Self-Assembling Injectable Hydrogel for Controlled Drug Delivery of Antimuscular Atrophy Drug Tilorone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
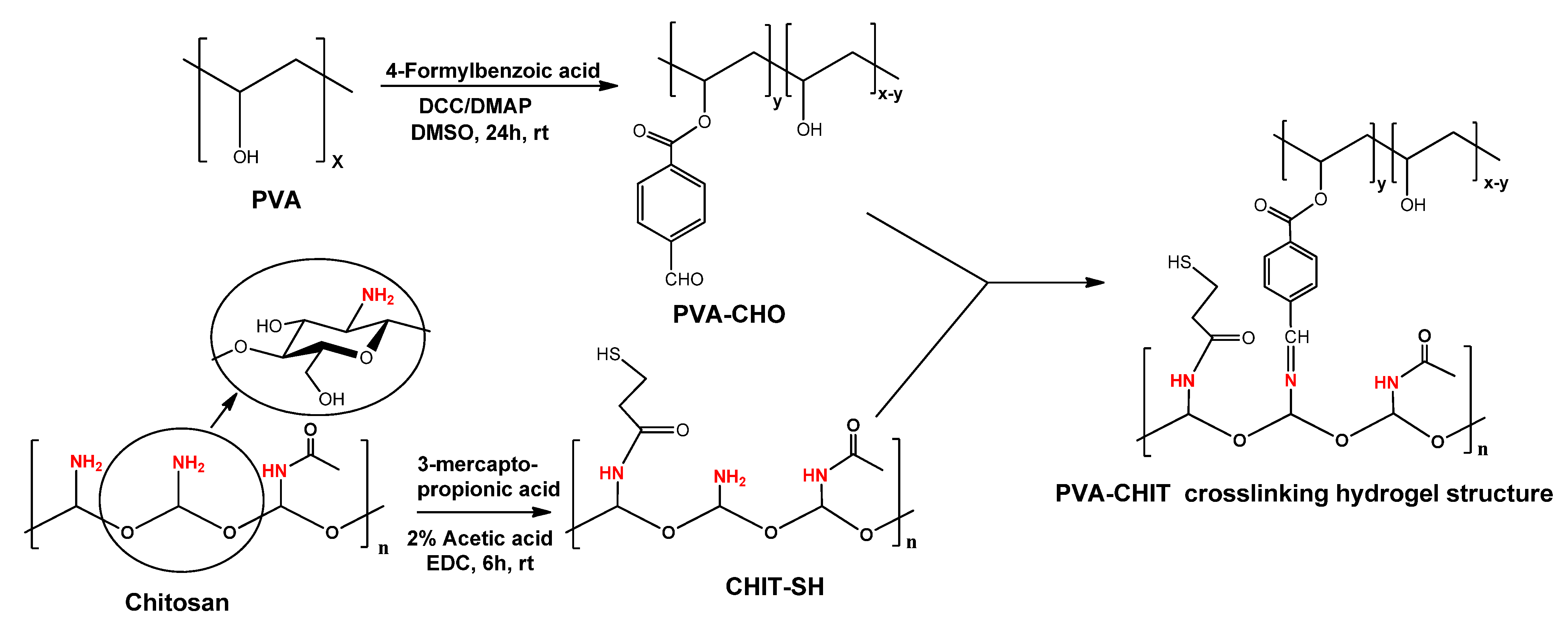
2.2. Synthesis of 4-Formylbenzoate PVA (PVA-CHO)
2.3. Synthesis of 3-Mercaptopropionate Chitosan
2.4. Preparation of PVA-CHO/CHIT-SH Hydrogels
2.5. Methods of Characterization
2.6. Statistical Analysis of the Results
3. Results and Discussion
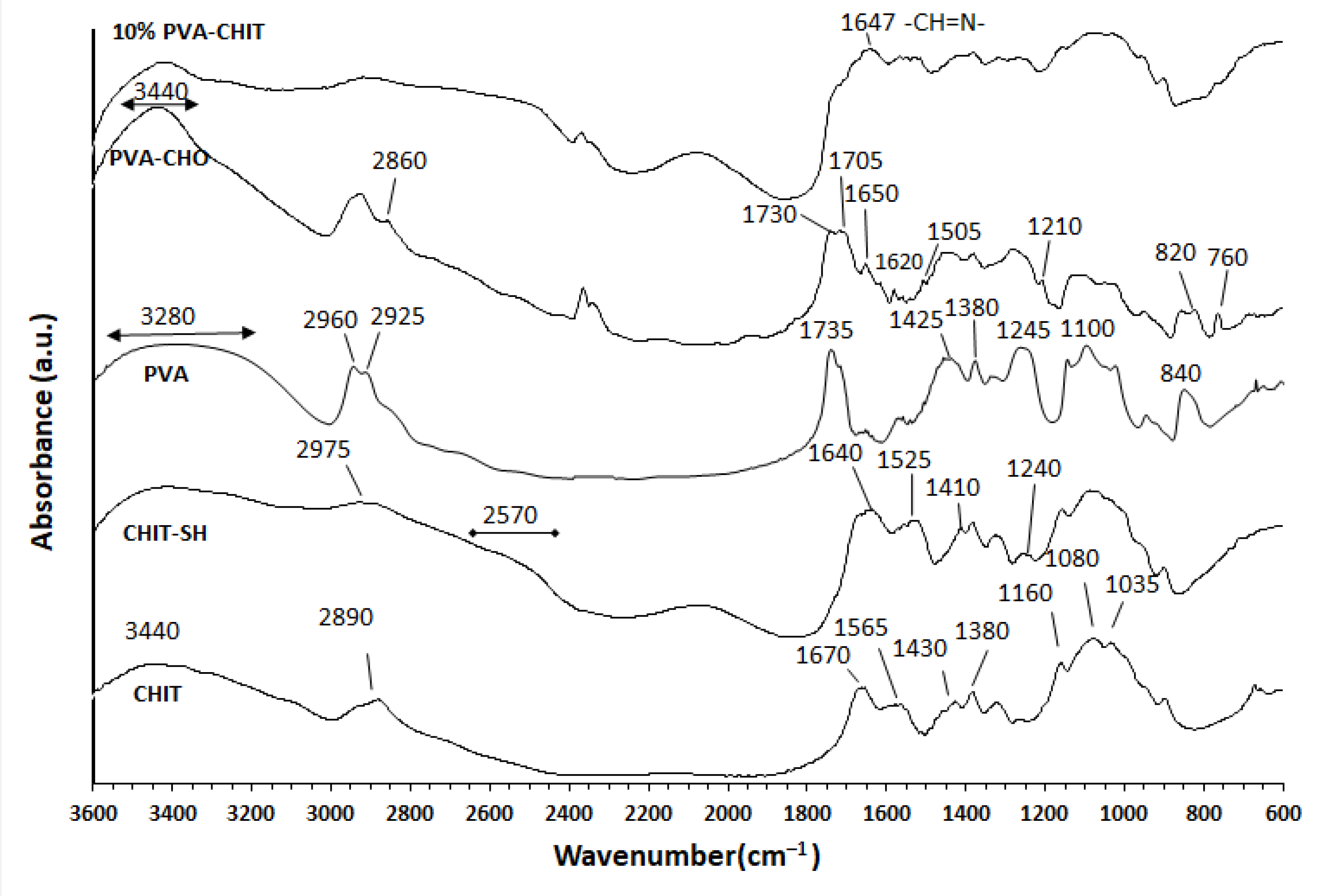
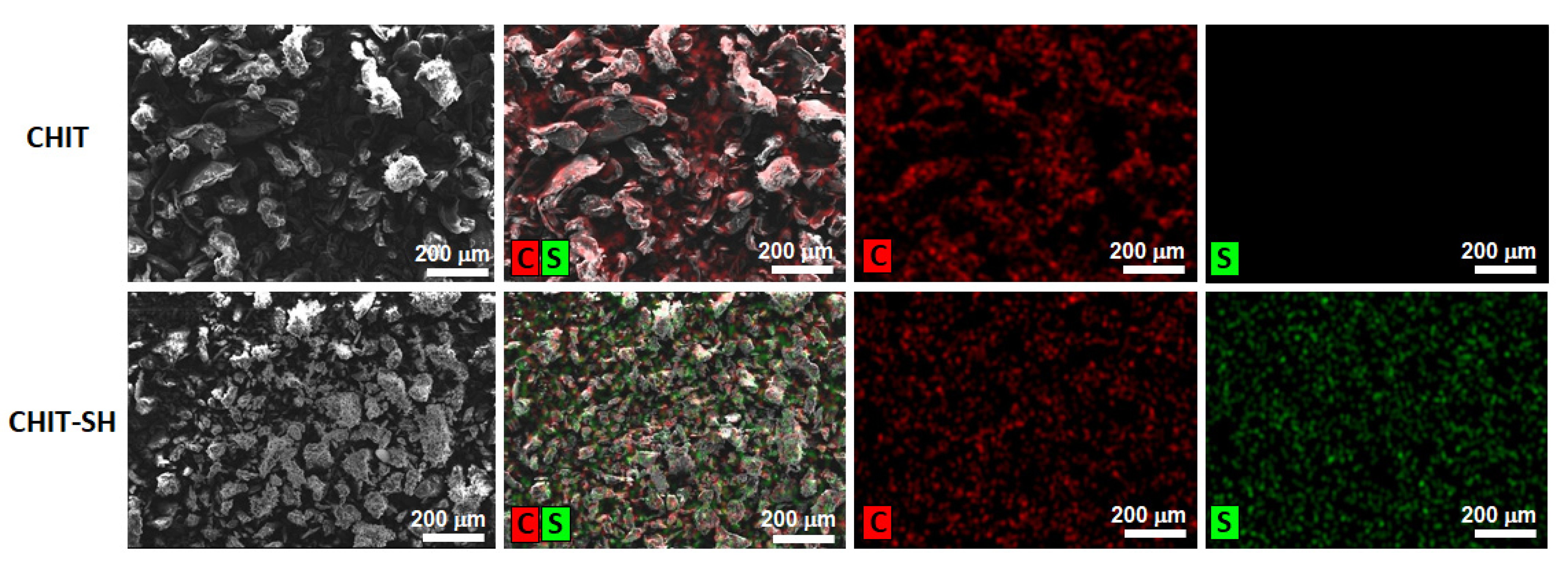
3.1. Structural Characterization of the Modified Polymers and Hydrogel
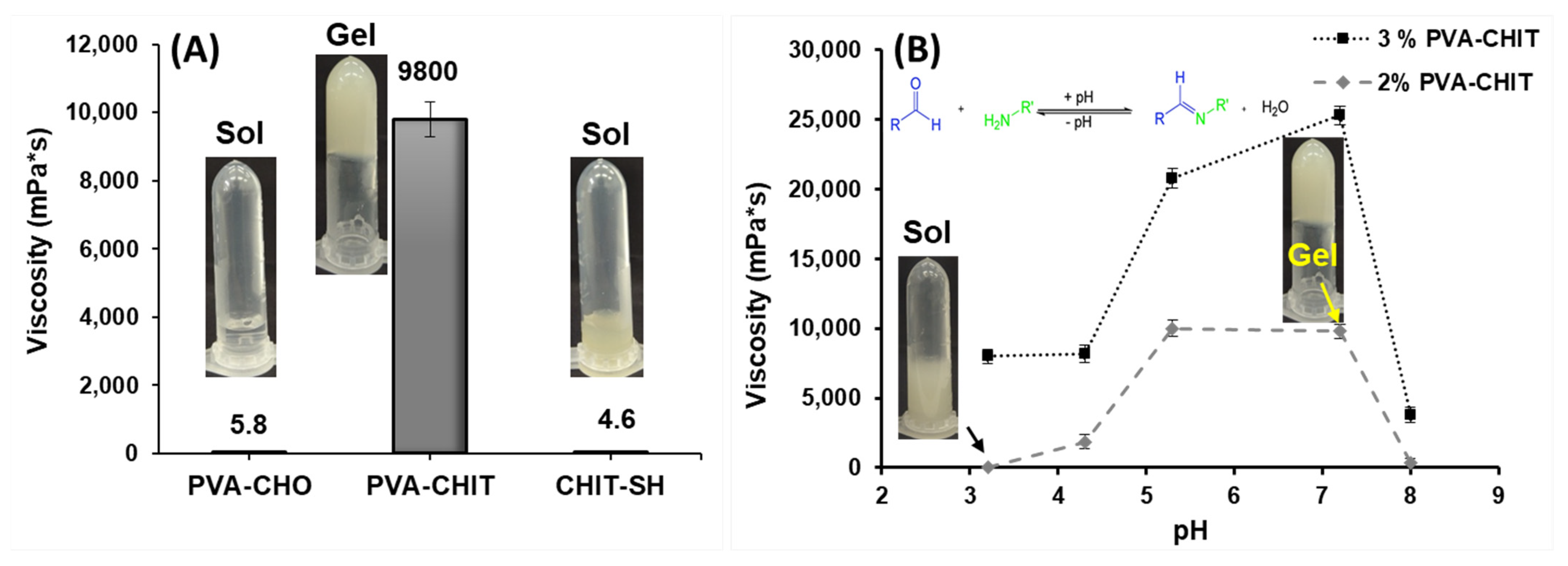
3.2. Physicochemical Characterization and pH-Dependent Sol–Gel Transition of PVA-CHO/CHIT-SH Hydrogel
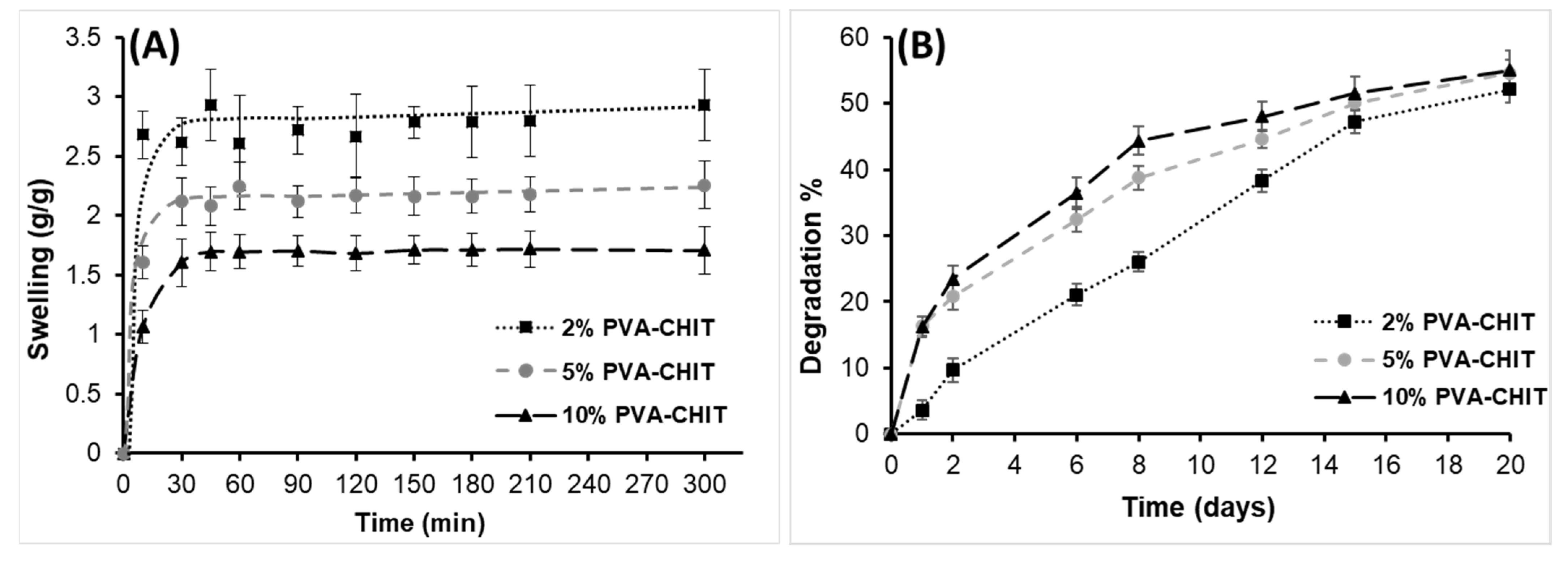
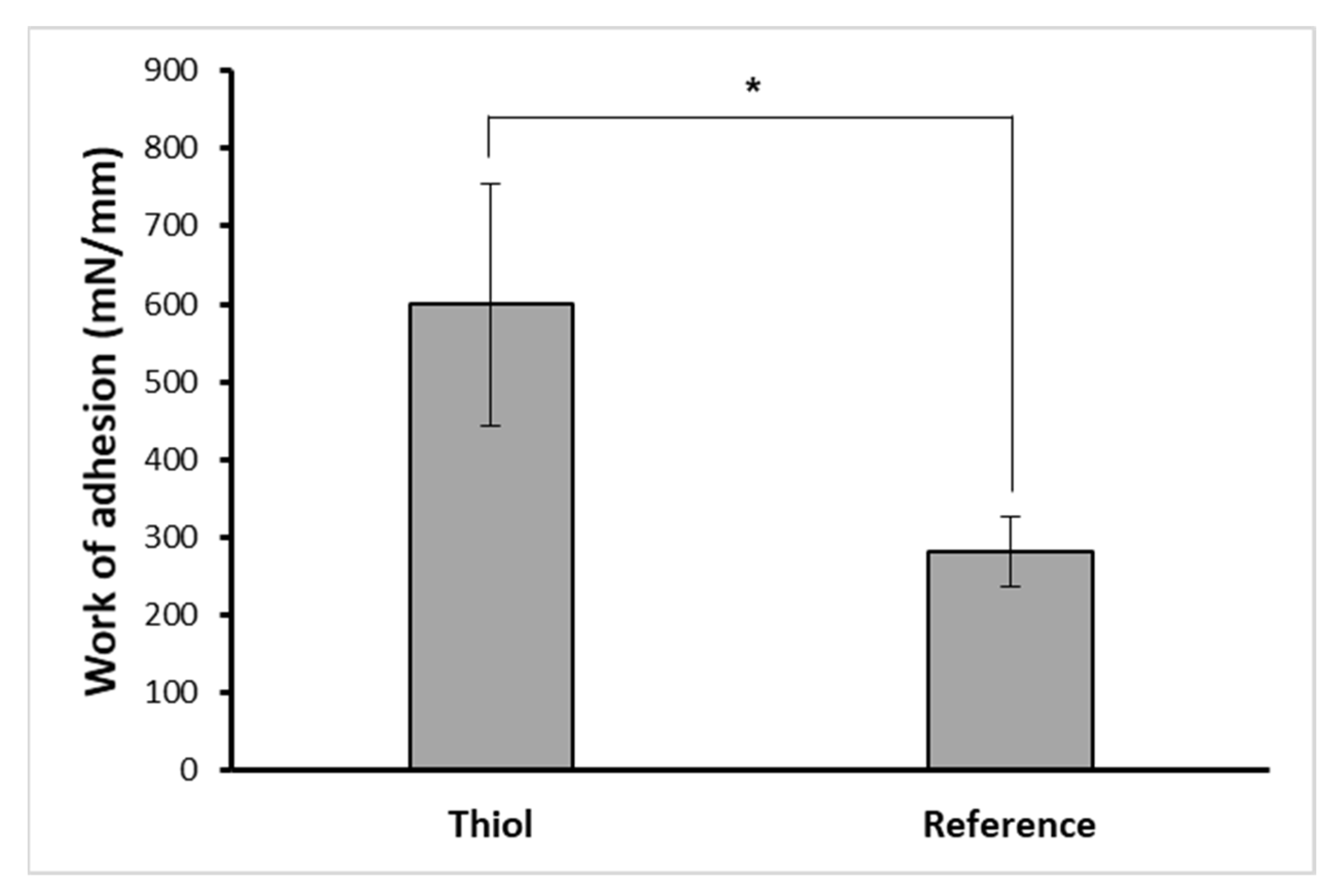
3.3. Mucoadhesive Properties of PVA-CHO/CHIT-SH Hydrogel
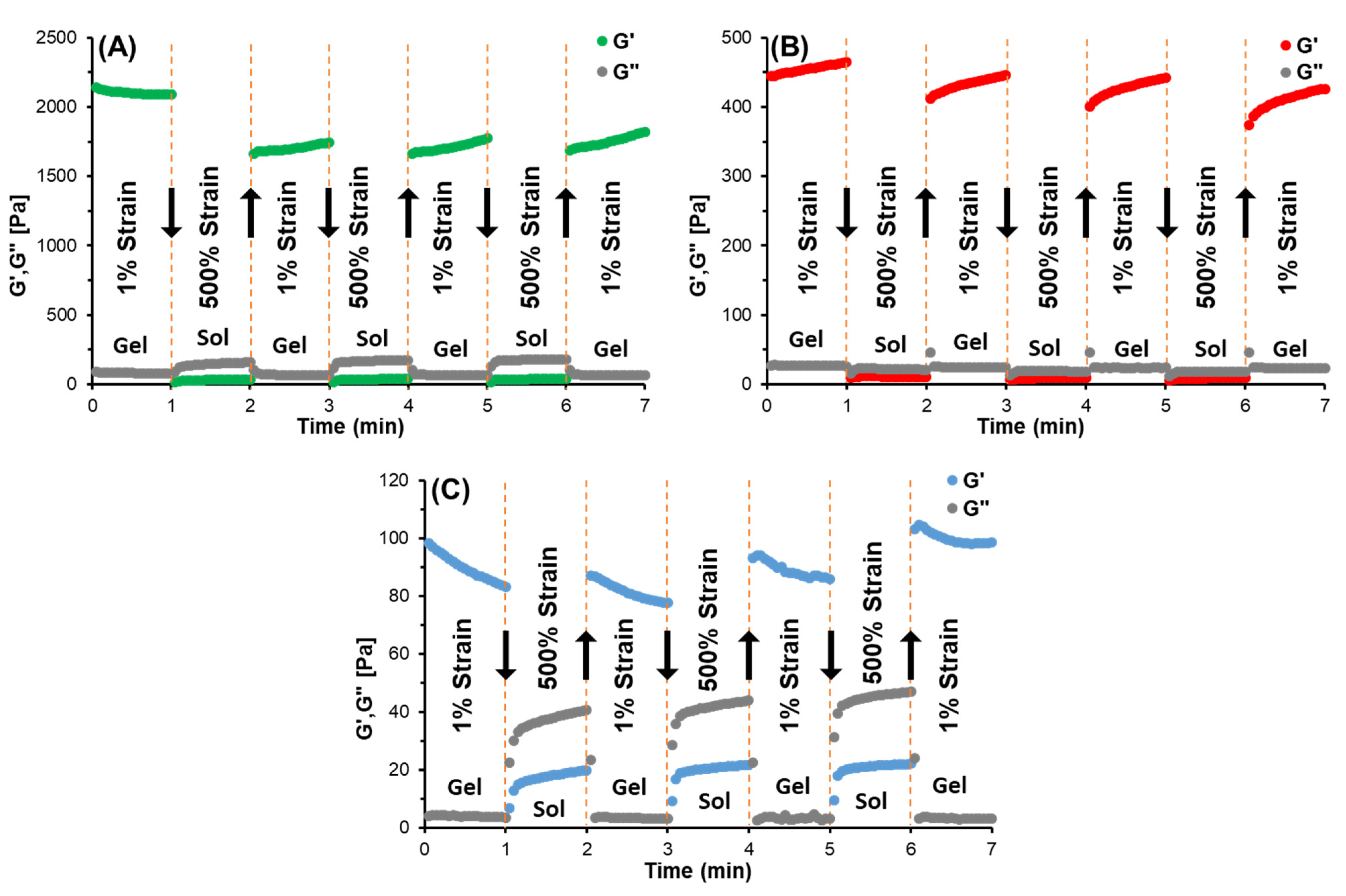
3.4. Self-Assembled and Self-Healing Hydrogel Formation
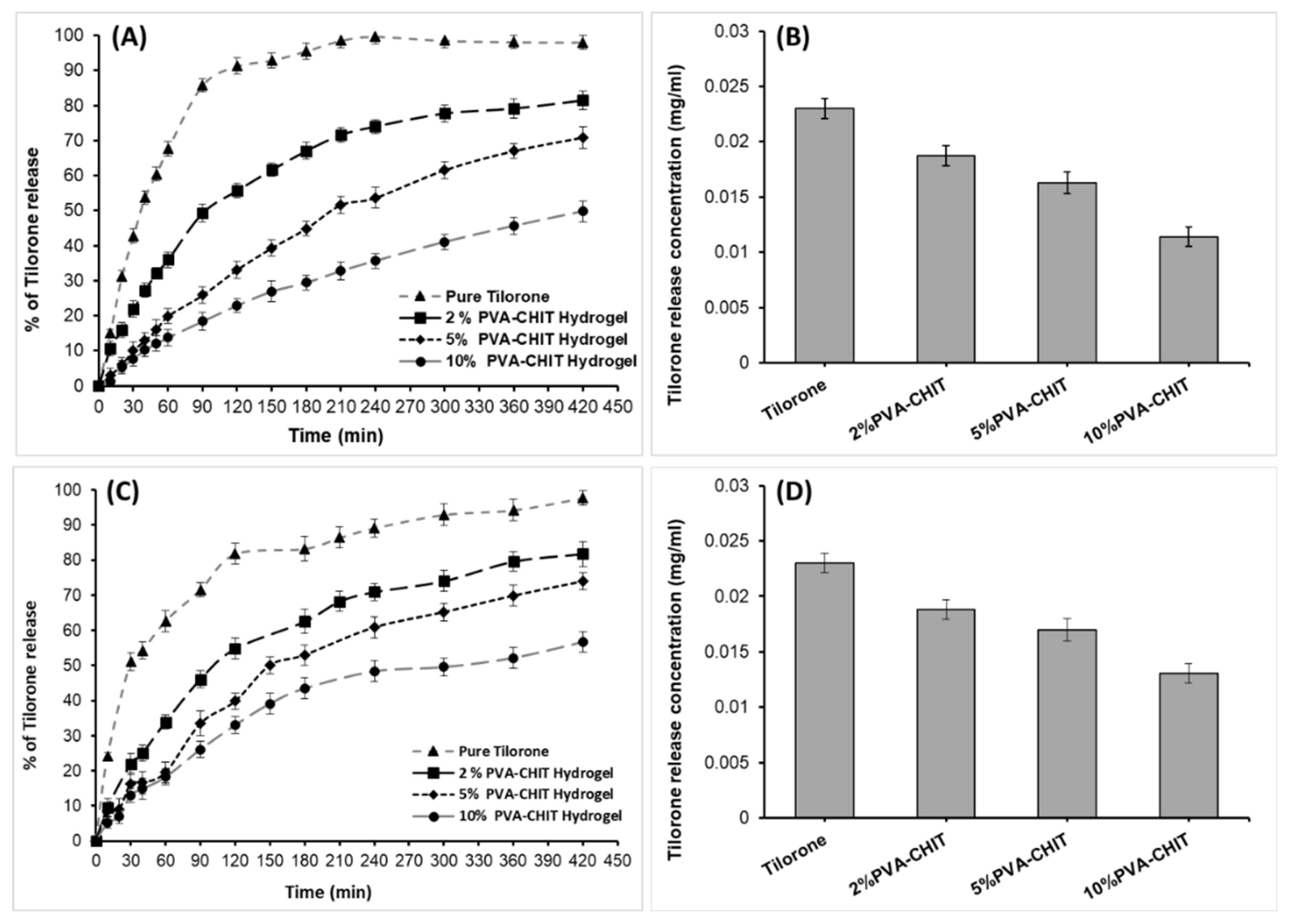
3.5. In Vitro Drug-Release Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Geng, Y.; Yue, B.; Lo, P.-C.; Huang, J.; Jin, H. Injectable Hydrogel as a Unique Platform for Antitumor Therapy Targeting Immunosuppressive Tumor Microenvironment. Front. Immunol. 2022, 12, 832942. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Yu, S.; He, C.; Chen, X. Injectable Hydrogels as Unique Platforms for Local Chemotherapeutics-Based Combination Antitumor Therapy. Macromol. Biosci. 2018, 18, e1800240. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Qin, S.; Zou, H.; Hai, Y.; You, L. Aggregation-induced emission luminogens and tunable multicolor polymer networks modulated by dynamic covalent chemistry. Chin. Chem. Lett. 2022, 33, 3267–3271. [Google Scholar] [CrossRef]
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv.Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef]
- Brandsch, J.; Piringer, O. Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd ed.; Wiley-VCH: Chichester, UK, 2008; p. 15. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Ikinci, G.; Senel, S.; Akincibay, H.; Kas, S.; Ercis, S.; Wilson, C.G.; Hincal, A.A. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int. J. Pharm. 2002, 235, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Patashnik, S.; Rabinovich, L.; Golomb, G. Preparation and evaluation of chitosan microspheres containing bisphosphonates. J. Drug Target. 1997, 4, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Furrer, P.; Mayer, J.M.; Plazonnet, B.; Buri, P.; Gurny, R. Topical use of chitosan in ophthalmology: Tolerance assessment and evaluation of precorneal retention. Int. J. Pharm. 1999, 180, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Suh, C.H.; Park, Y.B.; Lee, S.H.; Yoo, N.C.; Lee, J.D.; Kim, K.H.; Lee, S.K. A phase I/IIa study on intra-articular injection of holmium-166-chitosan complex for the treatment of knee synovitis of rheumatoid arthritis. Eur. J. Nucl. Med. 2001, 28, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, W.; Liu, Y.; Bai, S.; Wang, Q. Thermal melt processing to prepare halogen-free flame retardant poly (vinyl alcohol). Polym. Degrad. Stab. 2014, 109, 261–269. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Wu, J.; Xu, F.; Zuo, Y.; Jansen, J.A. In vitro and in vivo study to the biocompatibility and biodegradation of hydroxyapatite/poly(vinyl alcohol)/gelatin composite. J. Biomed. Mater. Res. A 2008, 85, 418–426. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Sathish, C.I.; Dhawale, D.S.; Pawar, S.H. Polyvinyl alcohol functionalized cobalt ferrite nanoparticles for biomedical applications. Appl. Surf. Sci. 2013, 264, 598–604. [Google Scholar] [CrossRef]
- Cavalieri, F.; Hamassi, A.E.; Chiessi, E.; Paradossi, G.; Villa, R.; Zaffaroni, N. Ligands tethering to biocompatible ultrasound active polymeric microbubbles surface. Macromol. Symp. 2006, 234, 94–101. [Google Scholar] [CrossRef]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Comparison of body distribution of poly(vinyl alcohol) with other water-soluble polymers after intravenous administration. J. Pharm. Pharmacol. 1995, 47, 479–486. [Google Scholar] [CrossRef]
- Kaneo, Y.; Hashihama, S.; Kakinoki, A.; Tanaka, T.; Nakano, T.; Ikeda, Y. Pharmacokinetics and biodisposition of poly(vinyl alcohol) in rats and mice. Drug Metab. Pharmacokinet. 2005, 20, 435–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerroni, B.; Cicconi, R.; Oddo, L.; Scimeca, M.; Bonfiglio, R.; Bernardini, R.; Palmieri, G.; Domenici, F.; Bonanno, E.; Mattei, M.; et al. In vivo biological fate of poly(vinylalcohol) microbubbles in mice. Heliyon 2018, 4, e00770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takács, T.; Abdelghafour, M.M.; Lamch, Ł.; Szenti, I.; Sebők, D.; Janovák, L.; Kukovecz, Á. Facile modification of hydroxyl group containing macromolecules provides autonomously self-healing polymers through the formation of dynamic Schiff base linkages. Eur. Polym. J. 2022, 168, 111086. [Google Scholar] [CrossRef]
- Rovó, L.; Madani, S.; Sztanó, B.; Majoros, V.; Smehák, G.; Szakács, L.; Jóri, J. A new thread guide instrument for endoscopic arytenoid lateropexy. Laryngoscope 2010, 120, 2002–2007. [Google Scholar] [CrossRef]
- Madani, S.; Bach, Á.; Matievics, V.; Erdélyi, E.; Sztanó, B.; Szegesdi, I.; Castellanos, P.F.; Rovó, L. A new solution for neonatal bilateral vocal cord paralysis: Endoscopic arytenoid abduction lateropexy. Laryngoscope 2017, 127, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Matievics, V.; Bach, A.; Sztano, B.; Bere, Z.; Tobias, Z.; Castellanos, P.F.; Mueller, A.H.; Rovo, L. Functional outcomes of endoscopic arytenoid abduction lateropexy for unilateral vocal cord paralysis with dyspnea. Eur. Arch. Otorhinolaryngol. 2017, 274, 3703–3710. [Google Scholar] [CrossRef]
- Ohno, S.; Hirano, S.; Yasumoto, A.; Ikeda, H.; Takebayashi, S.; Miura, M. Outcome of regenerative therapy for age-related vocal fold atrophy with basic fibroblast growth factor. Laryngoscope 2016, 126, 1844–1848. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Cheng, L.Y. Adipose-derived mesenchymal stem cells accelerate nerve regeneration and functional recovery in a rat model of recurrent laryngeal nerve injury. Neural. Regen. Res. 2017, 12, 1544–1550. [Google Scholar] [CrossRef]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP signaling controls muscle mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef]
- Winbanks, C.E.; Chen, J.L.; Qian, H.; Liu, Y.; Bernardo, B.C.; Beyer, C.; Watt, K.I.; Thomson, R.E.; Connor, T.; Turner, B.J.; et al. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J. Cell Biol. 2013, 203, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Leppäranta, O.; Tikkanen, J.M.; Bespalov, M.M.; Koli, K.; Myllärniemi, M. Bone morphogenetic protein-inducer tilorone identified by high-throughput screening is antifibrotic in vivo. Am. J. Respir. Cell Mol. Biol. 2013, 48, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Horlock, D.; Kaye, D.M.; Winbanks, C.E.; Gao, X.-M.; Kiriazis, H.; Donner, D.G.; Gregorevic, P.; McMullen, J.R.; Bernardo, B.C. Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart. Pharmaceuticals 2021, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Puhl, A.C.; Fritch, E.J.; Lane, T.R.; Tse, L.V.; Yount, B.L.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Tavella, T.A.; Maranhão Costa, F.T.; Weston, S.; et al. Repurposing the Ebola and Marburg Virus Inhibitors Tilorone, Quinacrine, and Pyronaridine: In Vitro Activity against SARS-CoV-2 and Potential Mechanisms. ACS Omega 2021, 6, 7454–7468. [Google Scholar] [CrossRef]
- Willson, J. The BMP pathway could curb cachexia. Nat. Rev. Cancer. 2021, 21, 612. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Sartori, R.; Baraldo, M.; Nogara, L.; Balmaceda, V.; Dumitras, G.A.; Ciciliot, S.; Scalabrin, M.; Nolte, H.; Blaauw, B. Activation of Akt-mTORC1 signalling reverts cancer-dependent muscle wasting. J. Cachexia Sarcopenia Muscle 2021, 13, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. Edn. Eng. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Borsagli, M.; Carvalho, I.C.; Mansur, H.S. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications. Int. J. Biol. Macromol. 2018, 114, 270–282. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Abdelghafour, M.M.; Deák, Á.; Szabó, D.; Dékány, I.; Rovó, L.; Janovák, L. Use of self-assembled colloidal prodrug nanoparticles for controlled drug delivery of anticancer, antifibrotic and antibacterial mitomycin. Int. J. Mol. Sci. 2022, 23, 6807. [Google Scholar] [CrossRef]
- Takács, T.; Abdelghafour, M.M.; Deák, Á.; Szabó, D.; Sebők, D.; Dékány, I.; Rovó, L.; Kukovecz, Á.; Janovák, L. Surface wetting driven release of antifibrotic Mitomycin-C drug from modified biopolymer thin films. Eur. Polym. J. 2020, 139, 109995. [Google Scholar] [CrossRef]
- Fornaro, T.; Burini, D.; Biczysko, M.; Barone, V. Hydrogen-bonding effects on infrared spectra from anharmonic computations: Uracil–water complexes and uracil dimers. J. Phys. Chem. A 2015, 119, 4224–4236. [Google Scholar] [CrossRef]
- Qi, J.-L.; Xu, W.; Zheng, Y.-Q. Synthesis, crystal structure and magnetic properties of a copper(II) p-formylbenzoate complex. Z. Für Nat. B 2012, 67, 1185–1190. [Google Scholar] [CrossRef]
- Kawamura, K.; Yasuda, T.; Hatanaka, T.; Hamahiga, K.; Matsuda, N.; Ueshima, M.; Nakai, K. Oxidation of aliphatic alcohols and benzyl alcohol by H2O2 under the hydrothermal conditions in the presence of solid-state catalysts using batch and flow reactors. Chem. Eng. J. 2016, 285, 49–56. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.; Saha, B.; Qadir, M.R.; Gafur, M.A.; Masum, S. Chromium (VI) ions removal from tannery effluent using chitosan-microcrystalline cellulose composite as adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Queiroz, M.; Melo, K.; Sabry, D.; Sassaki, G.; Rocha, H. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 41–158. [Google Scholar] [CrossRef]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol. Vis. 2012, 18, 1973–1982. [Google Scholar]
- Esquivel, R.; Juárez, J.; Almada, M.; Ibarra, J.; Valdez, M.A. Synthesis and characterization of new thiolated chitosan nanoparticles obtained by ionic gelation method. Int. J. Polym. Sci. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Vollhardt, K.P.C.; Schore, N.E. Organic Chemistry: Structure and Function, 5th ed.; W.H. Freeman: New York, NY, USA, 2005; pp. 779–784. [Google Scholar]
- Cavalieri, F.; El Hamassi, A.; Chiessi, E.; Paradossi, G. Stable polymeric microballoons as multifunctional device for biomedical uses: Synthesis and characterization. Langmuir 2005, 21, 8758–8764. [Google Scholar] [CrossRef]
- Rajpal, G.; Arvan, P. Disulfide bond formation. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1721–1729. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, C.; Wu, Z.; Teng, D.; Zhang, X.; Wang, Z.; Li, C. Chitosan-NAC nanoparticles as a vehicle for nasal absorption enhancement of insulin. J. Biomed. Mater. Res. B. Appl. Biomater. 2009, 88, 150–161. [Google Scholar] [CrossRef]
- Ways, T.M.; Lau, W.; Khutoryanskiy, V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, A.R.; Khodadadi, A. Mechanically Robust 3D Nanostructure Chitosan-Based Hydrogels with Autonomic Self-Healing Properties. ACS Appl. Mater. Interfaces 2016, 8, 27254–27263. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Soman, D.; Payanam, U.; Laurent, A.; Labarre, D.; Jayakrishnan, A. A novel injectable tissue adhesive based on oxidized dextran and chitosan. Acta Biomater. 2017, 53, 343–354. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Chen, Y.; Zhao, Y.; Zhang, Q.; Zheng, X.; Chen, L.; Zhang, L. Strong and rapidly self-healing hydrogels: Potential hemostatic materials. Adv. Healthcare Mater. 2016, 5, 2813–2822. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zheng, P.; Fei, G.; Wei, W.; Zhang, H.; Zhang, X.; Feng, X.; Liu, W. A mineralized high strength and tough hydrogel for skull bone regeneration. Adv. Funct. Mater. 2016, 27, 1604327. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Wang, H.; van der Stok, J.; Weinans, H.; Leeuwenburgh, S.C.; Öner, F.C.; Dhert, W.J.; Alblas, J. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS ONE 2013, 8, e72610. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Dynamic and Self-Healable Chitosan/Hyaluronic Acid-Based In Situ-Forming Hydrogels. Gels 2022, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Lee, D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control Release 2014, 193, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhao, L.; Liu, F.; Cheng, J.; Gu, J.; Shan-Dan, L.C.; Qu, X.; Yang, Z. Dually responsive injectable hydrogel prepared by in situ cross-linking of glycol chitosan and benzaldehyde-capped PEO-PPO-PEO. Biomacromolecules 2010, 11, 1043–1051. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomaterialia 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Qu, X.; Yang, Z. Benzoic-imine-based physiological pH-responsive materials for biomedical applications. Chem. Asian J. 2016, 11, 2633–2641. [Google Scholar] [CrossRef]
- Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and injectable hydrogel for drug delivery in soft tissues. Biomacromolecules 2019, 20, 149–163. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Schindeler, A.; Dehghani, F. Sustained protein release from a core-shell drug carrier system comprised of mesoporous nanoparticles and an injectable hydrogel. Macromol. Biosci. 2018, 18, e1800201. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 171. [Google Scholar] [CrossRef]
- Debnath, T.; Ghosh, S.; Potlapuvu, U.S.; Kona, L.; Kamaraju, S.R.; Sarkar, S.; Gaddam, S.; Chelluri, L.K. Proliferation and Differentiation Potential of Human Adipose-Derived Stem Cells Grown on Chitosan Hydrogel. PLoS ONE 2015, 10, e0120803. [Google Scholar] [CrossRef]
- Numata, K. Silk hydrogels for tissue engineering and dual-drug delivery. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 503–518. [Google Scholar] [CrossRef]


| Zero-Order Model | First-Order Model | Higuchi Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | r2 | k (h–1) | r2 | k (h–1) | r2 | k (h−1/2) | r2 | k (h−1/3) | n | r2 | k (h–n) |
| Pure Tilorone | 0.6602 | 12.496 | 0.8142 | 0.653 | 0.7841 | 35.375 | 0.5194 | 0.218 | 0.546 | 0.5745 | 41.029 |
| 2% PVA-CHIT | 0.8273 | 11.266 | 0.9403 | 0.252 | 0.962 | 34.553 | 0.5293 | 0.373 | 0.649 | 0.3946 | 25.834 |
| 5% PVA-CHIT | 0.9513 | 10.599 | 0.9946 | 0.181 | 0.9956 | 32.519 | 0.6979 | 0.428 | 0.906 | 0.6579 | 13.810 |
| 10% PVA-CHIT | 0.9643 | 6.911 | 0.9901 | 0.096 | 0.9997 | 21.662 | 0.8091 | 0.307 | 0.909 | 0.702 | 9.687 |
| Zero-Order Model | First-Order Model | Higuchi Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | r2 | k (h−1) | r2 | k (h−1) | r2 | k (h−1/2) | r2 | k (h−1/3) | n | r2 | k (h−n) |
| Pure Tilorone | 0.7444 | 8.3884 | 0.9717 | 0.442 | 0.8856 | 28.687 | 0.6367 | 0.1739 | 0.333 | 0.9153 | 57.042 |
| 2% PVA-CHIT | 0.855 | 11.029 | 0.9664 | 0.243 | 0.982 | 35.049 | 0.5476 | 0.3867 | 0.7018 | 0.3991 | 22.563 |
| 5% PVA-CHIT | 0.9108 | 10.55 | 0.9775 | 0.191 | 0.9794 | 33.389 | 0.6295 | 0.397 | 0.7272 | 0.4873 | 18.150 |
| 10% PVA-CHIT | 0.8861 | 7.5437 | 0.934 | 0.116 | 0.972 | 24.429 | 0.7782 | 0.2791 | 0.739 | 0.5351 | 14.467 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghafour, M.M.; Deák, Á.; Kiss, T.; Budai-Szűcs, M.; Katona, G.; Ambrus, R.; Lőrinczi, B.; Keller-Pintér, A.; Szatmári, I.; Szabó, D.; et al. Self-Assembling Injectable Hydrogel for Controlled Drug Delivery of Antimuscular Atrophy Drug Tilorone. Pharmaceutics 2022, 14, 2723. https://doi.org/10.3390/pharmaceutics14122723
Abdelghafour MM, Deák Á, Kiss T, Budai-Szűcs M, Katona G, Ambrus R, Lőrinczi B, Keller-Pintér A, Szatmári I, Szabó D, et al. Self-Assembling Injectable Hydrogel for Controlled Drug Delivery of Antimuscular Atrophy Drug Tilorone. Pharmaceutics. 2022; 14(12):2723. https://doi.org/10.3390/pharmaceutics14122723
Chicago/Turabian StyleAbdelghafour, Mohamed M., Ágota Deák, Tamás Kiss, Mária Budai-Szűcs, Gábor Katona, Rita Ambrus, Bálint Lőrinczi, Anikó Keller-Pintér, István Szatmári, Diána Szabó, and et al. 2022. "Self-Assembling Injectable Hydrogel for Controlled Drug Delivery of Antimuscular Atrophy Drug Tilorone" Pharmaceutics 14, no. 12: 2723. https://doi.org/10.3390/pharmaceutics14122723
APA StyleAbdelghafour, M. M., Deák, Á., Kiss, T., Budai-Szűcs, M., Katona, G., Ambrus, R., Lőrinczi, B., Keller-Pintér, A., Szatmári, I., Szabó, D., Rovó, L., & Janovák, L. (2022). Self-Assembling Injectable Hydrogel for Controlled Drug Delivery of Antimuscular Atrophy Drug Tilorone. Pharmaceutics, 14(12), 2723. https://doi.org/10.3390/pharmaceutics14122723











