposomes/biomolecular corona | Ex situ Stability after plasma incubation | Drug delivery efficiency | [44] | Commercial formulation stability assessment |
| mRNA transfection | Lipoplexes (DOTAP/RNA/Protamine) | Ex situ Formulation design | Physico-chemical particles characterization | [45] | Formulation improvement for better transfection efficiency |
| Lipoplexes/Polysarcosin | Ex situ Particles design | Improvement of stability | [46] | Optimized formulation | |
| DNA transfection | Lipoplexes (Cationic lipids/Cholesterol/DNA) | Ex situ Formulation design | Physico-chemical characterization | [47] | Optimized formulation |
| Lipoplexes (Cationic lipid/DNA/PEG) | Ex situ Formulation design | Microfluidics for NPs synthesis | [48] | Optimized pDNA loaded NPs production | |
| siRNA delivery | lipid shell/PLGA core/RNA | Ex situ Stability in physiological conditions | Design of aerosol drug delivery systems | [49] | Understanding the dynamics of lung cells–lipid NPs interactions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartori, B.; Marmiroli, B. Tailoring Lipid-Based Drug Delivery Nanosystems by Synchrotron Small Angle X-ray Scattering. Pharmaceutics 2022, 14, 2704. https://doi.org/10.3390/pharmaceutics14122704
Sartori B, Marmiroli B. Tailoring Lipid-Based Drug Delivery Nanosystems by Synchrotron Small Angle X-ray Scattering. Pharmaceutics. 2022; 14(12):2704. https://doi.org/10.3390/pharmaceutics14122704
Chicago/Turabian StyleSartori, Barbara, and Benedetta Marmiroli. 2022. "Tailoring Lipid-Based Drug Delivery Nanosystems by Synchrotron Small Angle X-ray Scattering" Pharmaceutics 14, no. 12: 2704. https://doi.org/10.3390/pharmaceutics14122704
APA StyleSartori, B., & Marmiroli, B. (2022). Tailoring Lipid-Based Drug Delivery Nanosystems by Synchrotron Small Angle X-ray Scattering. Pharmaceutics, 14(12), 2704. https://doi.org/10.3390/pharmaceutics14122704