Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi?
Abstract
1. Introduction
2. Fungal Pulmonary Diseases
3. Current Treatment for Fungal Pulmonary Diseases
3.1. Azoles
3.2. Polyenes
3.3. Echinocandin
4. Amphotericin B Mechanism of Action and Challenges
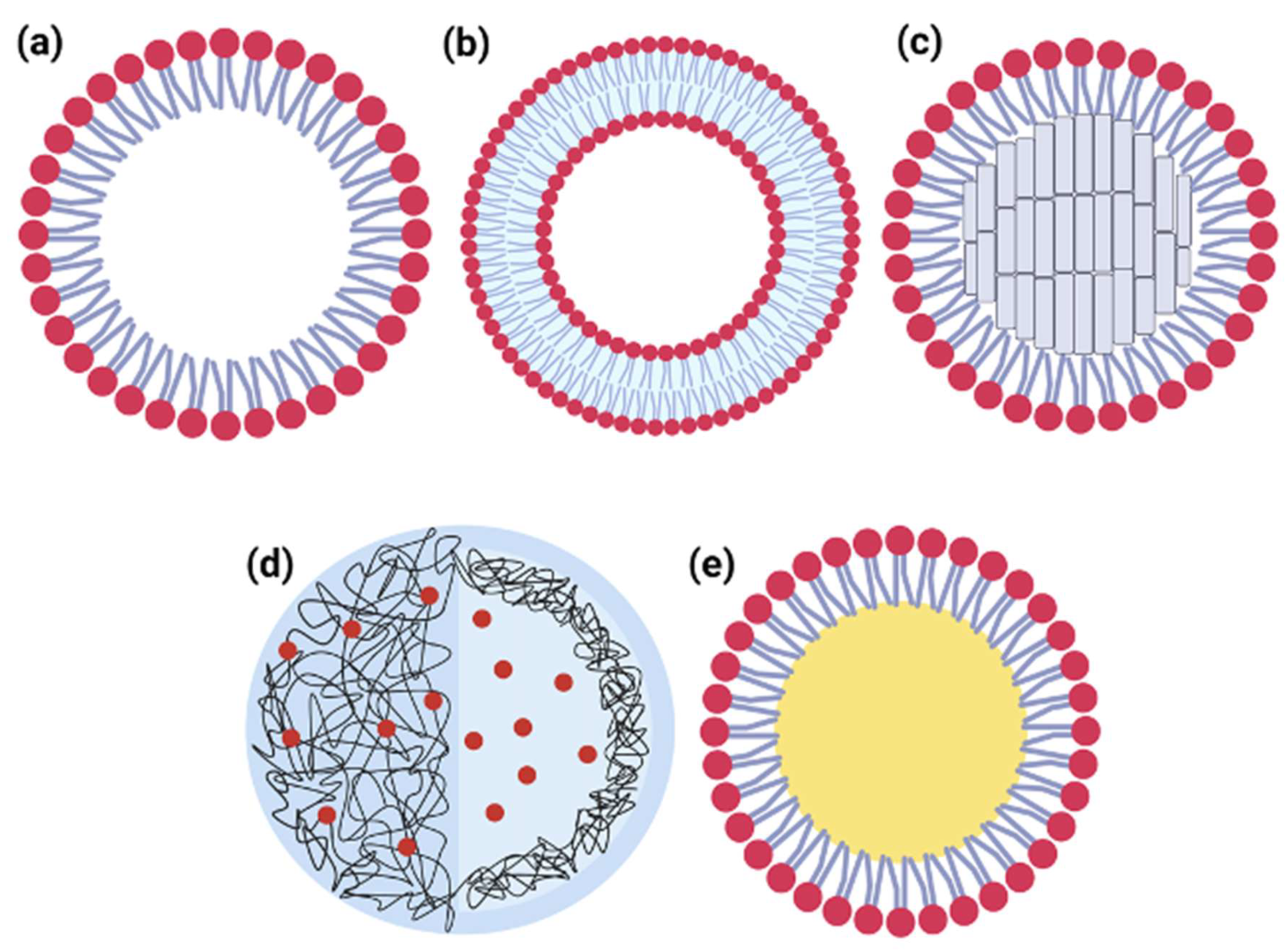
5. Strategies for Pulmonary Delivery
6. Use of AmB Formulation for Pulmonary Delivery
7. Technological Alternative to Delivery of AmB to the Lungs
| Formulation | Methodology | Lung Application | Drug Concentration | Average Size (µm) | Zeta Potential (mV) | VMD (µm) | MMAD (µm) | GSD (µm) | FPF (%) | RF (%) | In Vitro Release | In Vivo Test | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerosolized, non-ionic surfactant vesicle with cyclodextrin | Lipid film and then freeze-drying | Air-jet nebulization | 0.93–1.19 mg/mL | 1.677 ± 0.310 | −70.8 ± 2.9 | NA | NA | NA | NA | NA | No | Yes | [230] |
| Nanodisk composed of PL and Apo A-I | Lipid-film | NA | 0.6 mg/mL | 0.008–0.01 | NA | NA | NA | NA | NA | NA | No | No | [231,232] |
| Nano-emulsion Intralipid® | Sonication and vorttexing | Air-jet nebulization | 87.46 ± 2.21% (21.86 mg) | ~0.375 | −22 | 5.00 ± 0.07 | NA | NA | 57% | 88 | No | No | [156] |
| Nano-emulsion Clinoleic® | 80.7 ± 0.70% (20.19 mg) | ~0.325 | −34 | 4.41 ± 0.19 | 80% | 90 | |||||||
| Proliposomal microparticles/nanoparticles, aerosolized | Co-spray drying of the drug and the phospholipids | DPI/Handihaler (Boehringer Ingelheim) | 0.202 ± 0.118 mg/mg | 1.105 ± 0.461 | NA | NA | 12.1 ± 5.2 | 3.5 ± 0.5 | 13.0 ± 1.6 | 32.4 ± 9.6 | No | No | [226] |
| 0.146 ± 0.0.019 mg/mg | 1.110 ± 0.317 | NA | NA | 5.3 ± 1.0 | 2.7 ± 0.2 | 22.4 ± 4.1 | 46.0 ± 4.3 | ||||||
| 0.155 ± 0.060 mg/mg | 1.311 ± 0.581 | NA | NA | 2.2 ± 0.1 | 1.8 ± 0.1 | 46.8 ± 5.4 | 93.6 ± 0.7 | ||||||
| Solid lipid nanoparticles | Solvent emulsification-evaporation to obtain the SLN and then lyophilization with a solution of lactose | NA | NA | 0.187 ± 0.120 (SLN) | −30.16 ± 1.60 | NA | NA | NA | 35.71 ± 1.81 (1% lactose) 53.96 ± 3.67 (5% lactose) 72.57 ± 4.33 (10% lactose) 54.99 ± 3.04 (15% lactose) 22.03 ± 2.53 (20% lactose) | NA | Yes | No | [201] |
| Liposome (MLV) | Lipid film rehydration followed by sonication followed by the coating process | Ultrasonic jet nebulizer | 74.29 ± 2.3% | 0.413 ± 0.046 | −18.5 ± 3.8 | 0.285 ± 0.025 * | NA | NA | NA | NA | No | No | [158] |
| Emulsomes | 79.79 ± 3.1% | 0.384 ± 0.040 | −14.3 ± 2.1 | 0.286 ± 0.021 * | |||||||||
| Liposome coated OPM | 72.19 ± 2.1 % | 0.487 ± 0.039 | −26.3 ± 2.3 | 0.378 ± 0.033 * | |||||||||
| Liposome coated with EBA-2 mAb | 77.39 ± 2.7% | 0.435 ± 0.038 | −20.2 ± 1.9 | 0.280 ± 0.025 * | |||||||||
| Emulsomes coated with OPM | 71.89 ± 2.1% | 0.455 ± 0.042 | −24.2 ± 2.5 | 0.343 ± 0.030 * | |||||||||
| Emulsomes coated with EBA-2 mAb | 77.29 ± 1.9 % | 0.392 ± 0.040 | −21.7 ± 2.5 | 0.318 ± 0.028 * | |||||||||
| MLV | Lipid film and rehydration followed by recovering | Pressurized packed systems based on chlorofluorocarbon aerosol propellants | 78.2 ± 1.3% | 2.56 ± 0.32 | NA | 2.27 ± 0.25 | NA | NA | NA | NA | No | Yes | [159] |
| MLVs coated with OPM | 77.3 ± 2.5% | 3.15 ± 0.59 | 2.87 ± 0.47 | ||||||||||
| MLV coated with OPP | 76.8 ± 3.4% | 3.23 ± 0.62 | 2.96 ± 0.58 | ||||||||||
| MLV mixture with lactose | Modified reverse phase evaporation | 16.8 ± 2.2 | NA | No | No | [192] | |||||||
| Polymeric micelles | Solvent evaporation | Air-jet nebulization | 1780 ug | 0.150 ± 0.006 | 47.2 ± 6.5 | - | - | 41.2 ± 1.7 | NA | No | No | [205] | |
| MLV | Thin-film hydration followed by sonication | Air-jet nebulization | 0.067 mg/mL | 0.181 ± 0.0034 | −8.36 ± 2.8 | 3.43 ± 0.87 | 95.6 ± 1.2 | NA | 56 (188.6 ± 8 µg AmB) | NA | No | No | [157] |
| MLV coated with 0.2% of chitosan | 0.079 mg/mL | 0.196 ± 0.0016 | +11.5 ± 3.1 | 3.82 ± 1.66 | 93.8 ± 0.9 | 58 (229.7± 9 µg AmB) | |||||||
| Proliposomes liposome | Ethanol-based proliposomes followed by sonication | 0.047 mg/mL | 0.172 ± 0.0072 | −11.4 ± 5.9 | 2.46 ± 0.92 | 94.3 ± 2.1 | 53.1 ± 2.6 (177.1 ± 6 µg) | ||||||
| Proliposomes liposome coated with 0.3% of chitosan | 0.056 mg/mL | 0.211 ± 0.0033 | +22.9 ± 2.4 | 3.28 ± 0.87 | 93.1 ± 2.6 | 61.3 ± 1.9 (243.7 ± 11 µg) |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Salazar, F.; Bignell, E.; Brown, G.D.; Cook, P.C.; Warris, A. Pathogenesis of respiratory viral and fungal coinfections. Clin. Microbiol. Rev. 2022, 35, e0009421. [Google Scholar] [CrossRef]
- Hayes, D., Jr.; Murphy, B.S.; Lynch, J.E.; Feola, D.J. Aerosolized amphotericin for the treatment of allergic bronchopulmonary aspergillosis. Pediatr. Pulmonol. 2010, 45, 1145–1148. [Google Scholar] [CrossRef]
- Soubani, A.O.; Chandrasekar, P.H. The clinical spectrum of pulmonary aspergillosis. Chest 2002, 121, 1988–1999. [Google Scholar] [CrossRef]
- Kriengkauykiat, J.; Ito, J.I.; Dadwal, S.S. Epidemiology and treatment approaches in management of invasive fungal infections. Clin. Epidemiol. 2011, 3, 175. [Google Scholar]
- Koltsida, G.; Zaoutis, T. Fungal lung disease. Paediatr. Respir. Rev. 2021, 37, 99–104. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Gomes Marisa, Z.R.; Lewis Russell, E.; Kontoyiannis Dimitrios, P. Mucormycosis caused by unusual mucormycetes, non-rhizopus, -mucor, and -lichtheimia species. Clin. Microbiol. Rev. 2011, 24, 411–445. [Google Scholar] [CrossRef]
- Kuiper, L.; Ruijgrok, E.J. A review on the clinical use of inhaled amphotericin b. J. Aerosol. Med. Pulm. Drug. Deliv. 2009, 22, 213–227. [Google Scholar] [CrossRef]
- Wang, X.; Mohammad, I.S.; Fan, L.; Zhao, Z.; Nurunnabi, M.; Sallam, M.A.; Wu, J.; Chen, Z.; Yin, L.; He, W. Delivery strategies of amphotericin b for invasive fungal infections. Acta Pharm. Sin. B 2021, 11, 2585–2604. [Google Scholar] [CrossRef]
- Moldoveanu, B.; Gearhart, A.M.; Jalil, B.A.; Saad, M.; Guardiola, J.J. Pulmonary aspergillosis: Spectrum of disease. Am. J. Med. Sci. 2021, 361, 411–419. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical practice guidelines by the infectious diseases society of america: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin. Infect. Dis. 2019, 68, e1–e47. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill h1n1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Ku, Y.-H.; Chan, K.-S.; Yang, C.-C.; Tan, C.-K.; Chuang, Y.-C.; Yu, W.-L. Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J. Formos. Med. Assoc. 2017, 116, 660–670. [Google Scholar] [CrossRef]
- Beumer, M.C.; Koch, R.M.; van Beuningen, D.; OudeLashof, A.M.; van de Veerdonk, F.L.; Kolwijck, E.; van der Hoeven, J.G.; Bergmans, D.C.; Hoedemaekers, C.W.E. Influenza virus and factors that are associated with icu admission, pulmonary co-infections and icu mortality. J. Crit. Care 2019, 50, 59–65. [Google Scholar] [CrossRef]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in icu patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Zoumpourlis, V.; Goulielmaki, M.; Rizos, E.; Baliou, S.; Spandidos, D.A. [comment] The COVID-19 pandemic as a scientific and social challenge in the 21st century. Mol. Med. Rep. 2020, 22, 3035–3048. [Google Scholar] [CrossRef]
- Hussain, I.; Pervaiz, N.; Khan, A.; Saleem, S.; Shireen, H.; Wei, D.-Q.; Labrie, V.; Bao, Y.; Abbasi, A.A. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020, 21, 409–419. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Yuan, J.; Guo, Z.; Liu, J.; Liu, M. Global excess mortality during COVID-19 pandemic: A systematic review and meta-analysis. Vaccines 2022, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M. Invasive fungal disease complicating coronavirus disease 2019: When it rains, it spores. Clin. Infect. Dis. 2021, 73, e1645–e1648. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Ananthakrishnan, R.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Glampedakis, E.; Boillat-Blanco, N.; Oddo, M.; Pagani, J.L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1706–1708. [Google Scholar] [CrossRef]
- Calvo, V.; Borro, J.M.; Morales, P.; Morcillo, A.; Vicente, R.; Tarrazona, V.; Pariés, F.; Valencia Lung Transplant Group. Antifungal prophylaxis during the early postoperative period of lung transplantation. Chest 1999, 115, 1301–1304. [Google Scholar] [CrossRef]
- Oehling, A.G.M.S.M.; Giron, M.; Subira, M.L. Aerosol chemotherapy in bronchopulmonary candidiasis. Respiration 1975, 32, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rostami, N.; Alidadi, H.; Zarrinfar, H.; Salehi, P. Assessment of indoor and outdoor airborne fungi in an educational, research and treatment center. Ital. J. Med. 2016, 11, 52–56. [Google Scholar] [CrossRef][Green Version]
- Zarif, A.A.-O.; Thomas, A.; Vayro, A. Chronic pulmonary aspergillosis: A brief review. Yale J. Biol. Med. 2021, 94, 673–679. [Google Scholar]
- McDonald, E.G.; Butler-Laporte, G.; Del Corpo, O.; Hsu, J.M.; Lawandi, A.; Senecal, J.; Sohani, Z.N.; Cheng, M.P.; Lee, T.C. On the treatment of pneumocystis jirovecii pneumonia: Current practice based on outdated evidence. Open Forum Infect. Dis. 2021, 8, ofab545. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of hiv-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Young, J.H. Aspergillus infections. N. Engl. J. Med. 2021, 385, 1496–1509. [Google Scholar] [CrossRef]
- Latge, J.P.; Chamilos, G. Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.; Moss, R.B. Manifestations of pulmonary aspergillosis in pediatrics. Curr. Opin. Pediatr. 2020, 32, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Schanne, D.H.; Gunther, G.; Aebersold, D.M.; Elicin, O. Stereotactic body radiotherapy for recurrent hemoptysis due to chronic pulmonary aspergillosis: A case report and systematic review of the literature. Strahlenther. Onkol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Bories, P.; Moulin, J.C.; Ledoux, M.P.; Letscher-Bru, V. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann. N. Y. Acad. Sci. 2012, 1272, 23–30. [Google Scholar] [CrossRef]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef]
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for action: Invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin. Infect. Dis. 2018, 66, 140–148. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Florl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ecmm/isham consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Alvarez Fernandez, M.; Armando Melendez, D.; Aymerich de Franceschi, M. COVID-19 associated pulmonary aspergillosis. Med. Intensiv. 2022, S2173–S5727. [Google Scholar] [CrossRef]
- Feys, S.; Goncalves, S.M.; Khan, M.; Choi, S.; Boeckx, B.; Chatelain, D.; Cunha, C.; Debaveye, Y.; Hermans, G.; Hertoghs, M.; et al. Lung epithelial and myeloid innate immunity in influenza-associated or COVID-19-associated pulmonary aspergillosis: An observational study. Lancet Respir. Med. 2022, in press. [CrossRef]
- Shepardson, K.M.; Jhingran, A.; Caffrey, A.; Obar, J.J.; Suratt, B.T.; Berwin, B.L.; Hohl, T.M.; Cramer, R.A. Myeloid derived hypoxia inducible factor 1-alpha is required for protection against pulmonary aspergillus fumigatus infection. PLoS Pathog. 2014, 10, e1004378. [Google Scholar] [CrossRef]
- Espinosa, V.; Dutta, O.; McElrath, C.; Du, P.; Chang, Y.J.; Cicciarelli, B.; Pitler, A.; Whitehead, I.; Obar, J.J.; Durbin, J.E.; et al. Type iii interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2017, 2, eaan5357. [Google Scholar] [CrossRef] [PubMed]
- Osherov, N. Interaction of the pathogenic mold aspergillus fumigatus with lung epithelial cells. Front. Microbiol. 2012, 3, 346. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.; Hayes, G.E.; Icheoku, U.J.; van Rhijn, N.; Denning, D.W.; Osherov, N.; Bignell, E.M. Anti-aspergillus activities of the respiratory epithelium in health and disease. J. Fungi. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.C.; Fischer, G.J.; Sinha, M.; McCabe, O.; Palmer, J.M.; Choera, T.; Lim, F.Y.; Wimmerova, M.; Carrington, S.D.; Yuan, S.; et al. Flea expression in aspergillus fumigatus is recognized by fucosylated structures on mucins and macrophages to prevent lung infection. PLoS Pathog. 2016, 12, e1005555. [Google Scholar] [CrossRef] [PubMed]
- Beaussart, A.; El-Kirat-Chatel, S.; Fontaine, T.; Latge, J.P.; Dufrene, Y.F. Nanoscale biophysical properties of the cell surface galactosaminogalactan from the fungal pathogen aspergillus fumigatus. Nanoscale 2015, 7, 14996–15004. [Google Scholar] [CrossRef]
- Chaudhary, N.; Datta, K.; Askin, F.B.; Staab, J.F.; Marr, K.A. Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to aspergillus and resultant pulmonary inflammation. Am. J. Respir. Crit. Care Med. 2012, 185, 301–310. [Google Scholar] [CrossRef]
- Balloy, V.; Sallenave, J.M.; Wu, Y.; Touqui, L.; Latge, J.P.; Si-Tahar, M.; Chignard, M. Aspergillus fumigatus-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 mapk, and erk1/2 pathways and not by the toll-like receptor-myd88 pathway. J. Biol. Chem. 2008, 283, 30513–30521. [Google Scholar] [CrossRef]
- Fernandes, J.; Hamidi, F.; Leborgne, R.; Beau, R.; Castier, Y.; Mordant, P.; Boukkerou, A.; Latge, J.P.; Pretolani, M. Penetration of the human pulmonary epithelium by aspergillus fumigatus hyphae. J. Infect. Dis. 2018, 218, 1306–1313. [Google Scholar] [CrossRef]
- Cunha, C.; Aversa, F.; Lacerda, J.F.; Busca, A.; Kurzai, O.; Grube, M.; Loffler, J.; Maertens, J.A.; Bell, A.S.; Inforzato, A.; et al. Genetic ptx3 deficiency and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 2014, 370, 421–432. [Google Scholar] [CrossRef]
- Carrion Sde, J.; Leal, S.M., Jr.; Ghannoum, M.A.; Aimanianda, V.; Latge, J.P.; Pearlman, E. The roda hydrophobin on aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 2013, 191, 2581–2588. [Google Scholar] [CrossRef]
- Philippe, B.; Ibrahim-Granet, O.; Prevost, M.C.; Gougerot-Pocidalo, M.A.; Sanchez Perez, M.; Van der Meeren, A.; Latge, J.P. Killing of aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 2003, 71, 3034–3042. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.Y.; Tian, X.L.; Shao, H.T.; Wu, X.D.; Wang, Q.; Su, X.; Shi, Y. Activation of nf-kappab and respiratory burst following aspergillus fumigatus stimulation of macrophages. Immunobiology 2014, 219, 25–36. [Google Scholar] [CrossRef]
- Feldman, M.B.; Dutko, R.A.; Wood, M.A.; Ward, R.A.; Leung, H.M.; Snow, R.F.; De La Flor, D.J.; Yonker, L.M.; Reedy, J.L.; Tearney, G.J.; et al. Aspergillus fumigatus cell wall promotes apical airway epithelial recruitment of human neutrophils. Infect. Immun. 2020, 88, e00813-19. [Google Scholar] [CrossRef]
- Jhingran, A.; Kasahara, S.; Shepardson, K.M.; Junecko, B.A.; Heung, L.J.; Kumasaka, D.K.; Knoblaugh, S.E.; Lin, X.; Kazmierczak, B.I.; Reinhart, T.A.; et al. Compartment-specific and sequential role of myd88 and card9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog. 2015, 11, e1004589. [Google Scholar] [CrossRef]
- Caffrey, A.K.; Lehmann, M.M.; Zickovich, J.M.; Espinosa, V.; Shepardson, K.M.; Watschke, C.P.; Hilmer, K.M.; Thammahong, A.; Barker, B.M.; Rivera, A.; et al. Il-1alpha signaling is critical for leukocyte recruitment after pulmonary aspergillus fumigatus challenge. PLoS Pathog. 2015, 11, e1004625. [Google Scholar] [CrossRef]
- Jhingran, A.; Mar, K.B.; Kumasaka, D.K.; Knoblaugh, S.E.; Ngo, L.Y.; Segal, B.H.; Iwakura, Y.; Lowell, C.A.; Hamerman, J.A.; Lin, X.; et al. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep. 2012, 2, 1762–1773. [Google Scholar] [CrossRef]
- Zhang, W.; He, D.; Wei, Y.; Shang, S.; Li, D.; Wang, L. Suppression of aspergillus fumigatus germination by neutrophils is enhanced by endothelial-derived csf3 production. Front. Microbiol. 2022, 13, 837776. [Google Scholar] [CrossRef]
- Bruns, S.; Kniemeyer, O.; Hasenberg, M.; Aimanianda, V.; Nietzsche, S.; Thywissen, A.; Jeron, A.; Latge, J.P.; Brakhage, A.A.; Gunzer, M. Production of extracellular traps against aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin roda. PLoS Pathog. 2010, 6, e1000873. [Google Scholar] [CrossRef]
- Hirahara, K.; Kokubo, K.; Aoki, A.; Kiuchi, M.; Nakayama, T. The role of cd4(+) resident memory t cells in local immunity in the mucosal tissue—Protection versus pathology. Front. Immunol. 2021, 12, 616309. [Google Scholar] [CrossRef]
- Tao, J.; Segal, B.H.; Eppolito, C.; Li, Q.; Dennis, C.G.; Youn, R.; Shrikant, P.A. Aspergillus fumigatus extract differentially regulates antigen-specific cd4+ and cd8+ t cell responses to promote host immunity. J. Leukoc. Biol. 2006, 80, 529–537. [Google Scholar] [CrossRef]
- Kolwijck, E.; van de Veerdonk, F.L. The potential impact of the pulmonary microbiome on immunopathogenesis of aspergillus-related lung disease. Eur. J. Immunol. 2014, 44, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R. Angiogenesis at the mold-host interface: A potential key to understanding and treating invasive aspergillosis. Future Microbiol. 2013, 8, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Fliesser, M.; Morton, C.O.; Bonin, M.; Ebel, F.; Hunniger, K.; Kurzai, O.; Einsele, H.; Loffler, J. Hypoxia-inducible factor 1alpha modulates metabolic activity and cytokine release in anti-aspergillus fumigatus immune responses initiated by human dendritic cells. Int. J. Med. Microbiol. 2015, 305, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C.H.; Beattie, S.R.; Fuller, K.K.; McGurk, E.A.; Tang, Y.W.; Hohl, T.M.; Obar, J.J.; Cramer, R.A., Jr. Heterogeneity among isolates reveals that fitness in low oxygen correlates with aspergillus fumigatus virulence. mBio 2016, 7, e01515-16. [Google Scholar] [CrossRef] [PubMed]
- Tekaia, F.; Latge, J.P. Aspergillus fumigatus: Saprophyte or pathogen? Curr. Opin. Microbiol. 2005, 8, 385–392. [Google Scholar] [CrossRef]
- Brunel, S.F.; Willment, J.A.; Brown, G.D.; Devereux, G.; Warris, A. Aspergillus-induced superoxide production by cystic fibrosis phagocytes is associated with disease severity. ERJ Open Res. 2018, 4, 68. [Google Scholar] [CrossRef]
- Paris, S.; Wysong, D.; Debeaupuis, J.P.; Shibuya, K.; Philippe, B.; Diamond, R.D.; Latge, J.P. Catalases of aspergillus fumigatus. Infect. Immun. 2003, 71, 3551–3562. [Google Scholar] [CrossRef]
- Lambou, K.; Lamarre, C.; Beau, R.; Dufour, N.; Latge, J.P. Functional analysis of the superoxide dismutase family in aspergillus fumigatus. Mol. Microbiol. 2010, 75, 910–923. [Google Scholar] [CrossRef]
- Burns, C.; Geraghty, R.; Neville, C.; Murphy, A.; Kavanagh, K.; Doyle, S. Identification, cloning, and functional expression of three glutathione transferase genes from aspergillus fumigatus. Fungal Genet. Biol. 2005, 42, 319–327. [Google Scholar] [CrossRef][Green Version]
- Robinet, P.; Baychelier, F.; Fontaine, T.; Picard, C.; Debre, P.; Vieillard, V.; Latge, J.P.; Elbim, C. A polysaccharide virulence factor of a human fungal pathogen induces neutrophil apoptosis via nk cells. J. Immunol. 2014, 192, 5332–5342. [Google Scholar] [CrossRef]
- Lee, M.J.; Liu, H.; Barker, B.M.; Snarr, B.D.; Gravelat, F.N.; Al Abdallah, Q.; Gavino, C.; Baistrocchi, S.R.; Ostapska, H.; Xiao, T.; et al. The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog. 2015, 11, e1005187. [Google Scholar] [CrossRef]
- Gresnigt, M.S.; Bozza, S.; Becker, K.L.; Joosten, L.A.; Abdollahi-Roodsaz, S.; van der Berg, W.B.; Dinarello, C.A.; Netea, M.G.; Fontaine, T.; De Luca, A.; et al. A polysaccharide virulence factor from aspergillus fumigatus elicits anti-inflammatory effects through induction of interleukin-1 receptor antagonist. PLoS Pathog. 2014, 10, e1003936. [Google Scholar] [CrossRef]
- Brizendine, K.D.; Baddley, J.W.; Pappas, P.G. Pulmonary cryptococcosis. Semin. Respir. Crit. Care Med. 2011, 32, 727–734. [Google Scholar] [CrossRef]
- Chang, C.C.; Sorrell, T.C.; Chen, S.C. Pulmonary cryptococcosis. Semin. Respir. Crit. Care Med. 2015, 36, 681–691. [Google Scholar] [CrossRef]
- Speed, B.; Dunt, D. Clinical and host differences between infections with the two varieties of cryptococcus neoformans. Clin. Infect. Dis. 1995, 21, 28–34, discussion 35–26. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, Z.W.; Li, F.; Zhou, L.H.; Jiang, Y.S.; Yu, Y.; Ma, H.H.; Zhu, L.P.; Qu, J.M.; Jia, X.M. Inhibition of myeloid-derived suppressor cell arginase-1 production enhances t-cell-based immunotherapy against cryptococcus neoformans infection. Nat. Commun. 2022, 13, 4074. [Google Scholar] [CrossRef]
- Belda, W., Jr.; Casolato, A.T.S.; Luppi, J.B.; Passero, L.F.D.; Criado, P.R. Primary cutaneous cryptococcosis caused by cryptococcus gatti in an elderly patient. Trop. Med. Infect. Dis. 2022, 7, 206. [Google Scholar] [CrossRef]
- Idnurm, A.; Bahn, Y.S.; Nielsen, K.; Lin, X.; Fraser, J.A.; Heitman, J. Deciphering the model pathogenic fungus cryptococcus neoformans. Nat. Rev. Microbiol. 2005, 3, 753–764. [Google Scholar] [CrossRef]
- Santangelo, R.; Zoellner, H.; Sorrell, T.; Wilson, C.; Donald, C.; Djordjevic, J.; Shounan, Y.; Wright, L. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 2004, 72, 2229–2239. [Google Scholar] [CrossRef]
- Fu, M.S.; Coelho, C.; De Leon-Rodriguez, C.M.; Rossi, D.C.P.; Camacho, E.; Jung, E.H.; Kulkarni, M.; Casadevall, A. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal ph. PLoS Pathog. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Zaragoza, O. Basic principles of the virulence of cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Antachopoulos, C.; Walsh, T.J. Immunotherapy of cryptococcus infections. Clin. Microbiol. Infect. 2012, 18, 126–133. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.V.; Lei, S.; Radkov, A.; Volk, R.F.; Zaro, B.W.; Madhani, H.D. Secreted fungal virulence effector triggers allergic inflammation via tlr4. Nature 2022, 608, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Pneumocystis jiroveci. Semin. Respir. Crit. Care. Med. 2020, 41, 141–157. [Google Scholar] [CrossRef]
- Jeon, C.H.; Kim, S.H.; Kim, S.; Bae, M.; Lee, S.J.; Lim, S. Pneumocystis jirovecii pneumonia in patients with solid malignancies: A retrospective study in two hospitals. Pathogens 2022, 11, 1169. [Google Scholar] [CrossRef]
- Gioia, F.; Albasata, H.; Hosseini-Moghaddam, S.M. Concurrent infection with SARS-CoV-2 and pneumocystis jirovecii in immunocompromised and immunocompetent individuals. J. Fungi 2022, 8, 585. [Google Scholar] [CrossRef]
- Ezekowitz, R.A.; Williams, D.J.; Koziel, H.; Armstrong, M.Y.; Warner, A.; Richards, F.F.; Rose, R.M. Uptake of pneumocystis carinii mediated by the macrophage mannose receptor. Nature 1991, 351, 155–158. [Google Scholar] [CrossRef]
- Nandakumar, V.; Hebrink, D.; Jenson, P.; Kottom, T.; Limper, A.H. Differential macrophage polarization from pneumocystis in immunocompetent and immunosuppressed hosts: Potential adjunctive therapy during pneumonia. Infect. Immun. 2017, 85, e00939-16. [Google Scholar] [CrossRef]
- Beck, J.M.; Warnock, M.L.; Curtis, J.L.; Sniezek, M.J.; Arraj-Peffer, S.M.; Kaltreider, H.B.; Shellito, J.E. Inflammatory responses to pneumocystis carinii in mice selectively depleted of helper t lymphocytes. Am. J. Respir. Cell. Mol. Biol. 1991, 5, 186–197. [Google Scholar] [CrossRef]
- Shellito, J.; Suzara, V.V.; Blumenfeld, W.; Beck, J.M.; Steger, H.J.; Ermak, T.H. A new model of pneumocystis carinii infection in mice selectively depleted of helper t lymphocytes. J. Clin. Invest. 1990, 85, 1686–1693. [Google Scholar] [CrossRef]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Luyt, C.E.; Bouadma, L.; Morris, A.C.; Dhanani, J.A.; Kollef, M.; Lipman, J.; Martin-Loeches, I.; Nseir, S.; Ranzani, O.T.; Roquilly, A.; et al. Pulmonary infections complicating ards. Intensive Care Med. 2020, 46, 2168–2183. [Google Scholar] [CrossRef]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers—Therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J. Nanobiotechnol. 2022, 20, 101. [Google Scholar] [CrossRef]
- Brunet, K.; Martellosio, J.P.; Tewes, F.; Marchand, S.; Rammaert, B. Inhaled antifungal agents for treatment and prophylaxis of bronchopulmonary invasive mold infections. Pharmaceutics 2022, 14, 641. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Petraitis, V.; Walsh, T.J. Combination therapy for the treatment of pulmonary mold infections. Expert. Rev. Respir. Med. 2017, 11, 481–489. [Google Scholar] [CrossRef]
- Jaroszewski, D.E.; Webb, B.J.; Leslie, K.O. Diagnosis and management of lung infections. Thorac. Surg. Clin. 2012, 22, 301–324. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of america. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Fuentefria, A.M.; Pippi, B.; Dalla Lana, D.F.; Donato, K.K.; de Andrade, S.F. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2018, 66, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Lewis, R.E.; Dodds Ashley, E.S.; Ostrosky-Zeichner, L.; Zaoutis, T.; Thompson, G.R.; Andes, D.R.; Walsh, T.J.; Pappas, P.G.; Cornely, O.A.; et al. Core recommendations for antifungal stewardship: A statement of the mycoses study group education and research consortium. J. Infect. Dis. 2020, 222, S175–S198. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharm. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Jorda, T.; Puig, S. Regulation of ergosterol biosynthesis in saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Cadranel, J.; Flick, H.; Godet, C.; Hennequin, C.; Hoenigl, M.; Kosmidis, C.; Lange, C.; Munteanu, O.; Page, I.; et al. Treatment of chronic pulmonary aspergillosis: Current standards and future perspectives. Respiration 2018, 96, 159–170. [Google Scholar] [CrossRef]
- McBride, J.A.; Gauthier, G.M.; Klein, B.S. Clinical manifestations and treatment of blastomycosis. Clin. Chest Med. 2017, 38, 435–449. [Google Scholar] [CrossRef]
- Johnson, R.H.; Sharma, R.; Kuran, R.; Fong, I.; Heidari, A. Coccidioidomycosis: A review. J. Investig. Med. 2021, 69, 316–323. [Google Scholar] [CrossRef]
- Arauz, A.B.; Papineni, P. Histoplasmosis. Infect. Dis. Clin. North. Am. 2021, 35, 471–491. [Google Scholar] [CrossRef]
- Santos, L.A.; Grisolia, J.C.; Malaquias, L.C.C.; Paula, F.B.A.; Dias, A.L.T.; Burger, E. Medication association and immunomodulation: An approach in fungal diseases and in particular in the treatment of paracoccidioidomycosis. Acta Trop. 2020, 206, 105412. [Google Scholar] [CrossRef]
- Aung, A.K.; Spelman, D.W.; Thompson, P.J. Pulmonary sporotrichosis: An evolving clinical paradigm. Semin. Respir. Crit. Care Med. 2015, 36, 756–766. [Google Scholar] [CrossRef]
- Setianingrum, F.; Rautemaa-Richardson, R.; Denning, D.W. Pulmonary cryptococcosis: A review of pathobiology and clinical aspects. Med. Mycol. 2019, 57, 133–150. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
- Moss, R.B. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur. Respir. J. 2014, 43, 1487–1500. [Google Scholar] [CrossRef]
- Stathakis, A.; Lim, K.P.; Boan, P.; Lavender, M.; Wrobel, J.; Musk, M.; Heath, C.H. Penicillium marneffei infection in a lung transplant recipient. Transpl. Infect. Dis. 2015, 17, 429–434. [Google Scholar] [CrossRef]
- Denning, D.W.; Tucker, R.M.; Hansen, L.H.; Stevens, D.A. Treatment of invasive aspergillosis with itraconazole. Am. J. Med. 1989, 86, 791–800. [Google Scholar] [CrossRef]
- Kanj, A.; Abdallah, N.; Soubani, A.O. The spectrum of pulmonary aspergillosis. Respir. Med. 2018, 141, 121–131. [Google Scholar] [CrossRef]
- Chrdle, A.; Mustakim, S.; Bright-Thomas, R.J.; Baxter, C.G.; Felton, T.; Denning, D.W. Aspergillus bronchitis without significant immunocompromise. Ann. N. Y. Acad. Sci. 2012, 1272, 73–85. [Google Scholar] [CrossRef]
- Nivoix, Y.; Ledoux, M.P.; Herbrecht, R. Antifungal therapy: New and evolving therapies. Semin. Respir. Crit. Care Med. 2020, 41, 158–174. [Google Scholar] [CrossRef]
- Denning, D.W.; Hope, W.W. Therapy for fungal diseases: Opportunities and priorities. Trends Microbiol. 2010, 18, 195–204. [Google Scholar] [CrossRef]
- Denning, D.W.; Chakrabarti, A. Pulmonary and sinus fungal diseases in non-immunocompromised patients. Lancet Infect. Dis. 2017, 17, e357–e366. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Antifungal agents: Spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. North Am. 2016, 30, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, T.E. The structure and function of amphotericin b-cholesterol pores in lipid bilayer membranes. Ann. N. Y. Acad. Sci. 1974, 235, 448–468. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Delhom, R.; Nelson, A.; Laux, V.; Haertlein, M.; Knecht, W.; Fragneto, G.; Wacklin-Knecht, H.P. The antifungal mechanism of amphotericin b elucidated in ergosterol and cholesterol-containing membranes using neutron reflectometry. Nanomaterials 2020, 10, 2439. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Soutar, C.P.; Greenwood, A.I.; Nimerovsky, E.; De Lio, A.M.; Holler, J.T.; Hisao, G.S.; Khandelwal, A.; Zhang, J.; SantaMaria, A.M.; et al. Fungicidal amphotericin b sponges are assemblies of staggered asymmetric homodimers encasing large void volumes. Nat. Struct. Mol. Biol. 2021, 28, 972–981. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Bau-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin b: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Hartmann, C.A.; Aye, W.T.; Blair, J.E. Treatment considerations in pulmonary coccidioidomycosis. Expert. Rev. Respir. Med. 2016, 10, 1079–1091. [Google Scholar] [CrossRef]
- Marques, S.A. Paracoccidioidomycosis. Clin. Derm. 2012, 30, 610–615. [Google Scholar] [CrossRef][Green Version]
- Smith, J.A.; Kauffman, C.A. Pulmonary fungal infections. Respirology 2012, 17, 913–926. [Google Scholar] [CrossRef]
- Loh, B.S.; Ang, W.H. “Illuminating” echinocandins’ mechanism of action. ACS Cent. Sci. 2020, 6, 1651–1653. [Google Scholar] [CrossRef]
- Szymanski, M.; Chmielewska, S.; Czyzewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Huttel, W. Echinocandins: Structural diversity, biosynthesis, and development of antimycotics. Appl. Microbiol. Biotechnol. 2021, 105, 55–66. [Google Scholar] [CrossRef]
- Patil, A.; Majumdar, S. Echinocandins in antifungal pharmacotherapy. J. Pharm. Pharm. 2017, 69, 1635–1660. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shukla, D.; Mishra, B.; Singh, S. Lipid—An emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. 2009, 73, 1–15. [Google Scholar] [CrossRef]
- Araujo, I.B.; Brito, C.R.; Urbano, I.A.; Dominici, V.A.; Silva Filho, M.A.; Silveira, W.L.; Damasceno, B.P.; Medeiros, A.C.; Egito, E.S. Similarity between the in vitro activity and toxicity of two different fungizone/lipofundin admixtures. Acta Cir. Bras. 2005, 20 (Suppl. S1), 257–261. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin b. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef]
- Serrano, D.R.; Lalatsa, A. Oral amphotericin b: The journey from bench to market. J. Drug Deliv. Sci. Technol. 2017, 42, 75–83. [Google Scholar] [CrossRef]
- Torrado, J.J.; Espada, R.; Ballesteros, M.P.; Torrado-Santiago, S. Amphotericin b formulations and drug targeting. J. Pharm. Sci. 2008, 97, 2405–2425. [Google Scholar] [CrossRef]
- Deray, G. Amphotericin b nephrotoxicity. J. Antimicrob. Chemother. 2002, 49 (Suppl. S1), 37–41. [Google Scholar] [CrossRef]
- Benson, J.M.; Nahata, M.C. Clinical use of systemic antifungal agents. Clin. Pharm. 1988, 7, 424–438. [Google Scholar]
- Huang, W.; Li, T.; Zhou, C.; Wei, F.; Cao, C.; Jiang, J. Voriconazole versus amphotericin b as induction therapy for talaromycosis in hiv/aids patients: A retrospective study. Mycopathologia 2021, 186, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Kinh, N.V.; Cuc, N.T.K.; Tung, N.L.N.; Lam, N.T.; Thuy, P.T.T.; Cuong, D.D.; Phuc, P.T.H.; Vinh, V.H.; Hanh, D.T.H.; et al. A trial of itraconazole or amphotericin b for hiv-associated talaromycosis. N. Engl. J. Med. 2017, 376, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Donovick, R.; Gold, W.; Pagano, J.; Stout, H. Amphotericins a and b, antifungal antibiotics produced by a streptomycete. I. In vitro studies. Antibiot. Annu. 1955, 3, 579. [Google Scholar] [PubMed]
- Grela, E.; Wieczor, M.; Luchowski, R.; Zielinska, J.; Barzycka, A.; Grudzinski, W.; Nowak, K.; Tarkowski, P.; Czub, J.; Gruszecki, W.I. Mechanism of binding of antifungal antibiotic amphotericin b to lipid membranes: An insight from combined single-membrane imaging, microspectroscopy, and molecular dynamics. Mol. Pharm. 2018, 15, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Kakeya, H.; Izumikawa, K.; Obata, Y.; Nishino, T.; Takazono, T.; Kosai, K.; Morinaga, Y.; Kurihara, S.; Nakamura, S.; et al. Efficacy of aerosolized liposomal amphotericin b against murine invasive pulmonary mucormycosis. J. Infect. Chemother. 2014, 20, 104–108. [Google Scholar] [CrossRef]
- Bishara, J.; Weinberger, M.; Lin, A.Y.; Pitlik, S. Amphotericin b—Not so terrible. Ann. Pharmacother. 2001, 35, 308–310. [Google Scholar] [CrossRef]
- Drew, R. Potential role of aerosolized amphotericin b formulations in the prevention and adjunctive treatment of invasive fungal infections. Int. J. Antimicrob. Agents 2006, 27, 36–44. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Schweiger, C.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J. Control. Release 2012, 158, 329–335. [Google Scholar] [CrossRef]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Hastedt, J.E.; Bäckman, P.; Clark, A.R.; Doub, W.; Hickey, A.; Hochhaus, G.; Kuehl, P.J.; Lehr, C.-M.; Mauser, P.; McConville, J.; et al. Erratum to: Scope and relevance of a pulmonary biopharmaceutical classification system aaps/fda/usp workshop march 16–17th, 2015 in baltimore, md. AAPS Open 2016, 2, 4. [Google Scholar] [CrossRef]
- Jaspart, S.; Bertholet, P.; Piel, G.; Dogné, J.M.; Delattre, L.; Evrard, B. Solid lipid microparticles as a sustained release system for pulmonary drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 47–56. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.; Traini, D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Denning, D.W. Invasive aspergillosis. Clin. Infect. Dis. 1998, 26, 781–803. [Google Scholar] [CrossRef]
- Shaji, J.; Shaik, M. Current development in the evaluation methods of pulmonary drug delivery system. Indian J. Pharm. Sci. 2016, 78, 294–306. [Google Scholar] [CrossRef]
- Telko, M.J.; Hickey, A.J. Dry powder inhaler formulation. Respir. Care 2005, 50, 1209–1227. [Google Scholar]
- Nasr, M.; Nawaz, S.; Elhissi, A. Amphotericin b lipid nanoemulsion aerosols for targeting peripheral respiratory airways via nebulization. Int. J. Pharm. 2012, 436, 611–616. [Google Scholar] [CrossRef]
- Albasarah, Y.Y.; Somavarapu, S.; Stapleton, P.; Taylor, K.M.G. Chitosan-coated antifungal formulations for nebulisation. J. Pharm. Pharmacol. 2010, 62, 821–828. [Google Scholar] [CrossRef]
- Vyas, S.P.; Khatri, K.; Goyal, A.K. Functionalized nanocarrier(s) to image and target fungi infected immune cells. Med. Mycol. 2009, 47 (Suppl. S1), S362–S368. [Google Scholar] [CrossRef]
- Vyas, S.P.; Quraishi, S.; Gupta, S.; Jaganathan, K.S. Aerosolized liposome-based delivery of amphotericin b to alveolar macrophages. Int. J. Pharm. 2005, 296, 12–25. [Google Scholar] [CrossRef]
- Ferron, G.A.; Kerrebijn, K.F.; Weber, J. Properties of aerosols produced with three nebulizers. Am. Rev. Respir. Dis. 1976, 114, 899–908. [Google Scholar]
- McCallion, O.N.; Taylor, K.M.; Thomas, M.; Taylor, A.J. Nebulization of fluids of different physicochemical properties with air-jet and ultrasonic nebulizers. Pharm. Res. 1995, 12, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Tiddens, H.A.; Bos, A.C.; Mouton, J.W.; Devadason, S.; Janssens, H.M. Inhaled antibiotics: Dry or wet? Eur. Respir. J. 2014, 44, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Gebhart, J.; Just-Ntibling, G.; Eisenhart-Rothe, B.y.; Beinhauer-Reeb, I. Characterization of amphotericin b aerosols for inhalation treatment of pulmonary aspergillosis* summary. Infection 1996, 24, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, T.E.; Venkataramanan, R.; Mihelc, K.M.; Marcinkowski, A.L.; Ou, J.; McCook, B.M.; Weber, L.; Carey, M.E.; Paterson, D.L.; Pilewski, J.M.; et al. Aerosol deposition of lipid complex amphotericin-b (abelcet) in lung transplant recipients. Am. J. Transpl. 2006, 6, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Rijnders, B.J.; Cornelissen, J.J.; Slobbe, L.; Becker, M.J.; Doorduijn, J.K.; Hop, W.C.; Ruijgrok, E.J.; Lowenberg, B.; Vulto, A.; Lugtenburg, P.J.; et al. Aerosolized liposomal amphotericin b for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: A randomized, placebo-controlled trial. Clin. Infect. Dis. 2008, 46, 1401–1408. [Google Scholar] [CrossRef]
- CDER. Metered Dose Inhaler (mdi) and Dry Powder Inhaler (dpi) Products—Quality Considerations Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- EMA. Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products; EMA: Amsterdam, The Netherlands, 2006. [Google Scholar]
- ANVISA. Instrução Normativa n° 33, de 16 de Abril de 2019. Brasil, 2019; ANVISA: Brasília, Brazil, 2019; Volume 33, pp. 1689–1699. [Google Scholar]
- Mansour, H.M.; Rhee, Y.-S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.; et al. Pulmonary delivery nanomedicines towards circumventing physiological barriers: Strategies and characterization approaches. Adv. Drug. Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef]
- Torchilin, V. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef]
- Torchilin, V.P. Nanoparticulates as Drug Carries; Imperial College Press: London, UK, 2006. [Google Scholar]
- Rahman, Z.; Charoo, N.A.; Akhter, S.; Beg, S.; Reddy, I.K.; Khan, M.A. Nanotechnology-based drug products. In Nanoscale Fabrication, Optimization, Scale-uUp and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 619–655. [Google Scholar]
- Food and Drug Administration. Considering Whether an Fda-Regulated Product Involves the Application OF Nanotechnology. 2014; p. 13. Available online: https://www.regulations.gov/docket?D=FDA-2010-D-0530 (accessed on 1 October 2022).
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Bailey, M.M.; Berkland, C.J. Nanoparticle formulations in pulmonary drug delivery. Med. Res. Rev. 2009, 29, 196–212. [Google Scholar] [CrossRef]
- Yang, W.; Peters, J.I.; Williams, R.O., 3rd. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef]
- Andrew, L.; Dziubla, T.; Wattamwar, P.P. Polymeric Nanoparticles. In Engineering Polymer Systems for Improved Drug Delivery; Bader, R.A., Putnam, D.A., Eds.; Wiley Online Books: Hoboken, NJ, USA, 2013; pp. 117–189. [Google Scholar]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Duncan, R. Polymer therapeutics as nanomedicines: New perspectives. Curr. Opin. Biotechnol. 2011, 22, 492–501. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; De Lucca Freitas, L.; Pohlmann, A.R.; Freitas, L.d.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Química Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Di Gioia, S.; Conese, M. Polyethylenimine-mediated gene delivery to the lung and therapeutic applications. Dev. Ther. 2008, 2, 163–188. [Google Scholar]
- Filipović-Grcić, J.; Skalko-Basnet, N.; Jalsenjak, I. Mucoadhesive chitosan-coated liposomes: Characteristics and stability. J. Microencapsul. 2001, 18, 3–12. [Google Scholar] [CrossRef]
- Gao, W.; Hu, C.M.; Fang, R.H.; Zhang, L. Liposome-like nanostructures for drug delivery. J. Mater. Chem. B 2013, 1, 6569–6585. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first {fda-approved} nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Russo, A.; Peghin, M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs 2020, 80, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.J.; Kellaway, I.W.; Parry-Jones, D.R.; Woolfrey, S.G. 99m-technetium as a marker of liposomal deposition and clearance in the human lung. Int. J. Pharm. 1985, 26, 303–316. [Google Scholar] [CrossRef]
- Taylor, K.M.G.; Taylor, G.; Kellaway, I.W.; Stevens, J. The stability of liposomes to nebulisation. Int. J. Pharm. 1990, 58, 57–61. [Google Scholar] [CrossRef]
- Shah, S.P.; Misra, A. Development of liposomal amphotericin b dry powder inhaler formulation. Drug Deliv. 2004, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Spray-freeze-dried dry powder inhalation of insulin-loaded liposomes for enhanced pulmonary delivery. J. Drug Target. 2008, 16, 639–648. [Google Scholar] [CrossRef]
- Marcantonio, A.; Kugler, A.R.; Sahner, D.; Mufti, N.; Lee, J.D.; Samford, L.K.; Eldon, M.A. A randomized, placebo-controlled, dose-escalation, phase 1 study of the safety and pharmacokinetics of amphotericin b inhalation powder in healthy subjects. Blood 2007, 110, 4988. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2020, 13, 31. [Google Scholar] [CrossRef]
- Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC Trends Anal. Chem. 2016, 77, 100–108. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäeder, K.; Gohla, S. Solid lipid nanoparticles (sln) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fraceto, L.F. Polymeric and solid lipid nanoparticles for sustained release of carbendazim and tebuconazole in agricultural applications. Sci. Rep. 2015, 5, 13809. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef]
- Liu, J.; Gong, T.; Fu, H.; Wang, C.; Wang, X.; Chen, Q.; Zhang, Q.; He, Q.; Zhang, Z. Solid lipid nanoparticles for pulmonary delivery of insulin. Int. J. Pharm. 2008, 356, 333–344. [Google Scholar] [CrossRef]
- Mehrabani Yeganeh, E.; Bagheri, H.; Mahjub, R. Preparation, statistical optimization and in-vitro characterization of a dry powder inhaler (dpi) containing solid lipid nanoparticles encapsulating amphotericin b: Ion paired complexes with distearoyl phosphatidylglycerol. Iran J. Pharm. Res. 2020, 19, 45–62. [Google Scholar]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719. [Google Scholar] [CrossRef]
- Cheng, S.N.; Tan, Z.G.; Pandey, M.; Srichana, T.; Pichika, M.R.; Gorain, B.; Choudhury, H. A critical review on emerging trends in dry powder inhaler formulation for the treatment of pulmonary aspergillosis. Pharmaceutics 2020, 12, 1161. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Chapter 5—Polymeric Micelles for Drug Targeting and Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 167–202. [Google Scholar]
- Gilani, K.; Moazeni, E.; Ramezanli, T.; Amini, M.; Fazeli, M.R.; Jamalifar, H. Development of respirable nanomicelle carriers for delivery of amphotericin b by jet nebulization. J. Pharm. Sci. 2011, 100, 252–259. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Oudshoorn, M.; Romeijn, S.; van Meijgaarden, K.; Koerten, H.; van der Meulen, H.; Lambert, G.; Ottenhoff, T.; Benita, S.; Junginger, H.; et al. Cationic submicron emulsions for pulmonary DNA immunization. J. Control. Release 2004, 100, 145–155. [Google Scholar] [CrossRef]
- Myers, S.E.; Devine, S.M.; Topper, R.L.; Ondrey, M.; Chandler, C.; Otoole, K.; Williams, S.F.; Larson, R.A.; Geller, R.B. A pilot study of prophylactic aerosolized amphotericin b in patients at risk for prolonged neutropenia. Leuk. Lymphoma 1992, 8, 229–233. [Google Scholar] [CrossRef]
- Monforte, V.; Roman, A.; Gavaldá, J.; López, R.; Pou, L.; Simó, M.; Aguadé, S.; Soriano, B.; Bravo, C.; Morell, F. Nebulized amphotericin b concentration and distribution in the respiratory tract of lung-transplanted patients. Transplantation 2003, 75, 1571–1574. [Google Scholar] [CrossRef]
- Xia, D.; Sun, W.-K.; Tan, M.-M.; Zhang, M.; Ding, Y.; Liu, Z.-C.; Su, X.; Shi, Y. Aerosolized amphotericin b as prophylaxis for invasive pulmonary aspergillosis: A meta-analysis. Int. J. Infect. Dis. 2015, 30, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Yang, W.-J.; Zhao, X.-Q. Pulmonary aspergillosis treated with inhaled amphotericin b. Int. J. Infect. Dis. 2017, 54, 92–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarcinelli, M.A.; Martins da Silva, T.; Artico Silva, A.D.; Ferreira de Carvalho Patricio, B.; Mendes de Paiva, F.C.; Santos de Lima, R.; Leal da Silva, M.; Antunes Rocha, H.V. The pulmonary route as a way to drug repositioning in COVID-19 therapy. J. Drug Deliv. Sci. Technol. 2021, 63, 102430. [Google Scholar] [CrossRef] [PubMed]
- Ruijgrok, E.J.; Vulto, A.G.; Van Etten, E.W.M. Aerosol delivery of amphotericin b desoxycholate (fungizone) and liposomal amphotericin b (ambisome): Aerosol characteristics and in-vivo amphotericin b deposition in rats. J. Pharm. Pharmacol. 2000, 52, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.J.; Bernard, E.M.; Häuser, M.; Armstrong, D. Aerosol amphotericin b is effective for prophylaxis and therapy in a rat model of pulmonary aspergillosis. Antimicrob. Agents Chemother. 1988, 32, 1676–1679. [Google Scholar] [CrossRef]
- Olson, J.A.; Adler-Moore, J.P.; Schwartz, J.; Jensen, G.M.; Proffitt, R.T. Comparative efficacies, toxicities, and tissue concentrations of amphotericin b lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 2006, 50, 2122–2131. [Google Scholar] [CrossRef][Green Version]
- Ruijgrok, E.J.; Vulto, A.G.; Van Etten, E.W. Efficacy of aerosolized amphotericin b desoxycholate and liposomal amphotericin b in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats. J. Antimicrob. Chemother. 2001, 48, 89–95. [Google Scholar] [CrossRef]
- Kilburn, K. The innocuousness and possible therapeuticuse of aerosol amphotericin b. Am. Rev. Respir. Dis. 1959, 80, 441–442. [Google Scholar]
- Shirkhani, K.; Teo, I.; Armstrong-James, D.; Shaunak, S. Nebulised amphotericin b-polymethacrylic acid nanoparticle prophylaxis prevents invasive aspergillosis. Nanomedicine 2015, 11, 1217–1226. [Google Scholar] [CrossRef]
- Ray, A.; Manikanta, J.; Vyas, S. Nebulized amphotericin b vs oral itraconazole in pulmonary aspergilloma: A parallel group randomized controlled trial. Chest 2020, 158, A305. [Google Scholar] [CrossRef]
- Yashwant, K.O.; Salla, V.; Mydavolu, A.; Sarma, L. Successful treatment of pulmonary cunninghamella bertholletiae infection with liposomal amphotericin and posaconazole in an elderly male with psoriatic arthritis on immunosupression. Chest 2017, 152, A203–A204. [Google Scholar]
- Furco, A.; Mouchet, B.; Carbonnelle, M.; Vallerand, H. Pulmonary mucormycosis: Benefit of aerosol amphotericin b? Rev. Mal. Respir. 2001, 18, 309–313. [Google Scholar]
- Le, J.; Ashley, E.D.; Neuhauser, M.M.; Brown, J.; Gentry, C.; Klepser, M.E.; Marr, A.M.; Schiller, D.; Schwiesow, J.N.; Tice, S.; et al. Consensus summary of aerosolized antimicrobial agents: Application of guideline criteria. Pharmacotherapy 2010, 30, 62–584. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, C.; Liu, C.; Gao, Y. Amphotericin b nebulisation for invasive pulmonary aspergillosis prophylaxis: The conflict of ideality and reality. Int. J. Antimicrob. Agents 2017, 49, 263–264. [Google Scholar] [CrossRef]
- Dubois, J.; Bartter, T.; Gryn, J.; Pratter, M.R. The physiologic effects of inhaled amphotericin b. Chest 1995, 108, 750–753. [Google Scholar] [CrossRef]
- Gryn, J.; Goldberg, J.; Johnson, E.; Siegel, J.; Inzerillo, J. The toxicity of daily inhaled amphotericin b. Am. J. Clin. Oncol. 1993, 16, 43–46. [Google Scholar] [CrossRef]
- Griese, M.; Schams, A.; Lohmeier, K.P. Amphotericin b and pulmonary surfactant. Eur. J. Med. Res. 1998, 3, 383–386. [Google Scholar]
- Gomez, A.I.; Acosta, M.F.; Muralidharan, P.; Yuan, J.X.; Black, S.M.; Hayes, D., Jr.; Mansour, H.M. Advanced spray dried proliposomes of amphotericin b lung surfactant-mimic phospholipid microparticles/nanoparticles as dry powder inhalers for targeted pulmonary drug delivery. Pulm. Pharm. 2020, 64, 101975. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Barry, P.W. The science of nebulised drug delivery. Thorax 1997, 52, S31–S44. [Google Scholar] [CrossRef]
- Patricio, B.F.C.; Prado, L.D.; Rocha, H.V.A. O desafio de novas vias de administração para anfotericina b. Braz. J. Health Pharm. 2019, 1, 62–69. [Google Scholar]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remunan-Lopez, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, M.; Italia, J.L.; Mullen, A.B.; Ravi Kumar, M.N.; Candlish, A.A.; Williams, R.A.; Shaw, C.D.; Al Gawhari, F.; Coombs, G.H.; Wiese, M.; et al. The efficacy of aerosol treatment with non-ionic surfactant vesicles containing amphotericin b in rodent models of leishmaniasis and pulmonary aspergillosis infection. J. Control. Release 2012, 160, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.L.; Cavigiolio, G.; Fannucchi, M.V.; Illek, B.; Forte, T.M.; Oda, M.N. A phospholipid-apolipoprotein a-i nanoparticle containing amphotericin b as a drug delivery platform with cell membrane protective properties. Int. J. Pharm. 2010, 399, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.N.; Hargreaves, P.L.; Beckstead, J.A.; Redmond, K.A.; van Antwerpen, R.; Ryan, R.O. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin b. J. Lipid Res. 2006, 47, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kretschmar, M.; Amselem, S.; Zawoznik, E.; Mosbach, K.; Dietz, A.; Hof, H.; Nichterlein, T. Efficient treatment of murine systemic infection with candida albicans using amphotericin b incorporated in nanosize range particles (emulsomes). Mycoses 2001, 44, 281–286. [Google Scholar] [CrossRef]
- Kirkpatrick, W.R.; Najvar, L.K.; Vallor, A.C.; Wiederhold, N.P.; Bocanegra, R.; Pfeiffer, J.; Perkins, K.; Kugler, A.R.; Sweeney, T.D.; Patterson, T.F. Prophylactic efficacy of single dose pulmonary administration of amphotericin b inhalation powder in a guinea pig model of invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 2012, 67, 970–976. [Google Scholar] [CrossRef]
- Morris, G.; Kokki, M.H.; Anderson, K.; Richardson, M.D. Sampling of aspergillus spores in air. J. Hosp. Infect. 2000, 44, 81–92. [Google Scholar] [CrossRef]
- Alexander, B.D.; Dodds Ashley, E.S.; Addison, R.M.; Alspaugh, J.A.; Chao, N.J.; Perfect, J.R. Non-comparative evaluation of the safety of aerosolized amphotericin b lipid complex in patients undergoing allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2006, 8, 13–20. [Google Scholar] [CrossRef]
- Drew, R.H.; Dodds Ashley, E.; Benjamin, D.K., Jr.; Duane Davis, R.; Palmer, S.M.; Perfect, J.R. Comparative safety of amphotericin b lipid complex and amphotericin b deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation 2004, 77, 232–237. [Google Scholar] [CrossRef]
- Liao, Q.; Lam, J.K.W. Inhaled antifungal agents for the treatment and prophylaxis of pulmonary mycoses. Curr. Opin. Organ Transplant. 2008, 13, 91–123. [Google Scholar] [CrossRef]
- Kagan, L.; Gershkovich, P.; Wasan, K.M.; Mager, D.E. Physiologically based pharmacokinetic model of amphotericin b disposition in rats following administration of deoxycholate formulation (fungizone(r)): Pooled analysis of published data. AAPS J. 2011, 13, 255–264. [Google Scholar] [CrossRef]
- Hidalgo, A.; Garcia-Mouton, C.; Autilio, C.; Carravilla, P.; Orellana, G.; Islam, M.N.; Bhattacharya, J.; Bhattacharya, S.; Cruz, A.; Perez-Gil, J. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Control. Release 2021, 329, 205–222. [Google Scholar] [CrossRef]
- Guagliardo, R.; Perez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef]

| Drug | Dosage Range | Delivery System | Mechanism | Fungal Lung Infection |
|---|---|---|---|---|
| Itraconazole | 200–400 mg/day | Oral Intravenous | Inhibition of lanosterol 14α-demethylase | Aspergillosis [105] Fungal asthma (Allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitize) Blastomycosis [106] Coccidioidomycosis [107] Histoplasmosis [108] Paracoccidioidomycosis [109] Sporotrichosis [110] Second-line: Cryptococcal pneumonia [111] |
| Fluconazole | 50–800 mg/day | Oral Intravenous | Inhibition of lanosterol 14α-demethylase | Coccidioidomycosis [107] Cryptococcal pneumonia [111] Systemic candidiasis [112] |
| Voriconazole | 200–400 mg/day 4–6 mg/kg/day | Oral Intravenous | Inhibition of lanosterol 14α-demethylase | Aspergillosis [105] Candidemia [112] Second-line: Cryptococcal pneumonia |
| Posaconazole | 100–800 mg/day | Oral Intravenous | Inhibition of lanosterol 14α-demethylase | Second-line treatment: Invasive aspergillosis and coccidioidomycosis (refractory or intolerance to AmB or itraconazole or fluconazole). Prophylaxis of invasive Aspergillus and Candida infections [105,112] |
| Nebulized Amphotericin B | 5–40 mg/day | Intravenous | Binding to ergosterol (Fungal membrane disruptor) | Fungal asthma (Allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization) [113] |
| Liposomal AmB | 3–5 mg/kg/day | Intravenous | Binding to ergosterol (Fungal membrane disruptor) | Acute cavitary Histoplasmosis [108] Penicillium marneffei [114] Severe pulmonary sporotrichosis [110] Second-line: Chronic pulmonary aspergillosis [105] Chronic pulmonary Coccidioidomycosis [107] Paracoccidioidomycosis [109] Blastomycosis [106] |
| Echinocandins | 50–70 mg/day | Intravenous | Inhibition of β(1,3)-D-glucan synthase | Aspergillosis (refractory or intolerance to AmB or itraconazole or voriconazole) [105] Candidiasis [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho Patricio, B.F.; da Silva Lopes Pereira, J.O.; Sarcinelli, M.A.; de Moraes, B.P.T.; Rocha, H.V.A.; Gonçalves-de-Albuquerque, C.F. Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi? Pharmaceutics 2022, 14, 2707. https://doi.org/10.3390/pharmaceutics14122707
de Carvalho Patricio BF, da Silva Lopes Pereira JO, Sarcinelli MA, de Moraes BPT, Rocha HVA, Gonçalves-de-Albuquerque CF. Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi? Pharmaceutics. 2022; 14(12):2707. https://doi.org/10.3390/pharmaceutics14122707
Chicago/Turabian Stylede Carvalho Patricio, Beatriz Ferreira, Juliana Oliveira da Silva Lopes Pereira, Michelle Alvares Sarcinelli, Bianca Portugal Tavares de Moraes, Helvécio Vinicius Antunes Rocha, and Cassiano Felippe Gonçalves-de-Albuquerque. 2022. "Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi?" Pharmaceutics 14, no. 12: 2707. https://doi.org/10.3390/pharmaceutics14122707
APA Stylede Carvalho Patricio, B. F., da Silva Lopes Pereira, J. O., Sarcinelli, M. A., de Moraes, B. P. T., Rocha, H. V. A., & Gonçalves-de-Albuquerque, C. F. (2022). Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi? Pharmaceutics, 14(12), 2707. https://doi.org/10.3390/pharmaceutics14122707









