Exploring Freeze-Drying as Strategy to Enhance Viability of Faecalibacterium duncaniae DSM 17677 upon Aerobic Storage and Gastrointestinal Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Aerobic Environments Tolerance of Free Cells
2.3. Acid and Bile Susceptibility of Free Cells
2.4. Formulation Procedure
- 400 µL of a solution containing inulin [5% (m/v), Orafti Beneo, Mannheim, Germany], trehalose dihydrate [5% (m/v), Sigma], and 0.2% (m/v) cysteine prepared in PBS (ITCR);
- 400 µL of a solution containing inulin [5% (m/v)], sucrose [2.5% (m/v)], trehalose dihydrate [2.5% (m/v)], and 0.2% (m/v) cysteine prepared in PBS (ISTCR);
- 400 µL of a solution containing inulin [5% (m/v)], sucrose [5% (m/v), Sigma], and 0.2% (m/v) cysteine prepared in PBS (ISCR);
- 400 µL of a solution containing inulin [10% (m/v)] with 0.2% (m/v) cysteine prepared in PBS (ICR).
2.5. Viability and Stability of Freeze-Dried Formulations during Aerobic Storage
2.6. Viability of a Selected Freeze-Dried Formulation after Exposure to Acidic pH and Bile
2.7. Statistical Analysis
3. Results and Discussion
3.1. Oxygen Sensitivity of F. duncaniae DSM 17677 Free Cells
3.2. Acid and Bile Sensitivity of F. duncaniae DSM 17677 Free Cells
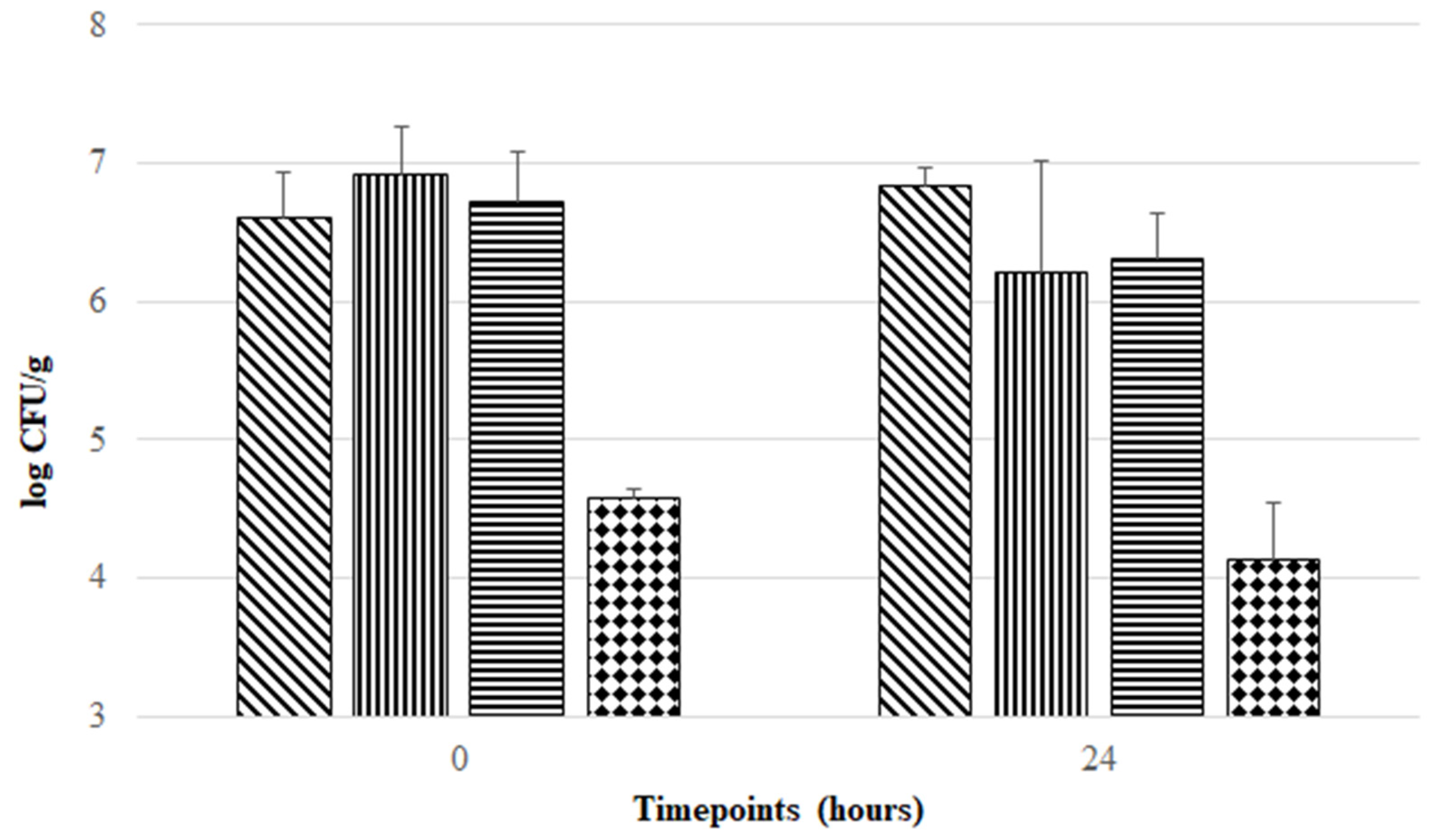
3.3. Aerobic Exposure of F. duncaniae Freeze-Dried Formulations at Room Temperature
3.4. Exposure of F. duncaniae Freeze-Dried in Inulin, Sucrose, Cysteine and Riboflavin Matrix to Acidic pH Values and Bile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.-W.; Singh, A.V.; Sitti, M. Bioengineered and Biohybrid Bacteria-Based Systems for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, J. Decorated Bacteria and the Application in Drug Delivery. Adv. Drug Deliv. Rev. 2022, 188, 114443. [Google Scholar] [CrossRef] [PubMed]
- Voss, G.B.; Machado, D.; Barbosa, J.C.; Campos, D.A.; Gomes, A.M.; Pintado, M. Interplay between Probiotics and Prebiotics for Human Nutrition and Health. In Probiotics for Human Nutrition in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–254. [Google Scholar]
- Almeida, D.; Machado, D.; Andrade, J.C.; Mendo, S.; Gomes, A.M.; Freitas, A.C. Evolving Trends in Next-Generation Probiotics: A 5W1H Perspective. Crit. Rev. Food Sci. Nutr. 2020, 60, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth Requirements and Fermentation Products of Fusobacterium Prausnitzii, and a Proposal to Reclassify It as Faecalibacterium Prausnitzii Gen. Nov., Comb. Nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, M.; Sakurai, N.; Tanno, H.; Iino, T.; Ohkuma, M.; Endo, A. Genome-Based, Phenotypic and Chemotaxonomic Classification of Faecalibacterium Strains: Proposal of Three Novel Species Faecalibacterium duncaniae sp. nov., Faecalibacterium hattorii sp. nov. and Faecalibacterium gallinarum sp. nov. Int. J. Syst. Evol. Microbiol. 2022, 72, 005379. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Machado, D.; Barbosa, J.C.; Domingos, M.; Almeida, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Revealing Antimicrobial Resistance Profile of the Novel Probiotic Candidate Faecalibacterium prausnitzii DSM 17677. Int. J. Food Microbiol. 2022, 363, 109501. [Google Scholar] [CrossRef]
- Andrade, J.C.; Almeida, D.; Domingos, M.; Seabra, C.L.; Machado, D.; Freitas, A.C.; Gomes, A.M. Commensal Obligate Anaerobic Bacteria and Health: Production, Storage, and Delivery Strategies. Front. Bioeng. Biotechnol. 2020, 8, 550. [Google Scholar] [CrossRef]
- Khan, M.T.; van Dijl, J.M.; Harmsen, H.J.M. Antioxidants Keep the Potentially Probiotic but Highly Oxygen-Sensitive Human Gut Bacterium Faecalibacterium prausnitzii Alive at Ambient Air. PLoS ONE 2014, 9, e96097. [Google Scholar] [CrossRef]
- Almeida, D.; Machado, D.; Sousa, S.; Seabra, C.L.; Barbosa, J.C.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Effect of Emulsification/Internal Gelation-Based Microencapsulation on the Viability of Akkermansia muciniphila upon Prolonged Storage and Simulated Gastrointestinal Passage. Food Hydrocoll. Health 2022, 2, 100084. [Google Scholar] [CrossRef]
- Machado, D.; Almeida, D.; Seabra, C.L.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Nanoprobiotics: When Technology Meets Gut Health. In Functional Bionanomaterials; Springer: Cham, Switzerland, 2020; pp. 389–425. [Google Scholar]
- Barbosa, J.; Almeida, D.; Machado, D.; Sousa, S.; Freitas, A.; Andrade, J.; Gomes, A. Spray-Drying Encapsulation of the Live Biotherapeutic Candidate Akkermansia muciniphila DSM 22959 to Survive Aerobic Storage. Pharmaceuticals 2022, 15, 628. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, A.; Liang, D.Y.; Guo, Y.; Pratap-Singh, A. Effect of the Formulation on Mucoadhesive Spray-Dried Microparticles Containing Iron for Food Fortification. Food Hydrocoll. 2023, 134, 107906. [Google Scholar] [CrossRef]
- Baldelli, A.; Boraey, M.A.; Oguzlu, H.; Cidem, A.; Rodriguez, A.P.; Ong, H.X.; Jiang, F.; Bacca, M.; Thamboo, A.; Traini, D.; et al. Engineered Nasal Dry Powder for the Encapsulation of Bioactive Compounds. Drug Discov. Today 2022, 27, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of Spray Drying, Freeze Drying and Convective Hot Air Drying for the Production of a Probiotic Orange Powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Torp, A.M.; Bahl, M.I.; Boisen, A.; Licht, T.R. Optimizing Oral Delivery of next Generation Probiotics. Trends Food Sci. Technol. 2022, 119, 101–109. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Kryeziu, T.L.; Nebija, D.; Salar-Behzadi, S.; Viernstein, H.; Mueller, M. Prebiotics as Excipients for Enhancement of Stability and Functionality of Bifidobacterium longum ssp. infantis with Potential Application as Symbiotics in Food and Pharmaceuticals. Pharmazie 2019, 74, 326–333. [Google Scholar] [CrossRef]
- Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ). Faecalibacterium Duncaniae 17677. Available online: https://www.dsmz.de/collection/catalogue/details/culture/DSM-17677 (accessed on 13 April 2021).
- Maier, E.; Anderson, R.; Roy, N. Live Faecalibacterium prausnitzii Does Not Enhance Epithelial Barrier Integrity in an Apical Anaerobic Co-Culture Model of the Large Intestine. Nutrients 2017, 9, 1349. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Somerville, G.A.; Proctor, R.A. Cultivation Conditions and the Diffusion of Oxygen into Culture Media: The Rationale for the Flask-to-Medium Ratio in Microbiology. BMC Microbiol. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of PH in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Khan, T.M.; Duncan, S.H.; Harmsen, H.J.M.; Garcia-Gil, L.J.; Flint, H.J. Cultured Representatives of Two Major Phylogroups of Human Colonic Faecalibacterium prausnitzii Can Utilize Pectin, Uronic Acids, and Host-Derived Substrates for Growth. Appl. Environ. Microbiol. 2012, 78, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foditsch, C.; Santos, T.M.A.; Teixeira, A.G.V.; Pereira, R.V.V.; Dias, J.M.; Gaeta, N.; Bicalho, R.C. Isolation and Characterization of Faecalibacterium prausnitzii from Calves and Piglets. PLoS ONE 2014, 9, e116465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Faecalibacterium prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. prausnitzii as a next-Generation Probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bircher, L.; Geirnaert, A.; Hammes, F.; Lacroix, C.; Schwab, C. Effect of Cryopreservation and Lyophilization on Viability and Growth of Strict Anaerobic Human Gut Microbes. Microb. Biotechnol. 2018, 11, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Marcial-Coba, M.S.; Cieplak, T.; Cahú, T.B.; Blennow, A.; Knøchel, S.; Nielsen, D.S. Viability of Microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during Freeze-Drying, Storage and in Vitro Simulated Upper Gastrointestinal Tract Passage. Food Funct. 2018, 9, 5868–5879. [Google Scholar] [CrossRef]
- Khan, M.T.; Duncan, S.H.; Stams, A.J.M.; van Dijl, J.M.; Flint, H.J.; Harmsen, H.J.M. The Gut Anaerobe Faecalibacterium prausnitzii Uses an Extracellular Electron Shuttle to Grow at Oxic-Anoxic Interphases. ISME J. 2012, 6, 1578–1585. [Google Scholar] [CrossRef]
- Marinova, V.Y.; Rasheva, I.K.; Kizheva, Y.K.; Dermenzhieva, Y.D.; Hristova, P.K. Microbiological Quality of Probiotic Dietary Supplements. Biotechnol. Biotechnol. Equip. 2019, 33, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Muangsin, N.; Shah, A.A.; Prapasarakul, N. The Efficacy of Three Double-Microencapsulation Methods for Preservation of Probiotic Bacteria. Sci. Rep. 2021, 11, 13753. [Google Scholar] [CrossRef]
- Raise, A.; Dupont, S.; Iaconelli, C.; Caliri, C.; Charriau, A.; Gervais, P.; Chambin, O.; Beney, L. Comparison of Two Encapsulation Processes to Protect the Commensal Gut Probiotic Bacterium Faecalibacterium prausnitzii from the Digestive Tract. J. Drug Deliv. Sci. Technol. 2020, 56, 101608. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, M.; You, X.; Sela, D.A.; Xiao, H.; McClements, D.J. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll. 2021, 116, 106634. [Google Scholar] [CrossRef]

| Oxygen Exposure Time (min) | log CFU/mL ± SD | |
|---|---|---|
| Exposure of Inoculated Plates | Exposure of Bacterial Suspensions | |
| 0 | 7.29 ± 0.25 | 7.29 ± 0.25 |
| 1 | <LOD 1,* | 7.52 ± 0.03 |
| 2 | <LOD 1,* | 7.45 ± 0.20 |
| 3 | <LOD 1,* | 7.23 ± 0.15 |
| 5 | <LOD 1,* | 7.40 ± 0.13 |
| Exposure Time (h) | log CFU/mL ± SD | ||
|---|---|---|---|
| Growth Control | pH Values | ||
| 3 | 5 | ||
| 0 | 7.35 ± 0.21 1 | 7.35 ± 0.21 | 7.35 ± 0.21 |
| 1 | 7.43 ± 0.13 | <LOD 2,* | 7.36 ± 0.15 |
| 2 | 7.40 ± 0.24 | <LOD 2,* | 7.17 ± 0.19 |
| Exposure | log CFU/g ± SD | |
|---|---|---|
| pH | Control before exposure (T = 0 h) | 6.96 ± 1.02 |
| Control after 2 h | 7.85 ± 0.47 | |
| pH 3 after 2 h | <LOD 1,* | |
| pH 5 after 2 h | 6.24 ± 0.97 | |
| Bile | Control before exposure (T = 0 h) | 7.23 ± 0.26 |
| Control after 3 h | 7.18 ± 0.71 | |
| Bile 0.25% (m/v) after 3 h | <LOD 1,* | |
| Bile 0.5% (m/v) after 3 h | <LOD 1,* | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, D.; Domingos, M.; Barbosa, J.C.; Almeida, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Exploring Freeze-Drying as Strategy to Enhance Viability of Faecalibacterium duncaniae DSM 17677 upon Aerobic Storage and Gastrointestinal Conditions. Pharmaceutics 2022, 14, 2735. https://doi.org/10.3390/pharmaceutics14122735
Machado D, Domingos M, Barbosa JC, Almeida D, Andrade JC, Freitas AC, Gomes AM. Exploring Freeze-Drying as Strategy to Enhance Viability of Faecalibacterium duncaniae DSM 17677 upon Aerobic Storage and Gastrointestinal Conditions. Pharmaceutics. 2022; 14(12):2735. https://doi.org/10.3390/pharmaceutics14122735
Chicago/Turabian StyleMachado, Daniela, Melany Domingos, Joana Cristina Barbosa, Diana Almeida, José Carlos Andrade, Ana Cristina Freitas, and Ana Maria Gomes. 2022. "Exploring Freeze-Drying as Strategy to Enhance Viability of Faecalibacterium duncaniae DSM 17677 upon Aerobic Storage and Gastrointestinal Conditions" Pharmaceutics 14, no. 12: 2735. https://doi.org/10.3390/pharmaceutics14122735
APA StyleMachado, D., Domingos, M., Barbosa, J. C., Almeida, D., Andrade, J. C., Freitas, A. C., & Gomes, A. M. (2022). Exploring Freeze-Drying as Strategy to Enhance Viability of Faecalibacterium duncaniae DSM 17677 upon Aerobic Storage and Gastrointestinal Conditions. Pharmaceutics, 14(12), 2735. https://doi.org/10.3390/pharmaceutics14122735










