Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies
Abstract
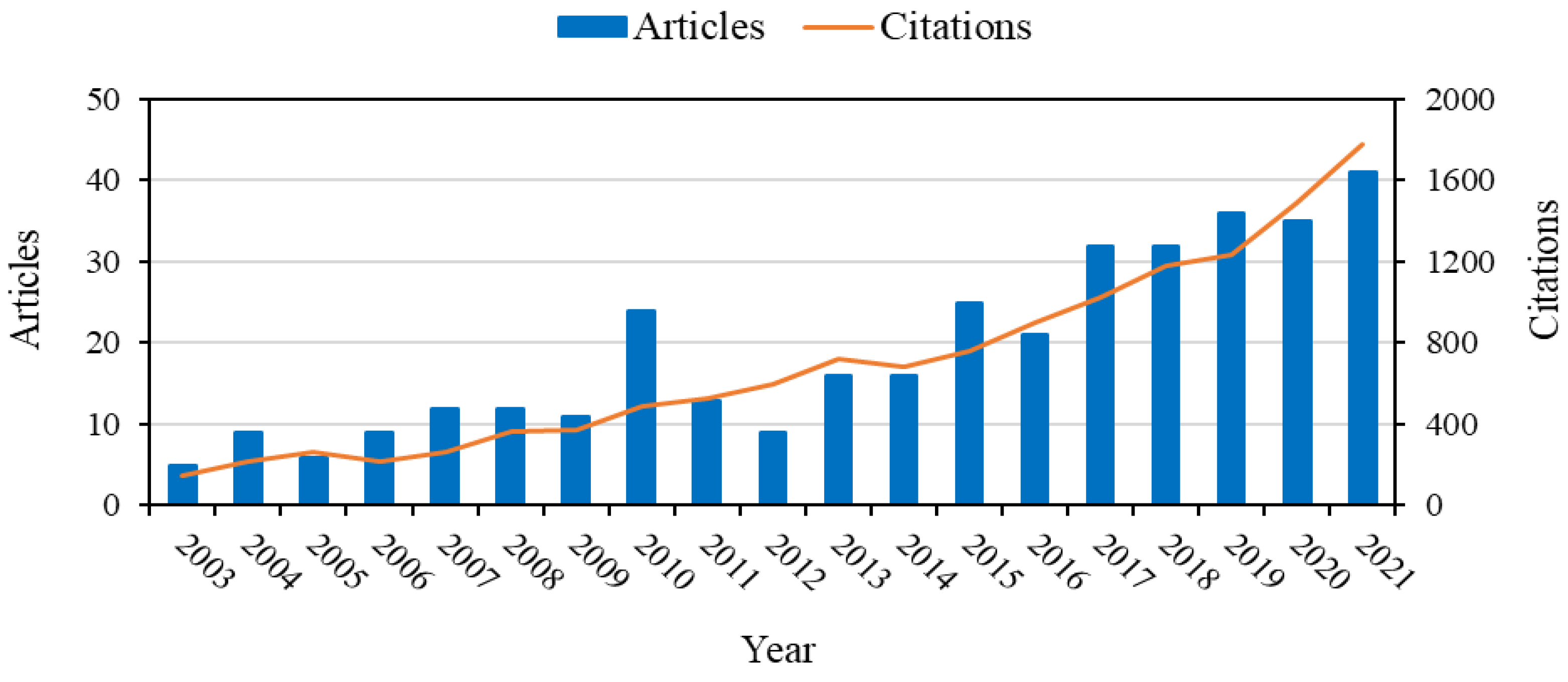
1. Introduction
2. Types of Polymeric Biomaterials Commonly Used in Drug Delivery in the Oral Cavity
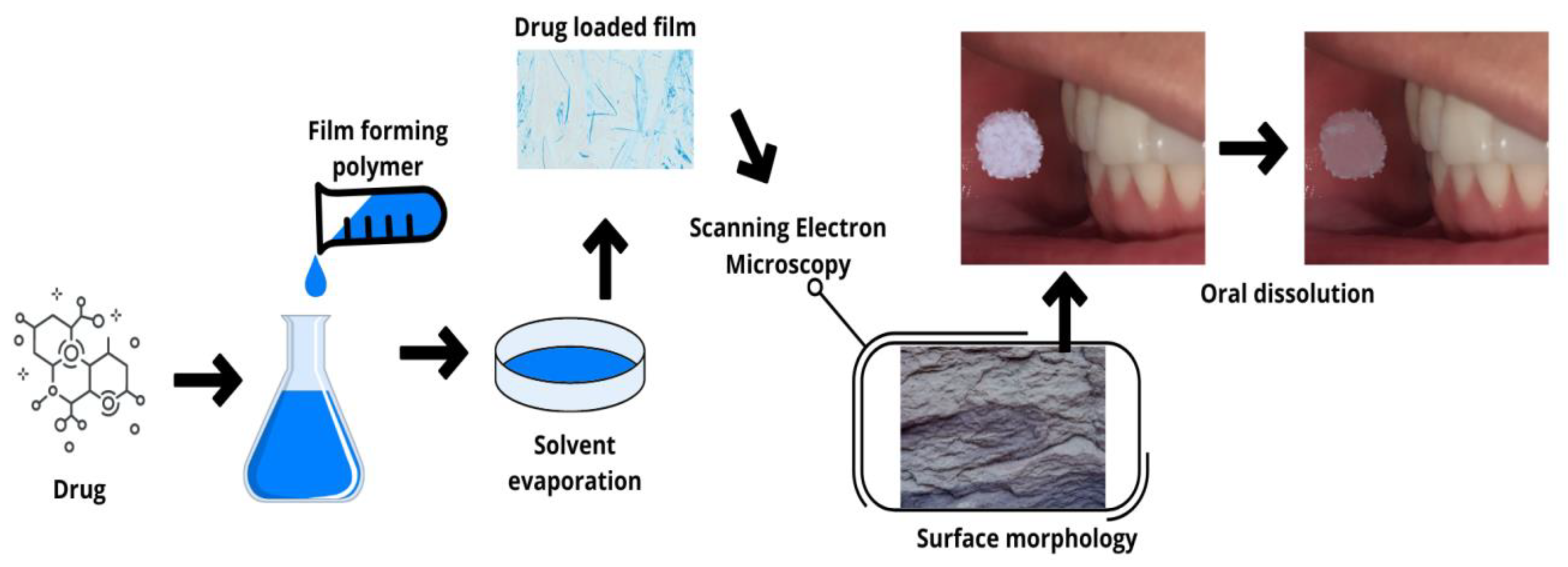
2.1. Fast-Dissolving Films (Oro-Dissolving)
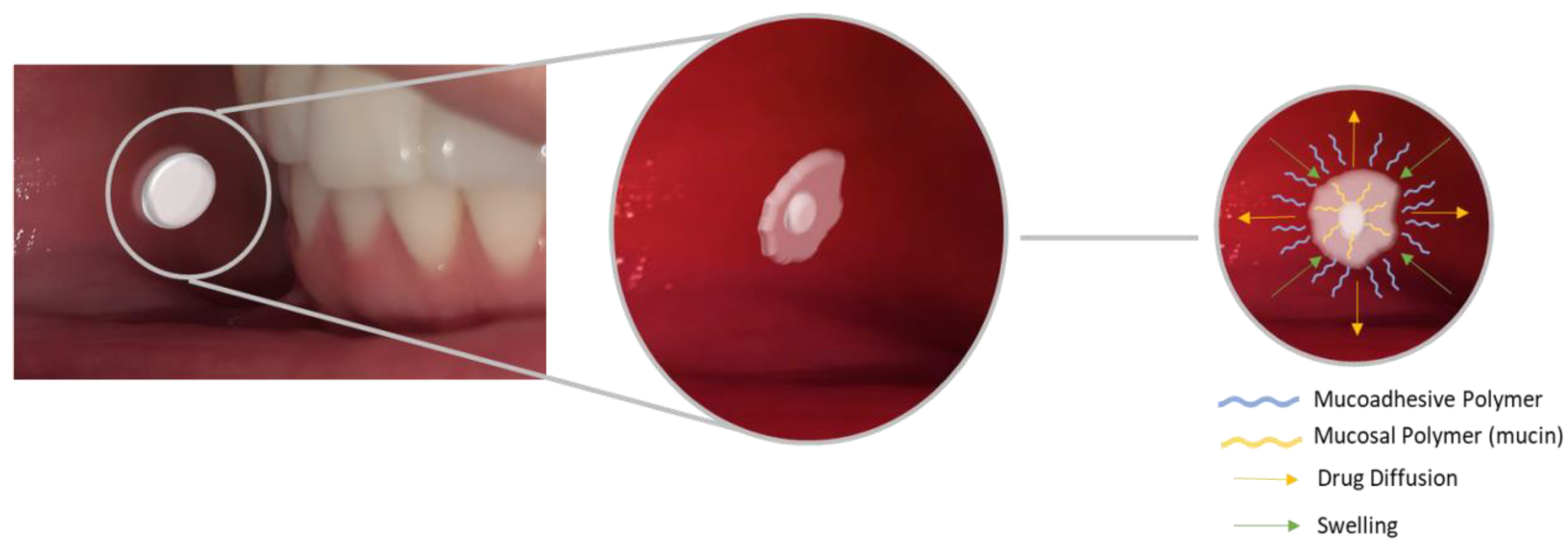
2.2. Mucoadhesive Biomaterials
2.2.1. Buccal Tablets
2.2.2. Mucoadhesive Films and Gels
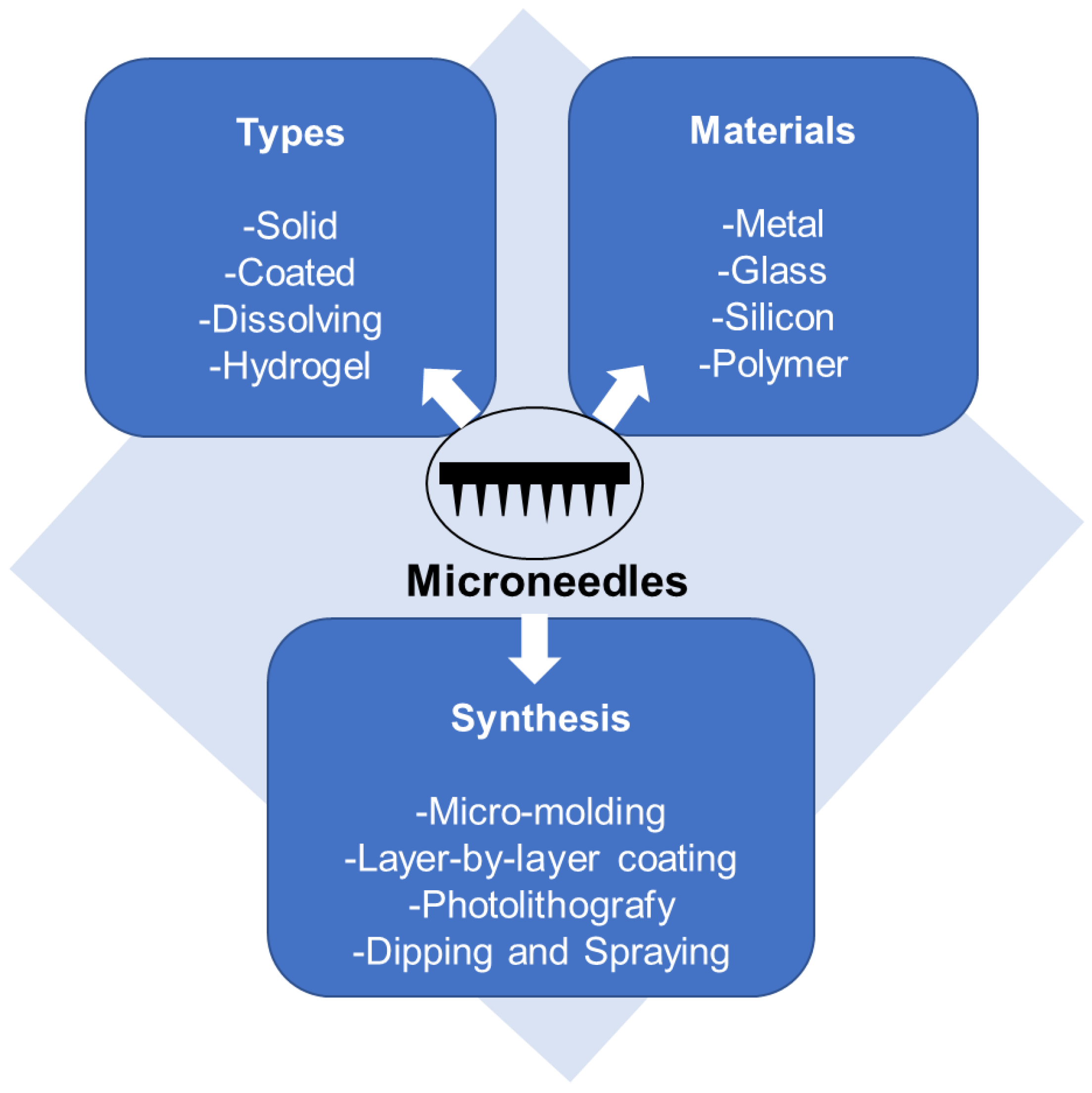
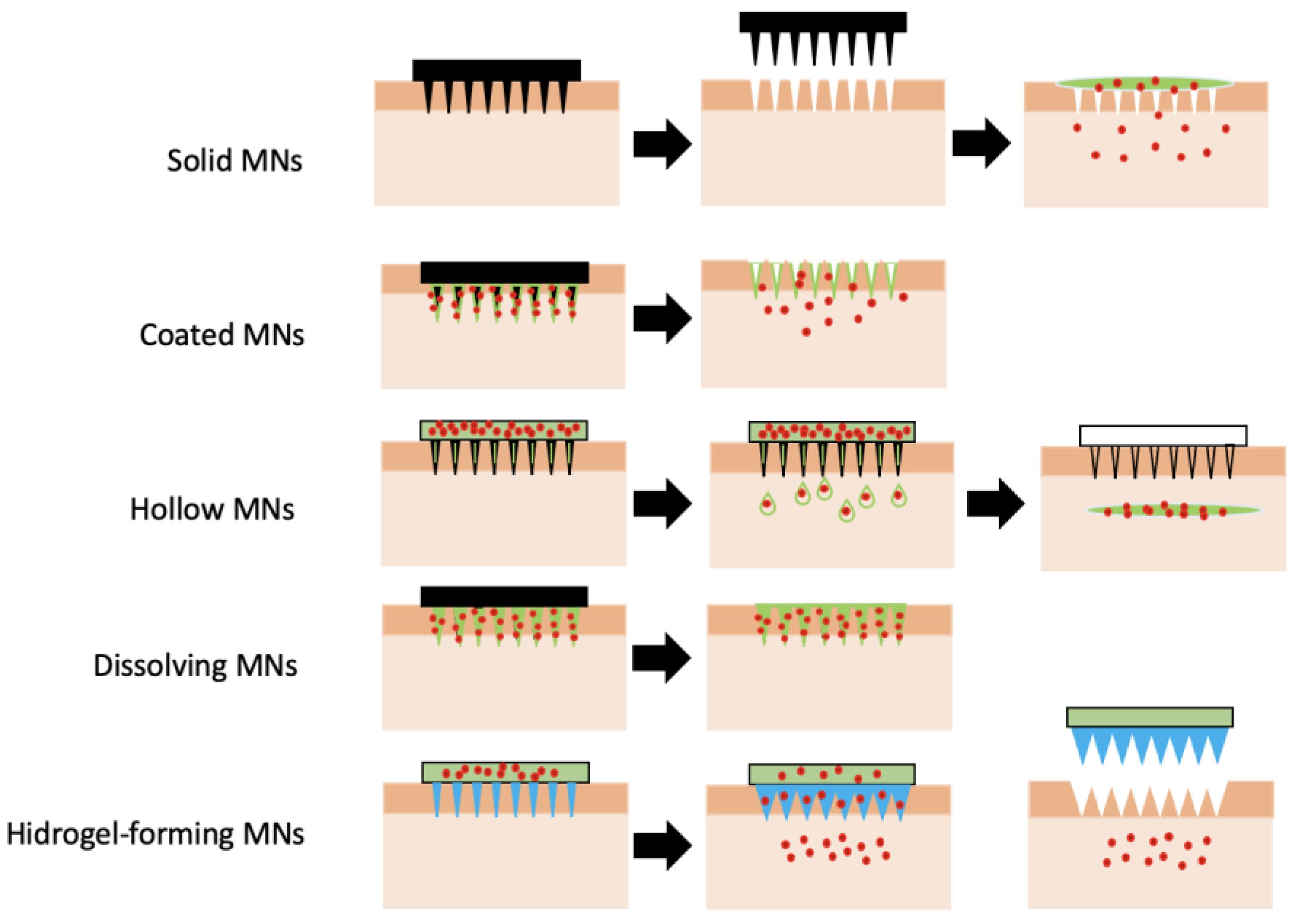
2.3. Microneedles (MNs)
2.3.1. Types of Microneedles Concerning the Technique of Drug Loading and Delivery
2.3.2. Polymeric Microneedles
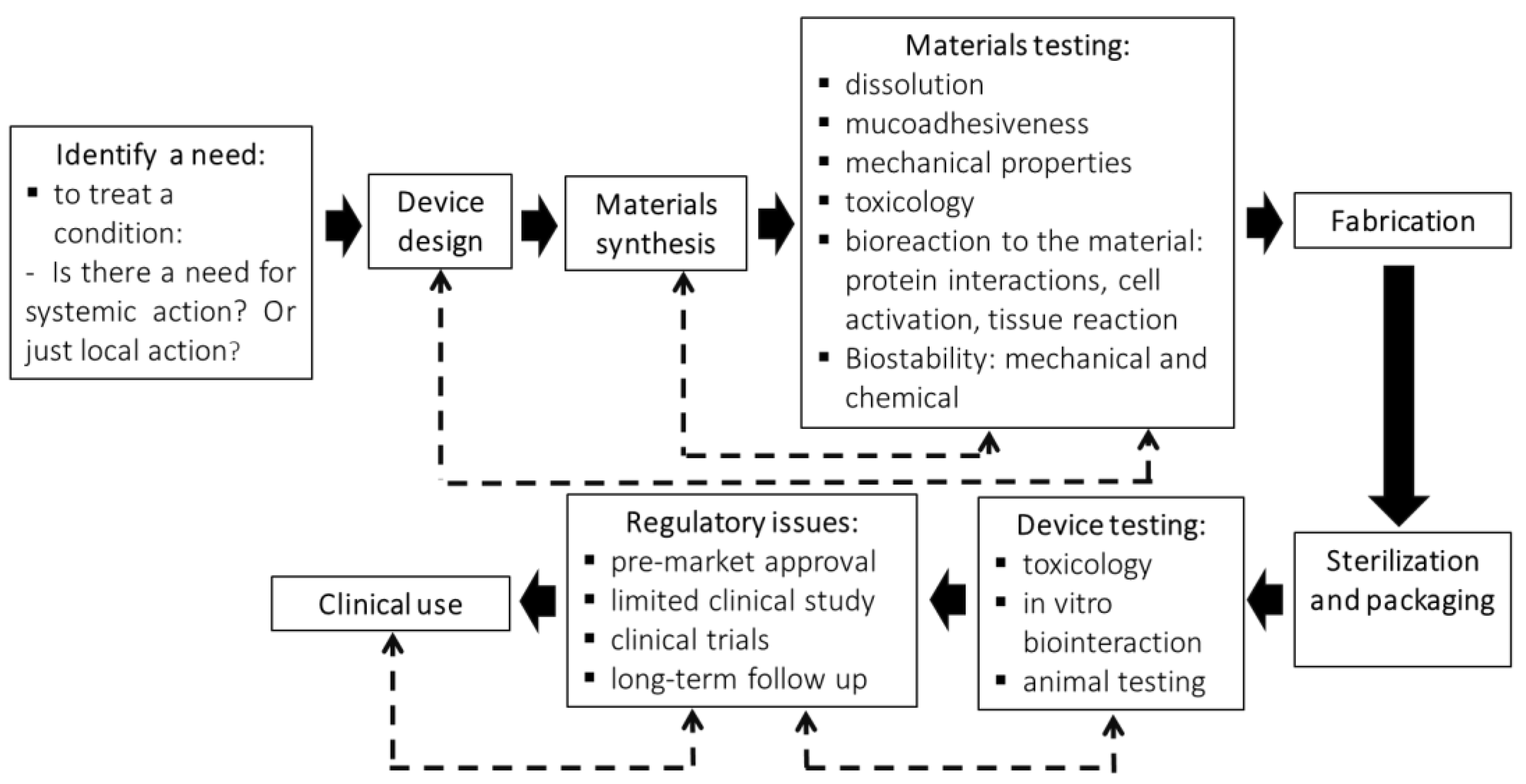
3. Steps Involved in the Production of an Oral Dispositive to Drug Delivery
4. Polymers Used to Produce Biomaterials for the Use in Oral Cavity
4.1. Natural Polymers
4.2. Synthetic Polymers
4.3. Smart Polymers
4.3.1. Temperature-Responsive Polymers
4.3.2. pH-Responsive Polymers
4.3.3. Bioresponsive Polymers
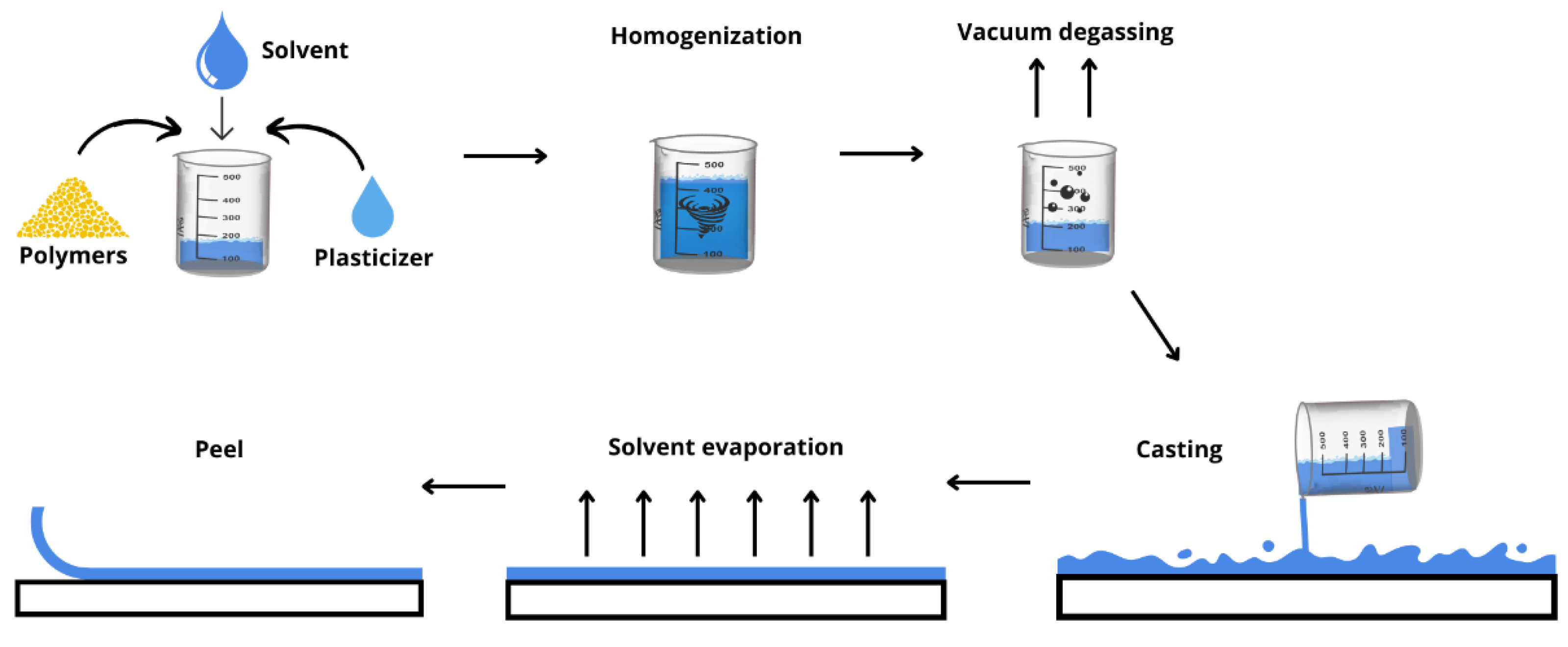
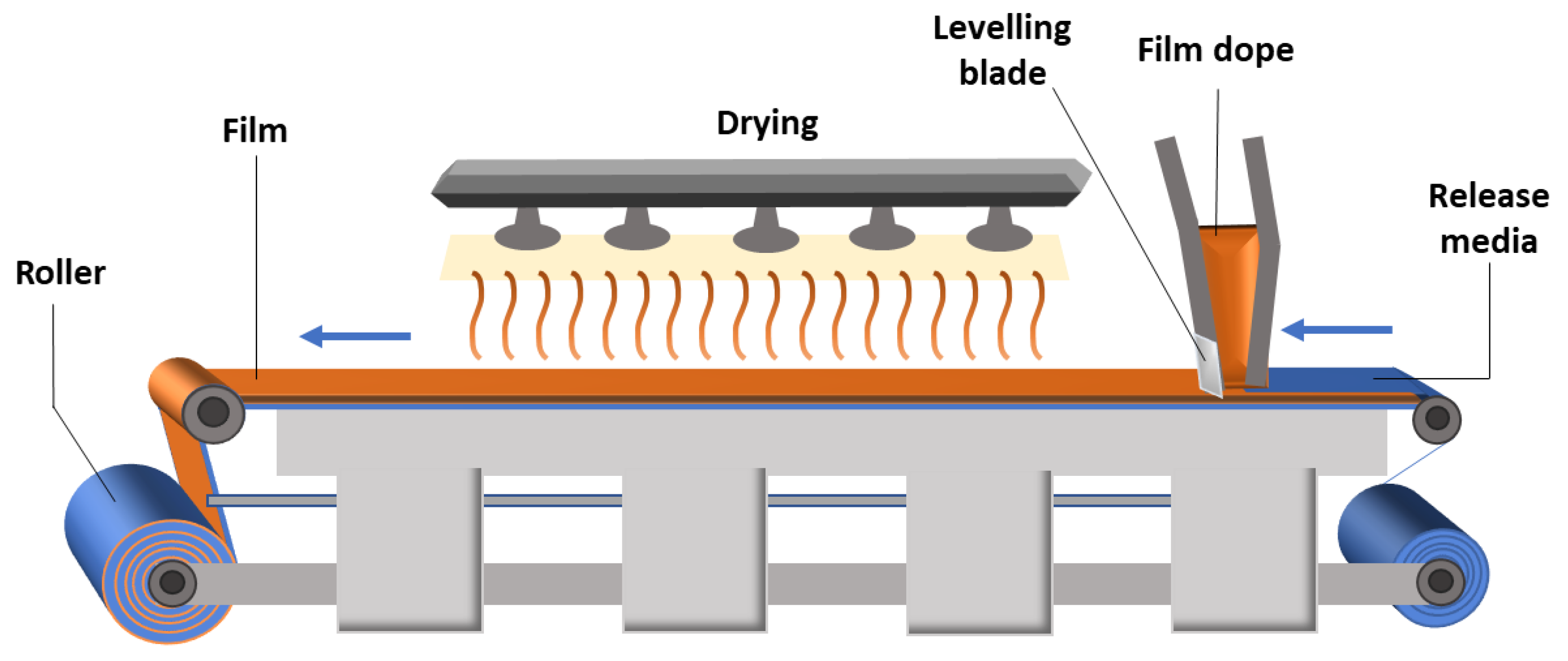
5. Methods for the Production of Drug Delivery Systems
5.1. Solvent Casting Method
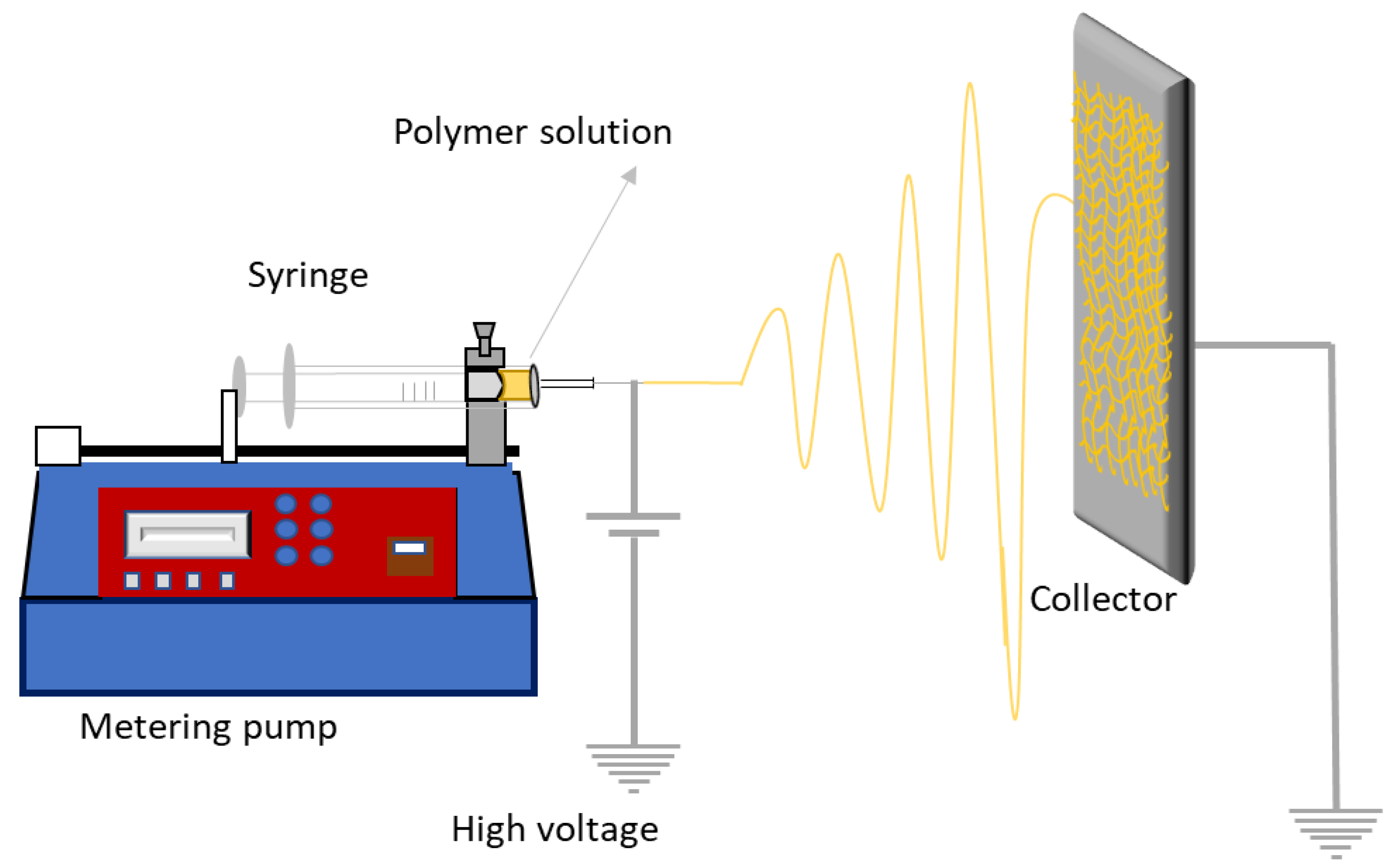
5.2. Electrospinning
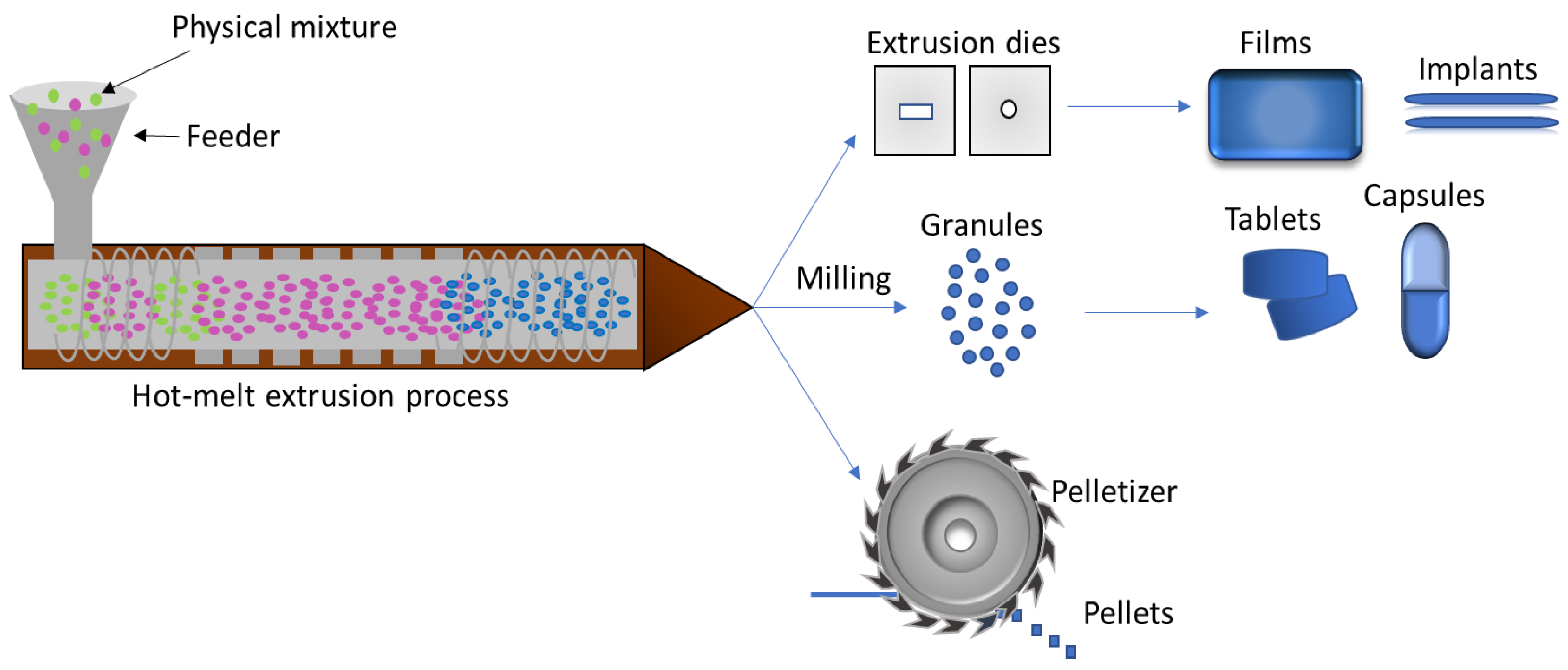
5.3. Hot Melt Extrusion
5.4. 3D Printing Method
6. Market Perspectives of Drug Delivery Biomaterials for the Oral Cavity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwatra, S.; Taneja, G.; Nasa, N. Alternative routes of drug administration—Transdermal, pulmonary & parenteral. Indo Glob. J. Pharm. Sci. 2012, 2, 409–426. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.M.T.; Cassiani, R.D.A.; Dos Santos, C.M.; Dantas, R.O. Gender effect on the clinical measurement of swallowing. Arq. Gastroenterol. 2007, 44, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef]
- Camargo, L.G.; Remiro, P.D.F.R.; Rezende, G.S.; Santos, S.D.C.; Franz-Montan, M.; Moraes, Â.M. Development of bioadhesive polysaccharide-based films for topical release of the immunomodulatory agent imiquimod on oral mucosa lesions. Eur. Polym. J. 2021, 151, 110422. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Shaikh, R.; Singh, R.R.T.; Garland, M.J.; Woolfson, A.D. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- Franz-Montan, M.; de Araújo, D.R.; de Morais Ribeiro, L.N.; de Melo, N.F.S.; de Paula, E. Nanostructured systems for transbuccal drug delivery. In Nanostructures for Oral Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–121. [Google Scholar]
- Kulkarni, U.; Mahalingam, R.; Pather, S.I.; Li, X.; Jasti, B. Porcine buccal mucosa as an in vitro model: Relative contribution of epithelium and connective tissue as permeability barriers. J. Pharm. Sci. 2009, 98, 471–483. [Google Scholar] [CrossRef]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 97–112. [Google Scholar] [CrossRef]
- Paderni, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef]
- Sanz, R.; Calpena, A.C.; Mallandrich, M.; Clares, B. Enhancing topical analgesic administration: Review and prospect for transdermal and transbuccal drug delivery systems. Curr. Pharm. Des. 2015, 21, 2867–2882. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Ribeiro, L.N.D.M.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; Araújo, D.R.; Santi, P.; Padula, C.; de Paula, E. Recent advances and perspectives in topical oral anesthesia. Expert Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Triggle David, J.; Taylor John, B. (Eds.) Comprehensive Medicinal Chemistry II; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Guilherme, V.A.; Ribeiro, L.N.; Tofoli, G.R.; Franz-Montan, M.; de Paula, E.; de Jesus, M.B. Current challenges and future of lipid nanoparticles formulations for topical drug application to oral mucosa, skin, and eye. Curr. Pharm. Des. 2017, 23, 6659–6675. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Khanna, S.; Pawar, P.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Natural Polymers vs. Synthetic Polymer. In Natural Polymer Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The Impact of the Preparation Method on the Properties of Orodispersible Films with Aripiprazole: Electrospinning vs. Casting and 3D Printing Methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef]
- Salawi, A. An Insight into Preparatory Methods and Characterization of Orodispersible Film—A Review. Pharmaceuticals 2022, 15, 844. [Google Scholar] [CrossRef]
- Kalyan, S.; Bansal, M. Recent Trends in the Development of Oral dissolving Film. Int. J. Pharm. Tech. Res. 2012, 4, 725–733. [Google Scholar]
- Mostafa, D.A.E. Fast dissolving oral film: Overview. Eur. J. Biomed. Pharm. Sci. 2018, 5, 86–101. [Google Scholar]
- Bhyan, B.; Jangra, S.; Kaur, M.; Singh, H. Orally fast dissolving films. Innovations in formulation and technology. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 009. [Google Scholar]
- Gavaskar, B.; Vijaya Kumar, S.; Sharan, G.; Madhusudan Rao, Y. Overview on fast dissolving films. Int. J. Pharm. Pharm Sci. 2010, 2, 0975–1491. [Google Scholar]
- Prabhu, P.; Malli, R.; Koland, M.; Vijaynarayana, K.; D′souza, U.; Harish, N.; Shastry, C.; Charyulu, R. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int. J. Pharm. Investig. 2011, 1, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Londhe, V.Y.; Umalkar, K.B. Formulation Development and Evaluation of Fast Dissolving Film of Telmisartan. Indian J. Pharm. Sci. 2012, 74, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Sheela, A.; Haque, S.E. Development of polymer-bound fast-dissolving metformin buccal film with disintegrants. Int. J. Nanomed. 2015, 10, 199–205. [Google Scholar] [CrossRef][Green Version]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef]
- Nippani, A.; Vijendar, C.; Dindigala, A.; Kandhula, A.G.; Chandra, S.K.; Alabadri, A. Preparation and In-Vitro Evaluation of Mirtazapine Oral Films. Res. Rev. J. Pharm. Sci. 2016, 5, 96–103. [Google Scholar]
- Kim, B.-S.; Park, G.-T.; Park, M.-H.; Shin, Y.G.; Cho, C.-W. Preparation and evaluation of oral dissolving film containing local anesthetic agent, lidocaine. J. Pharm. Investig. 2016, 47, 575–581. [Google Scholar] [CrossRef]
- Sallam, N.M.; Sanad, R.A.; Kharshoom, R.M.; Zeneldin, M.A. Development of Salbutamol Sulphate Sublingual Films in Pullulan Matrix for Enhanced Bioavailability & Clinical Efficacy. Curr. Drug. Deliv. 2017, 14, 503–515. [Google Scholar] [CrossRef]
- Garcia, V.A.D.S.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; Lapa-Guimaraes, J.D.G.; Vanin, F.M.; de Carvalho, R.A. Gelatin/starch orally disintegrating films as a promising system for vitamin C delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Wannaphatchaiyong, S.; Heng, P.W.S.; Suksaeree, J.; Boonme, P.; Pichayakorn, W. Lidocaine loaded gelatin/gelatinized tapioca starch films for buccal delivery and the irritancy evaluation using chick chorioallantoic membrane. Saudi Pharm. J. 2019, 27, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Al-Mogherah, A.I.; Ibrahim, M.A.; Hassan, M.A. Optimization and evaluation of venlafaxine hydrochloride fast dissolving oral films. Saudi Pharm. J. 2020, 28, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef]
- Celik, B. Risperidone mucoadhesive buccal tablets: Formulation design, optimization and evaluation. Drug Des. Dev. Ther. 2017, 11, 3355–3365. [Google Scholar] [CrossRef]
- Gajdziok, J.; Bajerová, M.; Chalupová, Z.; Rabišková, M. Oxycellulose as mucoadhesive polymer in buccal tablets. Drug Dev. Ind. Pharm. 2010, 36, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M.C.; Luppi, B. Mucoadhesive Buccal Tablets Based on Chitosan/Gelatin Microparticles for Delivery of Propranolol Hydrochloride. J. Pharm. Sci. 2015, 104, 4365–4372. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef]
- Pham, Q.D.; Nöjd, S.; Edman, M.; Lindell, K.; Topgaard, D.; Wahlgren, M. Mucoadhesion: Mucin-polymer molecular interactions. Int. J. Pharm. 2021, 610, 121245. [Google Scholar] [CrossRef]
- Laffleur, F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 591–598. [Google Scholar] [CrossRef]
- Boyapally, H.; Nukala, R.K.; Bhujbal, P.; Douroumis, D. Controlled release from directly compressible theophylline buccal tablets. Colloids Surf. B Biointerfaces 2010, 77, 227–233. [Google Scholar] [CrossRef]
- Koirala, S.; Nepal, P.; Ghimire, G.; Basnet, R.; Rawat, I.; Dahal, A.; Pandey, J.; Parajuli-Baral, K. Formulation and evaluation of mucoadhesive buccal tablets of aceclofenac. Heliyon 2021, 7, e06439. [Google Scholar] [CrossRef] [PubMed]
- Chandira, M.; Sachin, D.B.; Jayakar, B. Formulation and evaluation of mucoadhesive oral tablet of Clarithromycin. Pharm. Res. 2009, 2, 30–42. [Google Scholar]
- Salehi, S.; Boddohi, S. Design and optimization of kollicoat ® IR based mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 97, 230–244. [Google Scholar] [CrossRef]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive gelatin buccal films with propranolol hydrochloride: Evaluation of mechanical, mucoadhesive, and biopharmaceutical properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef]
- Fini, A.; Bergamante, V.; Ceschel, G.C. Mucoadhesive Gels Designed for the Controlled Release of Chlorhexidine in the Oral Cavity. Pharmaceutics 2011, 3, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Tangsuksan, P.; Chuerduangphui, J.; Yupanqui, C.T.; Srichana, T.; Hitakomate, E.; Pientong, C.; Ekalaksananan, T.; Nittayananta, W. Mucoadhesive film containing α-mangostin shows potential role in oral cancer treatment. BMC Oral Health 2021, 21, 512. [Google Scholar] [CrossRef]
- del Consuelo, I.D.; Falson, F.; Guy, R.H.; Jacques, Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J. Control. Release 2007, 122, 135–140. [Google Scholar] [CrossRef]
- Nair, A.; Shah, J.; Jacob, S.; Al-Dhubiab, B.; Patel, V.; Sreeharsha, N.; Shinu, P. Development of Mucoadhesive Buccal Film for Rizatriptan: In Vitro and In Vivo Evaluation. Pharmaceutics 2021, 13, 728. [Google Scholar] [CrossRef]
- Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H. Clinical evaluation of an oral mucoadhesive film containing chitosan for the treatment of recurrent aphthous stomatitis: A randomized, double-blind study. J. Dermatol. Treat. 2020, 31, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; Ribeiro, L.N.M.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C.; et al. Hybrid hydrogel composed of polymeric nanocapsules co-loading lidocaine and prilocaine for topical intraoral anesthesia. Sci. Rep. 2018, 8, 17972. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Franz-Montan, M.; Alcântara, A.C.S.; Breitkreitz, M.C.; Castro, S.R.; Guilherme, V.A.; Muniz, B.V.; da Silva, G.H.R.; De Paula, E. Hybrid nanofilms as topical anesthetics for pain-free procedures in dentistry. Sci. Rep. 2020, 10, 11341. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Zuccheri, G.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Film-nanoparticle composite for enhanced oral delivery of alpha-casozepine. Colloids Surf. B Biointerfaces 2019, 181, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef]
- Adams, D. Penetration of Water through Human and Rabbi Oral Mucosa in vitro. Arch. Oral Biol. 1974, 19, 865–872. [Google Scholar] [CrossRef]
- Santos, S.D.C.; Fávaro-Moreira, N.C.; Abdalla, H.B.; Augusto, G.G.X.; Costa, Y.M.; Volpato, M.C.; Groppo, F.C.; Gill, H.S.; Franz-Montan, M. A crossover clinical study to evaluate pain intensity from microneedle insertion in different parts of the oral cavity. Int. J. Pharm. 2020, 592, 120050. [Google Scholar] [CrossRef]
- Ma, Y.; Boese, S.E.; Luo, Z.; Nitin, N.; Gill, H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices 2015, 17, 44. [Google Scholar] [CrossRef]
- Zhao, X.; Coulman, S.A.; Hanna, S.; Wong, F.S.; Dayan, C.M.; Birchall, J.C. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J. Control. Release 2017, 265, 2–13. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Quan, F.-S.; Yoo, D.-G.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 2009, 27, 6932–6938. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated Microneedles for Drug Delivery to the Eye. Investig. Opthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Grossniklaus, H.E.; Edelhauser, H.F.; Prausnitz, M.R. Intrastromal Delivery of Bevacizumab Using Microneedles to Treat Corneal Neovascularization. Investig. Opthalmol. Vis. Sci. 2014, 55, 7376–7386. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Gadeela, P.R.; Thathireddy, P.; Venuganti, V.V.K. Microneedle-based drug delivery: Materials of construction. J. Chem. Sci. 2019, 131, 90. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N.; Nisar, A. Fabrication and analysis of tapered tip silicon microneedles for mems based drug delivery system. Sens. Transducer 2010, 122, 158–173. [Google Scholar]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro Electromechanical Systems (MEMS) Based Microfluidic Devices for Biomedical Applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef]
- Cahill, E.M.; Keaveney, S.; Stuettgen, V.; Eberts, P.; Ramos-Luna, P.; Zhang, N.; Dangol, M.; O’Cearbhaill, E.D. Corrigendum to “Metallic microneedles with interconnected porosity: A scalable platform for biosensing and drug delivery”. Acta Biomater. 2018, 86, 493–495. [Google Scholar] [CrossRef]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, C.M.; Kim, G.M. Porous polymer coatings on metal microneedles for enhanced drug delivery. R. Soc. Open Sci. 2018, 5, 171609. [Google Scholar] [CrossRef]
- Ma, Y.; Tao, W.; Krebs, S.J.; Sutton, W.F.; Haigwood, N.L.; Gill, H.S. Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm. Res. 2014, 31, 2393–2403. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise Microinjection into Skin Using Hollow Microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef]
- Bolton, C.J.W.; Howells, O.; Blayney, G.J.; Eng, P.F.; Birchall, J.C.; Gualeni, B.K.; Roberts, K.; Ashraf, H.; Guy, O.J. Hollow silicon microneedle fabrication using advanced plasma etch technologies for applications in transdermal drug delivery. Lab Chip 2020, 20, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.P.; Raghavan, S. Solid silicon microneedles for drug delivery applications. Int. J. Adv. Manuf. Technol. 2016, 93, 407–422. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, R.; Laffitte, Y.; Schmill, U.; Hu, W.; Kaddoura, M.; Blondeel, E.J.M.; Cui, B. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst. Nanoeng. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Battisti, M.; Vecchione, R.; Casale, C.; Pennacchio, F.A.; Lettera, V.; Jamaledin, R.; Profeta, M.; Di Natale, C.; Imparato, G.; Urciuolo, F.; et al. Non-invasive Production of Multi-Compartmental Biodegradable Polymer Microneedles for Controlled Intradermal Drug Release of Labile Molecules. Front. Bioeng. Biotechnol. 2019, 7, 296. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, G.; Lee, H.; Bao, L.; Park, J.; Takama, N.; Kim, B. Comparison of polymers to enhance mechanical properties of microneedles for bio-medical applications. Micro Nano Syst. Lett. 2020, 8, 13. [Google Scholar] [CrossRef]
- Demir, Y.K.; Kerimoglu, O. Novel use of pectin as a microneedle base. Chem. Pharm. Bull. 2015, 63, 300–304. [Google Scholar] [CrossRef]
- Vallhov, H.; Xia, W.; Engqvist, H.; Scheynius, A. Bioceramic microneedle arrays are able to deliver OVA to dendritic cells in human skin. J. Mater. Chem. B 2018, 6, 6808–6816. [Google Scholar] [CrossRef]
- Jiang, J.; Moore, J.; Edelhauser, H.F.; Prausnitz, M.R. Intrascleral Drug Delivery to the Eye Using Hollow Microneedles. Pharm. Res. 2008, 26, 395–403. [Google Scholar] [CrossRef]
- Lyon, B.J.; Aria, A.I.; Gharib, M. Fabrication of carbon nanotube-polyimide composite hollow microneedles for transdermal drug delivery. Biomed. Microdevices 2014, 16, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, R.R.T.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, Materials, Production Methods and Commercial Development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.J. Micro-Injection Moulded Microneedles for Drug Delivery; University of Bradford: Bradford, UK, 2014. [Google Scholar]
- Ingrole, R.S.J.; Gill, H.S. Microneedle Coating Methods: A Review with a Perspective. J. Pharmacol. Exp. Ther. 2019, 370, 555–569. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Ita, K. Dissolving microneedles for transdermal drug delivery: Advances and challenges. Biomed. Pharm. 2017, 93, 1116–1127. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, C.; Zhang, S.; Yang, G.; He, M.; Gao, Y. Systemic delivery of artemether by dissolving microneedles. Int. J. Pharm. 2016, 508, 1–9. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, W.; Weng, J.; Zhang, Z.; Yin, L.; Yang, Q.; Guo, F.; Wang, X.; Chen, F.; Yang, G. Dissolving microneedles for transdermal delivery of huperzine A for the treatment of Alzheimer’s disease. Drug Deliv. 2020, 27, 1147–1155. [Google Scholar] [CrossRef]
- McCrudden, M.T.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving Microneedle Patch for Transdermal Delivery of Human Growth Hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Seon-Woo, H.-S.; Kim, H.J.; Roh, J.Y.; Park, J.-H. Dissolving Microneedle Systems for the Oral Mucosal Delivery of Triamcinolone Acetonide to Treat Aphthous Stomatitis. Macromol. Res. 2018, 27, 282–289. [Google Scholar] [CrossRef]
- Caffarel-Salvador, E.; Kim, S.; Soares, V.; Tian, R.Y.; Stern, S.R.; Minahan, D.; Yona, R.; Lu, X.; Zakaria, F.R.; Collins, J.; et al. A microneedle platform for buccal macromolecule delivery. Sci. Adv. 2021, 7, eabe2620. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2020, 21, e2000307. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wu, Z.; Chen, L.; Wu, F.; Wei, L.; Yuan, W. Hydrogel Microneedle Arrays for Transdermal Drug Delivery. Nano-Micro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Chu, L.Y.; Choi, S.-O.; Prausnitz, M.R. Fabrication of Dissolving Polymer Microneedles for Controlled Drug Encapsulation and Delivery: Bubble and Pedestal Microneedle Designs. J. Pharm. Sci. 2010, 99, 4228–4238. [Google Scholar] [CrossRef]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Dev. Ther. 2013, 7, 945–952. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, X.; Yi, X.; Guo, X.; Li, L.; Hao, Y.; Wang, W. Lidocaine-Loaded Hyaluronic Acid Adhesive Microneedle Patch for Oral Mucosal Topical Anesthesia. Pharmaceutics 2022, 14, 686. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.Y.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv. Mater. 2016, 28, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; Singh, T.R.R.; Migalska, K.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev. Ind. Pharm. 2009, 35, 1242–1254. [Google Scholar] [CrossRef]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, X.; Liu, L.; Zhao, L.; Xie, H.; Yang, Z. Dissolving Polymer Microneedles for Transdermal Delivery of Insulin. Front. Pharmacol. 2021, 12, 719905. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Cole, G.; McCaffrey, J.; Ali, A.A.; McBride, J.W.; McCrudden, C.M.; Vincente-Perez, E.M.; Donnelly, R.; McCarthy, H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and RALA complexation. Hum. Vaccines Immunother. 2017, 13, 50–62. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Zhang, P.; Du, J.; Wang, Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J. Control. Release 2018, 286, 201–209. [Google Scholar] [CrossRef]
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic acid-based dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Han, M.-R.; Kim, Y.-H.; Shin, S.-W.; Nam, S.-Y.; Park, J.-H. Tip-loaded dissolving microneedles for transdermal delivery of donepezil hydrochloride for treatment of Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 105, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm. Res. 2011, 28, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Hassan, S.; Hussain, A.; Abbas, N.; Kucuk, I.; Nazari, K.; Ali, R.; Ramzan, S.; Alqahtani, A.; Andriotis, E.G.; et al. Improved transdermal delivery of cetirizine hydrochloride using polymeric microneedles. DARU J. Pharm. Sci. 2019, 27, 673–681. [Google Scholar] [CrossRef]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of Polymeric Microneedle Arrays for Transdermal Drug Delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Carlton, H.; Gonzalez-Nino, D.; Miranda-Muñoz, K.A.; Daneshpour, R.; Huitink, D.; Prinz, G.; Powell, J.; Greenlee, L.; Almodovar, J. Design, characterization, and modeling of a chitosan microneedle patch for transdermal delivery of meloxicam as a pain management strategy for use in cattle. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111544. [Google Scholar] [CrossRef]
- Na, Y.-G.; Kim, M.; Han, M.; Huh, H.W.; Kim, J.-S.; Kim, J.C.; Park, J.-H.; Lee, H.-K.; Cho, C.-W. Characterization of Hepatitis B Surface Antigen Loaded Polylactic Acid-Based Microneedle and Its Dermal Safety Profile. Pharmaceutics 2020, 12, 531. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of Methotrexate and Characterization of Skin Treated by Fabricated PLGA Microneedles and Fractional Ablative Laser. Pharm. Res. 2018, 35, 68. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, D.D.; Chen, Y.; Guo, X.D. A fabrication method of microneedle molds with controlled microstructures. Mater. Sci. Eng. C 2016, 65, 135–142. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2020, 24, 102012. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.L.R.; Bierhalz, A.C.; Moraes, A.M. Biomaterials: Types, applications, and market. Química Nova 2015, 38, 957–971. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Shoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- ECHA. Available online: https://echa.europa.eu/documents/10162/23036412/polymers_en.pdf/9a74545f-05be-4e10-8555-4d7cf051bbed. (accessed on 4 November 2021).
- Muhamad, I.; Selvakumaran, S.; Lazim, N. Designing polymeric nanoparticles for targeted drug delivery system. In Nanomedicine, 1st ed.; Seifalian, A., De Mel, A., Kalaskar, D.M., Eds.; One Central Press: Manchester, UK, 2014; pp. 287–313. [Google Scholar]
- Ogaji, I.J.; Nep, E.; Audu-Peter, J.D. Advances in Natural Polymers as Pharmaceutical Excipients. Pharm. Anal. Acta 2012, 3, 146. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef]
- Kaur, N.; Gupta, A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Nazari, K.; Kontogiannidou, E.; Ahmad, R.H.; Gratsani, A.; Rasekh, M.; Arshad, M.S.; Sunar, B.S.; Armitage, D.; Bouropoulos, N.; Chang, M.-W.; et al. Development and characterisation of cellulose based electrospun mats for buccal delivery of non-steroidal anti-inflammatory drug (NSAID). Eur. J. Pharm. Sci. 2017, 102, 147–155. [Google Scholar] [CrossRef]
- Alves, M.C.; Chaves, D.S.; Benevenuto, B.R.; De Farias, B.O.; Coelho, S.M.; Ferreira, T.P.; Pereira, G.A.; Dos Santos, G.C.; Moreira, L.O.; De Freitas, J.P.; et al. Chitosan gels for buccal delivery of Schinus molle L. essential oil in dogs: Characterization and antimicrobial activity in vitro. An. Da Acad. Bras. De Ciências 2020, 92, e20200562. [Google Scholar] [CrossRef]
- Lakshmi, V.S.; Menon, R.B.; Raju, K.; Aiswaryam, M.U.; Nair, S.C. Formulation and evaluation of lorazepam encapsulated collagen/pectin buccal patch. Int. J. Appl. Pharm. 2019, 11, 200–209. [Google Scholar] [CrossRef]
- Fernandes, F.P.; Fortes, A.C.; Fonseca, S.G.D.C.; Breitkreutz, J.; Ferraz, H.G. Manufacture and Characterization of Mucoadhesive Buccal Films Based on Pectin and Gellan Gum Containing Triamcinolone Acetonide. Int. J. Polym. Sci. 2018, 1–10. [Google Scholar] [CrossRef]
- Shrivastava, A. Introduction to Plastics Engineering; William Andrew: Norwich, NY, USA, 2018; pp. 1–16. [Google Scholar]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Shin, C.Y.; Kim, K.-B. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; San Román, J. Smart polymers and their applications as biomaterials. In Topics in Tissue Engineering e-Book; Ashammakhi, N., Reis, R.L., Chiellini, E., Eds.; Expertissues: Alicante, Spain, 2007. [Google Scholar]
- James, H.P.; John, R.; Alex, A.; Anoop, K. Smart polymers for the controlled delivery of drugs – a concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Miar, S.; Ong, J.L.; Bizios, R.; Guda, T. Electrically stimulated tunable drug delivery from polypyrrole-coated polyvinylidene fluoride. Front. Chem. 2021, 9, 599631. [Google Scholar] [CrossRef] [PubMed]
- Frachini, E.; Petri, D. Magneto-Responsive Hydrogels: Preparation, Characterization, Biotechnological and Environmental Applications. J. Braz. Chem. Soc. 2019, 30, 2010–2028. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.; Rousseau, I.A. Shape memory polymer research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Banerjee, S.; Kar, K.K. Introduction to Liquid Crystalline Polymers. In Polymers and Polymeric Composites: A Reference Series; Kar, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Hoogenboom, R. Temperature-responsive polymers: Properties, synthesis, and applications. In Smart Polymers and Their Applications; Woodhead Publishing: Cambridge, UK, 2019; pp. 13–44. [Google Scholar]
- Gao, Y.; Ren, F.; Ding, B.; Sun, N.; Liu, X.; Ding, X.; Gao, S. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J. Drug Target. 2010, 19, 516–527. [Google Scholar] [CrossRef]
- Gil Choi, S.; Lee, S.-E.; Kang, B.-S.; Ng, C.L.; Davaa, E.; Park, J.-S. Thermosensitive and Mucoadhesive Sol-Gel Composites of Paclitaxel/Dimethyl-β-Cyclodextrin for Buccal Delivery. PLoS ONE 2014, 9, e109090. [Google Scholar] [CrossRef]
- Shin, B.K.; Baek, E.J.; Choi, S.G.; Davaa, E.; Nho, Y.C.; Lim, Y.M.; Park, J.S.; Huh, K.M.; Park, J.S. Preparation and irradiation of Pluronic F127-based thermoreversible and mucoadhesive hydrogel for local delivery of naproxen. Drug Dev. Ind. Pharm. 2013, 39, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F. pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 45–92. [Google Scholar]
- Kocak, G.; Tuncer, C.A.N.S.E.L.; Bütün, V.J.P.C. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagniere, E.; Mangin, D.; Elaïssari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Hu, W.Y.; Wu, Z.M.; Yang, Q.Q.; Liu, Y.J.; Li, J.; Zhang, C.Y. Smart pH-responsive polymeric micelles for programmed oral delivery of insulin. Colloids Surf. B Biointerfaces 2019, 183, 110443. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Aghajanzadeh, M.; Rostamizadeh, K.; Manjili, H.K.; Fridoni, M.; Danafar, H. In vivo study of poly (ethylene glycol)-poly (caprolactone)-modified folic acid nanocarriers as a pH responsive system for tumor-targeted co-delivery of tamoxifen and quercetin. J. Drug Deliv. Sci. Technol. 2019, 54, 101283. [Google Scholar] [CrossRef]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Sericin/RBA embedded gellan gum based smart nanosystem for pH responsive drug delivery. Int. J. Biol. Macromol. 2018, 120 Pt B, 1561–1571. [Google Scholar] [CrossRef]
- Sherje, A.P.; Londhe, V. Development and Evaluation of pH-Responsive Cyclodextrin-Based in situ Gel of Paliperidone for Intranasal Delivery. AAPS PharmSciTech 2017, 19, 384–394. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Jia, O.Y.; Ahmad, I. pH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers 2020, 12, 1932. [Google Scholar] [CrossRef]
- Jamshidzadeh, F.; Mohebali, A.; Abdouss, M. Three-ply biocompatible pH-responsive nanocarriers based on HNT sandwiched by chitosan/pectin layers for controlled release of phenytoin sodium. Int. J. Biol. Macromol. 2020, 150, 336–343. [Google Scholar] [CrossRef]
- Sawant, S.N. Development of biosensors from biopolymer composites. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–383. [Google Scholar]
- Radhakrishnan, K.; Tripathy, J.; Datey, A.; Chakravortty, D.; Raichur, A.M. Mesoporous silica–chondroitin sulphate hybrid nanoparticles for targeted and bio-responsive drug delivery. New J. Chem. 2015, 39, 1754–1760. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.; Todd, S.J.; Mart, R.; Smith, A.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Maitz, M.F.; Freudenberg, U.; Tsurkan, M.; Fischer, M.; Beyrich, T.; Werner, C. Bio-responsive polymer hydrogels homeostatically regulate blood coagulation. Nat. Commun. 2013, 4, 2168. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous manufacturing and analytical characterization of fixed-dose, multilayer orodispersible films. Eur. J. Pharm. Sci. 2018, 117, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2019, 576, 118963. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of electrospinning: Market overview of products and devices for pharmaceutical and biomedical purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Wilson, M.; A Williams, M.; Jones, D.S.; Andrews, G.P. Hot-melt extrusion technology and pharmaceutical application. Ther. Deliv. 2012, 3, 787–797. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Madan, S. Hot melt extrusion and its pharmaceutical applications. Asian J. Pharm. 2012, 7, 123–133. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Tambe, S.; Jain, D.; Agarwal, Y.; Amin, P. Hot-melt extrusion: Highlighting recent advances in pharmaceutical applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102452. [Google Scholar] [CrossRef]
- ASTM F2792-12A; Standard Terminology for Additive Manufacturing Technologies. ASTM International: West Conshohocken, PA, USA, 2012.
- Flynn, J.M.; Shokrani, A.; Newman, S.T.; Dhokia, V. Hybrid additive and subtractive machine tools – Research and industrial developments. Int. J. Mach. Tools Manuf. 2016, 101, 79–101. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Egan, P.F. Integrated Design Approaches for 3D Printed Tissue Scaffolds: Review and Outlook. Materials 2019, 12, 2355. [Google Scholar] [CrossRef]
- Arefin, A.; Khatri, N.; Kulkarni, N.; Egan, P. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Walker, J.L.; Santoro, M. Processing and production of bioresorbable polymer scaffolds for tissue engineering. In Bioresorbable Polymers for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 181–203. [Google Scholar]
- Huang, J.; Qin, Q.; Wang, J. A review of stereolithography: Processes and systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Selimis, A.; Mironov, V.; Farsari, M. Direct laser writing: Principles and materials for scaffold 3D printing. Microelectron. Eng. 2015, 132, 83–89. [Google Scholar] [CrossRef]
- Zahid, U.M.; Aamir, M.N.; Zaman, S.; Rehman, M.; Javaid, Z.; Hussain, H.; Mohsin, N.U.A. Preparation, Optimization and Ex Vivo Release Studies of Risperidone Orodispersible Films. Lat. Am. J. Pharm. 2020, 39, 686–693. [Google Scholar]
- Ali, M.S.; Vijendar, C.; Kumar, S.D.; Krishnaveni, J. Formulation and evaluation of fast dissolving oral films of diazepam. J. Pharmacovigil. 2016, 4, 210. [Google Scholar] [CrossRef]
- Raju, P.N.; Kumar, M.S.; Reddy, C.M.; Ravishankar, K. Formulation and evaluation of fast dissolving films of loratidine by solvent casting method. Pharma Innov. 2013, 2, 31–35. [Google Scholar]
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: in vitro and in vivo studies. Pharm. Dev. Technol. 2017, 24, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M.G.; Shankar, R.K.; Yang, H. Chitosan nanofibers for transbuccal insulin delivery. J. Biomed. Mater. Res. Part A 2017, 105, 1252–1259. [Google Scholar] [CrossRef]
- Clitherow, K.H.; Murdoch, C.; Spain, S.G.; Handler, A.M.; Colley, H.E.; Stie, M.B.; Nielsen, H.M.; Janfelt, C.; Hatton, P.V.; Jacobsen, J. Mucoadhesive Electrospun Patch Delivery of Lidocaine to the Oral Mucosa and Investigation of Spatial Distribution in a Tissue Using MALDI-Mass Spectrometry Imaging. Mol. Pharm. 2019, 16, 3948–3956. [Google Scholar] [CrossRef]
- Li, X.; Kanjwal, M.A.; Lin, L.; Chronakis, I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces 2013, 103, 182–188. [Google Scholar] [CrossRef]
- Alkahtani, M.; Aodah, A.; Abu Asab, O.; Basit, A.; Orlu, M.; Tawfik, E. Fabrication and Characterization of Fast-Dissolving Films Containing Escitalopram/Quetiapine for the Treatment of Major Depressive Disorder. Pharmaceutics 2021, 13, 891. [Google Scholar] [CrossRef]
- Ponrasu, T.; Chen, B.-H.; Chou, T.-H.; Wu, J.-J.; Cheng, Y.-S. Fast Dissolving Electrospun Nanofibers Fabricated from Jelly Fig Polysaccharide/Pullulan for Drug Delivery Applications. Polymers 2021, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Possemiers, S.; Boone, M.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bhagurkar, A.M.; Darji, M.; Lakhani, P.; Thipsay, P.; Bandari, S.; Repka, M.A. Effects of formulation composition on the characteristics of mucoadhesive films prepared by hot-melt extrusion technology. J. Pharm. Pharmacol. 2018, 71, 293–305. [Google Scholar] [CrossRef]
- Palem, C.R.; Battu, S.K.; Maddineni, S.; Gannu, R.; Repka, M.A.; Yamsani, M.R. Oral transmucosal delivery of domperidone from immediate release films produced via hot-melt extrusion technology. Pharm. Dev. Technol. 2012, 18, 186–195. [Google Scholar] [CrossRef]
- Palem, C.R.; Dudhipala, N.; Battu, S.K.; Goda, S.; Repka, M.A.; Yamsani, M.R. Combined dosage form of pioglitazone and felodipine as mucoadhesive pellets via hot melt extrusion for improved buccal delivery with application of quality by design approach. J. Drug Deliv. Sci. Technol. 2015, 30, 209–219. [Google Scholar] [CrossRef]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Xue, L.; Liu, S.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef]
- Tagami, T.; Yoshimura, N.; Goto, E.; Noda, T.; Ozeki, T. Fabrication of Muco-Adhesive Oral Films by the 3D Printing of Hydroxypropyl Methylcellulose-Based Catechin-Loaded Formulations. Biol. Pharm. Bull. 2019, 42, 1898–1905. [Google Scholar] [CrossRef]
- Tian, P.; Yang, F.; Xu, Y.; Lin, M.-M.; Yu, L.-P.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Oral disintegrating patient-tailored tablets of warfarin sodium produced by 3D printing. Drug Dev. Ind. Pharm. 2018, 44, 1918–1923. [Google Scholar] [CrossRef]
- Cho, H.W.; Baek, S.H.; Lee, B.J.; Jin, H.E. Orodispersible Polymer Films with the Poorly Water-Soluble Drug, Olanzapine: Hot-Melt Pneumatic Extrusion for Single-Process 3D Printing. Pharmaceutics 2020, 22, 692. [Google Scholar] [CrossRef] [PubMed]
- Databridge Market. Available online: https://www.databridgemarketresearch.com/reports/global-oral-drug-delivery-market#:~:text=The%20oral%20drug%20delivery%20market,period%20of%202022%20to%202029 (accessed on 20 November 2022).
- Future Marketinsights. Available online: https://www.futuremarketinsights.com/reports/oral-controlled-release-drug-delivery-technology-market (accessed on 20 November 2022).
- Grandview Research. Available online: https://www.grandviewresearch.com/industry-analysis/buccal-drug-delivery-systems-market-report#:~:text=The%20global%20buccal%20drug%20delivery,and%20development%20in%20this%20field (accessed on 20 November 2022).
- Transparency Market Research. Available online: https://www.transparencymarketresearch.com/oral-transmucosal-drugs-market.html (accessed on 20 November 2022).
- Biospace. Available online: https://www.biospace.com/article/oral-transmucosal-drugs-market-buccal-mucosa-segment-held-major-share-of-the-market/ (accessed on 20 November 2022).


| Polymer | Drug | Therapeutic Use | Tested Model | References |
|---|---|---|---|---|
| HPMC/PVA | Levocetirizine dihydrochloride | Antihistamine | In vitro dissolution tests, and in vivo studies in rats | [24] |
| HPMC/PVA | Telmisartan | Hypertension | In vitro dissolution test | [25] |
| PVP | Paracetamol/caffeine | Analgesic and antipyretic | In vitro dissolution test | [26] |
| Chitosan | Metformin | Diabetes | In vitro dissolution test | [27] |
| PEG/400 | Lercanidipine | Hypertension, and angina pectoris | In vitro Dissolution test and ex vivo drug permeation through porcine buccal mucosa | [28] |
| HPMC | Mirtazapine | Depression | In vitro dissolution test | [29] |
| HPMC/Alginate | Lidocaine | Anesthetic | In vitro dissolution test | [30] |
| Pullullan | Salbutamol sulfate | Asthma | In vitro dissolution tests, and in vivo studies on humans | [31] |
| Gelatin/Starch | Vitamin C | Assists in numerous functions | In vitro dissolution tests, and in vivo studies on humans | [32] |
| Chitosan/Pullullan | Aspirin | Minor aches, pains, and fever | In vitro dissolution test | [33] |
| Gelatin/gelatinized tapioca starch | Lidocaine | Anesthetic | In vitro dissolution test and ex vivo drug permeation through chick chorioallantoic membrane (CAM) | [34] |
| HPMC | Venlafaxine | Depression | In vitro dissolution test | [35] |
| Pectin/CMC | Paroxetin | Depression and anxiety | In vitro dissolution test and ex vivo drug permeation through chicken buccal pouch | [36] |
| Natural Polymer | Main Characteristic | Examples of Use | Tested Model | References |
|---|---|---|---|---|
| Alginate | Ability to form reversible hydrogels through interaction of carboxylic acid functional groups with metal cations | Film for the delivery of cetirizine dihydrochloride | In vitro release test | [134] |
| Cellulose | Tunable mechanical properties | Biomaterials for buccal delivery of non-steroidal anti-inflammatory drugs | In vitro release test | [135] |
| Chitosan | Cationic nature, a property that allows the formation of electrostatic complexes with negatively charged polymers | Gel for the delivery of the antimicrobial Schinus molle L essential oil | No models have been tested | [136] |
| Collagen | Excellent biocompatibility, flexibility and ability to absorb body fluids for delivery of nutrients | Buccal patch for the delivery of lorazepan | In vitro release test and ex vivo drug permeation through bovine buccal mucosa | [137] |
| Gellan Gum | Excellent gelation capability | Film for the delivery of triamcinolone acetonide | In vitro release test | [138] |
| Guar Gum | Excellent ability to hydrate rapidly, generating highly viscous solutions | Film-nanoparticle composite for the delivery of alpha-casozepine | In vitro release test | [57] |
| Gelatin | Thermoresponsiveness | Film for the delivery of propranolol hydrochloride | In vitro and in silico release test | [48] |
| Hyaluronic acid | Biocompatibility, hydrophilicity, low immunogenicity and excellent viscoelasticity | Mucoadhesive microneedles for the delivery of lidocaine | In vivo drug permeation through rat buccal mucosa | [104] |
| Pectin | Excellent biodegradability, biocompatibility and possibility of ionic crosslinking | Film for the delivery of triamcinolone acetonide | In vitro release test | [139] |
| Type of Process | Basic Description |
|---|---|
| Material extrusion | Material is extruded through a nozzle or orifice and deposited on the surface |
| Material jetting | Drops of material are deposited on the surface until the desired layers are formed |
| Binder jetting | A liquid bonding agent is deposited to join powder materials |
| Sheet lamination | Material sheets are deposited to form the final desired object |
| Vat photopolymerization | Liquid photopolymer in a vat is selectively cured by light-activated polymerization |
| Powder bed fusion | Thermal energy selectively fuses regions of a powder bed |
| Directed energy deposition | Focused thermal energy is used to fuse materials by melting as the material is deposited |
| Biomaterial Production Technique | Biomaterial Application | Matrix Composition | Active Agent | Main Conclusion | Reference |
|---|---|---|---|---|---|
| Solvent casting | Oral fast-dissolving film | HPMC | Risperidone | Satisfactory physicochemical properties and in vitro behavior | [188] |
| Oral fast-dissolving film | HPMC | Diazepam | Good mechanical strength, drug release, disintegration time and stability | [189] | |
| Oral fast-dissolving films | HPMC | Loratadine | Good physico chemical properties. The solvent casting method can be adopted for the preparation of films | [190] | |
| Mucoadhesive buccal films | EC/HPMC | Ornidazole/ dexamethasone | Desirable physical characteristics and mucoadhesive properties | [191] | |
| Microneedles | PVP | POXA1b laccase enzyme | The microneedles were able to control the release kinetics of the compound incorporated | [77] | |
| Electrospinning | Oral film | Chitosan and PEO | Insulin | Fiber morphology, film mechanical properties, and in vitro stability dependent on PEO feed ratio. Lower PEO content formulations produced smaller diameter fibers with significantly faster insulin release kinetics | [192] |
| Mucoadhesive buccal film | PVP, Eudragit RS100 and PEO (mucoadhesive layer) and PCL | Lidocaine | Analysis of ex vivo diffusion through porcine buccal mucosa suggested that lidocaine permeated the oral mucosa, enabling its use to reduce pain in the oral cavity. | [193] | |
| Oral fast-dissolving films | PVA | Caffeine and riboflavin | Burst released of both drugs (caffeine to an extent of 100% and riboflavin to an extent of 40% within 60 s) from PVA nanofibrous matrices | [194] | |
| Oral fast-dissolving films | Chitosan/ Pullulan | Aspirin | Fast film dissolution and efficient aspirin encapsulation indicated potential use for oral mucosal drug release | [33] | |
| Oral fast-dissolving films | PVP | Escitalopram and quetiapine | The drug-loaded fibers exhibited a disintegration time of 2 s, which accelerated the release of both drugs (50% after 5 min) making it an attractive formulation for oral mucosal delivery | [195] | |
| Fast-dissolving drug delivery system | Jelly fig polysaccharide/Pullulan | Hydrophobic drugs | Formulation consisting of a promising carrier to encapsulate hydrophobic drugs for fast-dissolving/disintegrating delivery applications. | [196] | |
| Hot melt extrusion | Oral tablets | EVA | Metropolol tartrate | Drug release dependent on drug loading and extrusion temperature | [197] |
| Oral fast dissolving film | Lycoat® RS 780 (modified starch) | Chlorpheniramine maleate | Films showed immediate disintegration and dissolution, due to the presence hydrophilic excipients. The formulation showed to be a good option to produce solvent-free thin films | [198] | |
| Mucoadhesive buccal film | HPC/HPMC/PEG | Salbutamol sulphate | Evidence provided to support the selection of formulation compositions to produce hot-melt extruded mucoadhesive films | [199] | |
| Mucoadhesive buccal film | PEO N10/HPMC/Eudragit RL100 | Domperidone | HME is a viable technique for the preparation of buccal-adhesive films with improved drug bioavailability | [200] | |
| Mucoadhesive oral tablet | PEO/HPMC | Pioglitazone/felodipine | The optimized formulation showed adequate in vitro drug release, ex vivo permeation, and bioadhesive properties | [201] | |
| 3D printing | Oral film | Pullulan/HPMC | Caffeine | Effective spatial deposition control of films and successful determination of orientation to maximize the mechanical properties of the hybrid films obtained through 3D printing | [202] |
| Microneedles | Alginate and hydroxyapatite | Glucose -responsive insulin | Microneedles exhibited sufficient mechanical strength to penetrate the skin of mice and responsively released insulin according to the glucose levels both in glucose solution and in type 1 diabetic mice | [203] | |
| Mucoadhesive oral film | HPMC | Catechin hydrate | Flexible application of 3D bioprinters (semi-solid extrusion-type 3D printers) to prepare film formulations | [204] | |
| Oral disintegrating tablets | PVP/Starch/ Microcrystalline Cellulose | Warfarin sodium | Tablets prepared by the 3D technique showed uniform drug content, good mechanical properties, and presented fast disintegration and fast dissolution | [205] | |
| Oral fast-dissolving films | PEO/PVP/ Poloxamer (P 407 and P188) | Olanzapine | The films showed increased dissolution rates of the poorly water-soluble drug, consisting in a suitable formulation for fast drug absorption | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remiro, P.d.F.R.; Nagahara, M.H.T.; Azoubel, R.A.; Franz-Montan, M.; d’Ávila, M.A.; Moraes, Â.M. Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies. Pharmaceutics 2023, 15, 12. https://doi.org/10.3390/pharmaceutics15010012
Remiro PdFR, Nagahara MHT, Azoubel RA, Franz-Montan M, d’Ávila MA, Moraes ÂM. Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies. Pharmaceutics. 2023; 15(1):12. https://doi.org/10.3390/pharmaceutics15010012
Chicago/Turabian StyleRemiro, Paula de Freitas Rosa, Mariana Harue Taniguchi Nagahara, Rafael Abboud Azoubel, Michelle Franz-Montan, Marcos Akira d’Ávila, and Ângela Maria Moraes. 2023. "Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies" Pharmaceutics 15, no. 1: 12. https://doi.org/10.3390/pharmaceutics15010012
APA StyleRemiro, P. d. F. R., Nagahara, M. H. T., Azoubel, R. A., Franz-Montan, M., d’Ávila, M. A., & Moraes, Â. M. (2023). Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies. Pharmaceutics, 15(1), 12. https://doi.org/10.3390/pharmaceutics15010012








