Mesenchymal Stem/Stromal Cells and Their Paracrine Activity—Immunomodulation Mechanisms and How to Influence the Therapeutic Potential
Abstract
:1. Introduction
2. Mesenchymal Stem Cells (MSCs) Immune Action Mechanisms
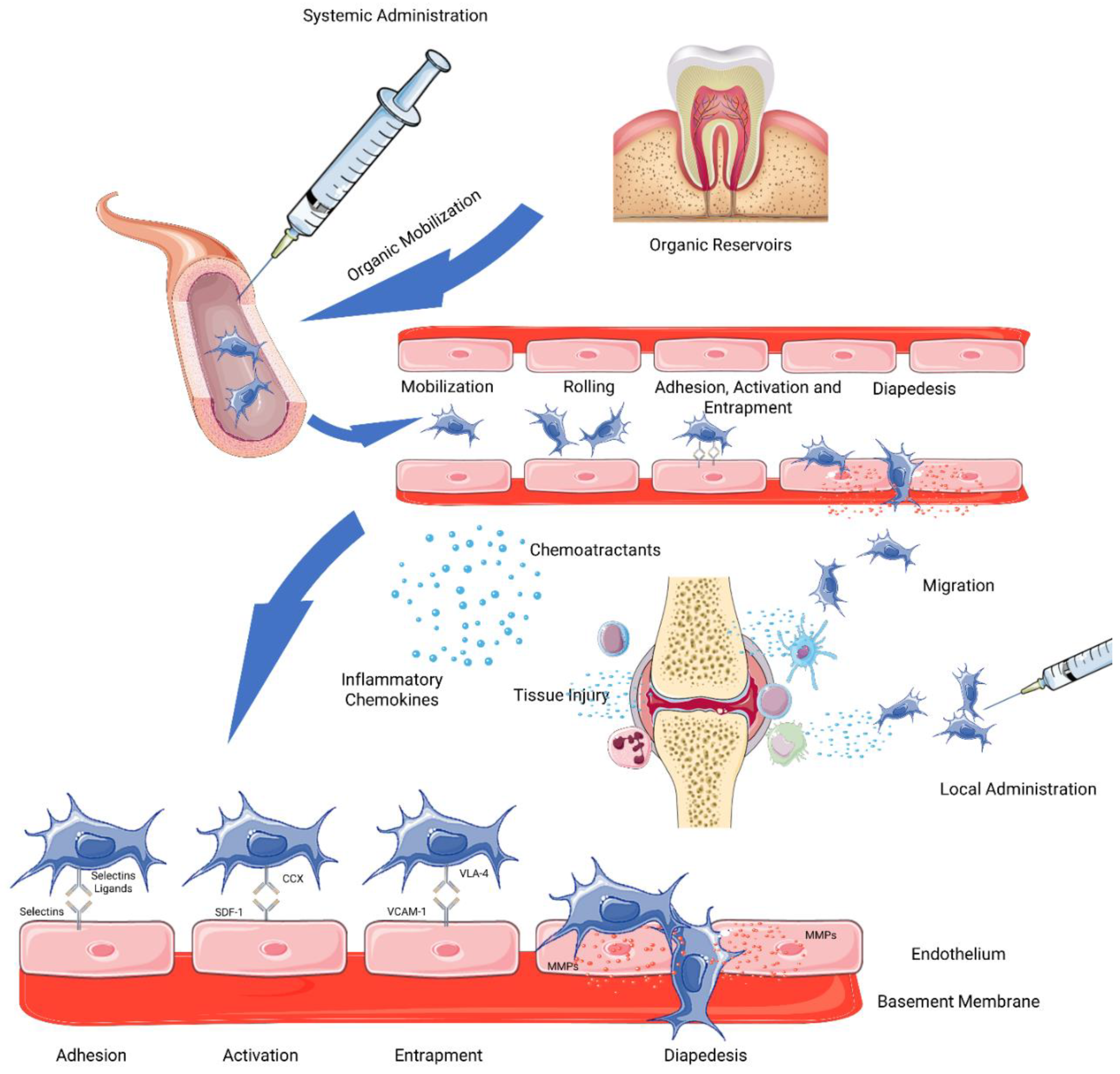
2.1. Delivery and Mobilization
2.2. Homing
Homing Improvement Techniques
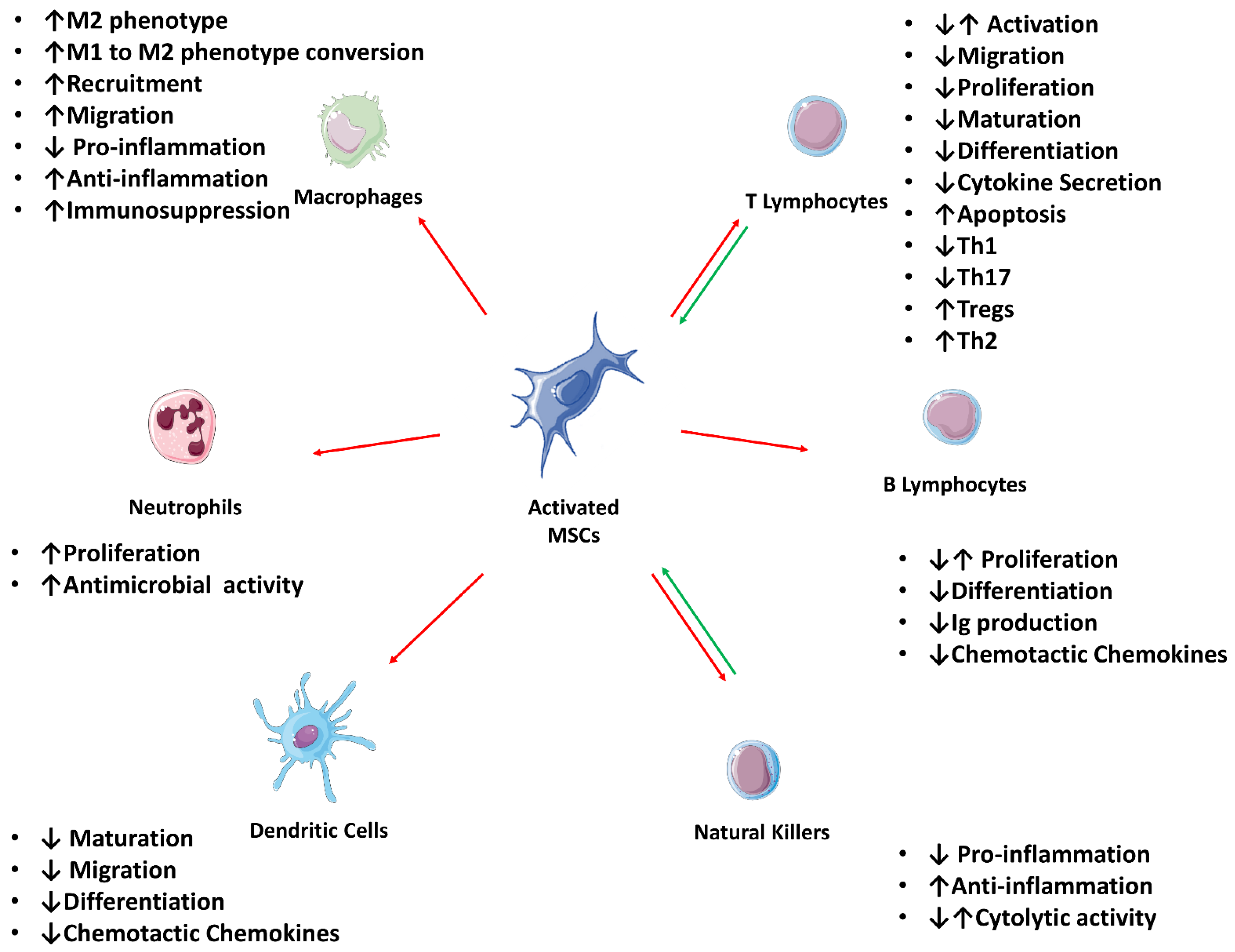
2.3. Direct Immunomodulation
2.3.1. Influence over the Adaptive Immune System
T-Cells
B-Cells
2.3.2. Influence over the Innate Immune System
Natural Killers
Dendritic Cells
Macrophages
Neutrophils
2.4. Alternative Immunomodulation Methods
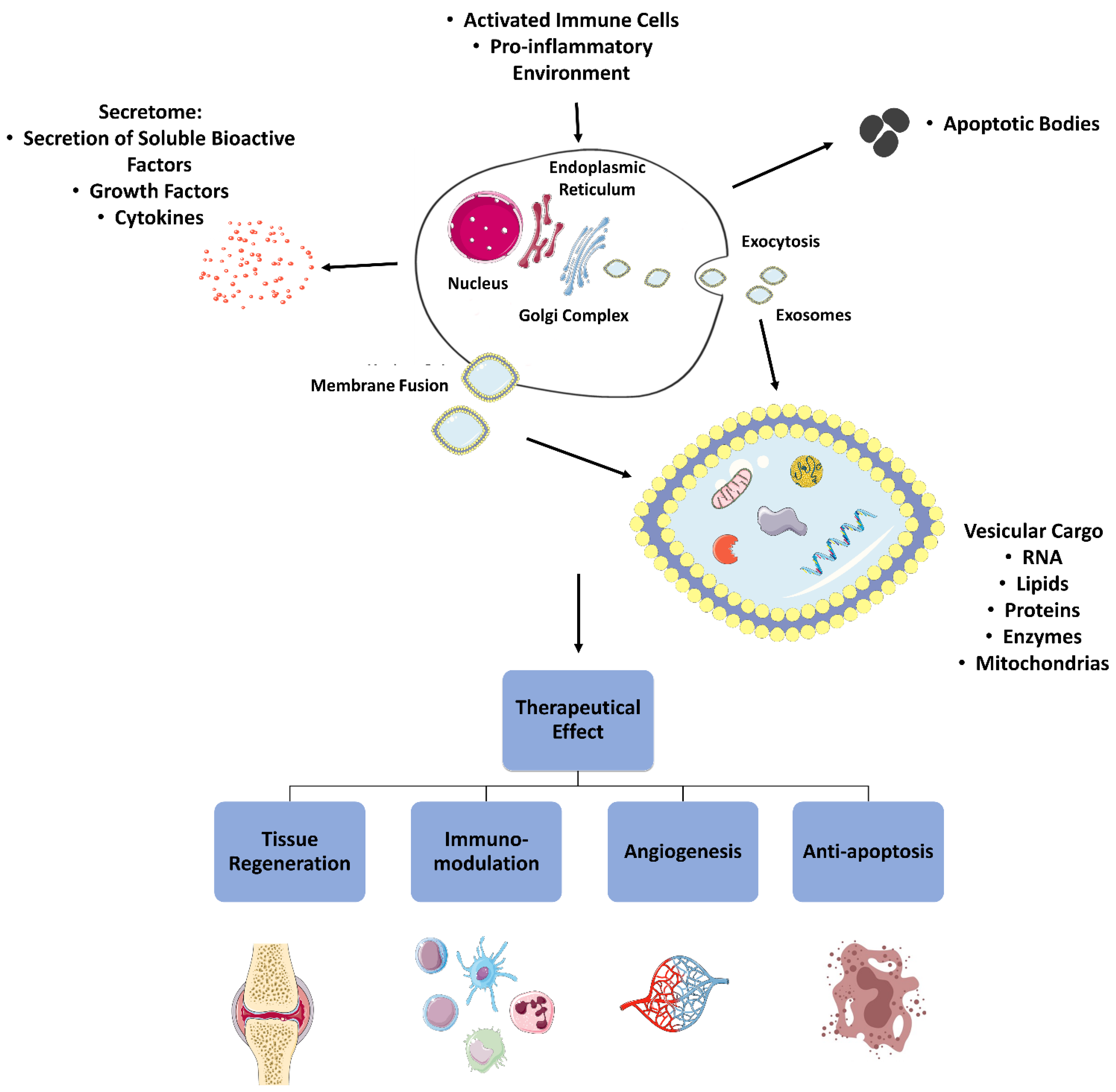
2.5. Paracrine Immunomodulation
2.5.1. Secretome
2.5.2. Extracellular Vesicles
3. MSCs, Immunomodulatory Activity and Clinical Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aMSCs | Animal Mesenchymal Stem/Stromal Cells; |
| ATMPs | Advanced Therapies Medicinal Products; |
| bFBF | Basic fibroblast growth factors |
| CCR | Chemokine receptors |
| CM | Conditioned medium |
| CD | Cluster of differentiation |
| COX2 | Cyclooxygenase 2 |
| CXCL | Chemokine ligand |
| CXCR | Chemokine receptors |
| DC | Dendritic Cells; |
| EGF | Epidermal Growth Factor |
| EMA | European Medicine Agency; |
| EV | Extracellular Vesicles |
| FasL | Fas Ligand |
| FDA | Food and Drug Administration; |
| FGFR | Fibroblast growth factor receptors |
| HCAM | Homing Cell Adhesion Molecule |
| HCT/Ps | Human Cellular and Tissue-based Products; |
| HGF | Hepatocyte growth Factor |
| HLA | Human Leucocyte Antigen |
| HLA-G | Human Leucocyte Antigen G; |
| hMSCs | Human Mesenchymal Stem/Stromal Cells; |
| ICAM | Intercellular Adhesion Molecule |
| IDO | Indoleamine 2, 3-dioxygenase |
| IFN | Interferon |
| IGF | insulin-like growth factor |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| ISCT | International Society for Cellular Therapy |
| KGF | Keratinocyte Growth Factor |
| LPS | Lipopolysaccharides |
| miRNA | MicroRNA |
| MMP | Metalloproteinases |
| MSCs | Mesenchymal Stem/Stromal Cells; |
| NK | Natural Killers; |
| NO | Nitric oxide |
| PCL | Phospholipase |
| PDGF | Platelet-derived growth factor |
| PD-L | Programmed death-ligand |
| PD | Programmed death |
| PGE | Prostaglandins E |
| PSGL | P-selectin glycoprotein ligand |
| ROS | Reactive Oxygen Species |
| SDF | Stromal cell-derived factor |
| TGF | Transforming Growth Factor |
| TGF | Transforming Growth Factor |
| TIMP | Tissue inhibitors of metalloproteinases |
| TLR | Toll-like receptors |
| TNF | Tumor Necrosis Factor |
| Treg | Regulatory T Lymphocytes |
| VCAM | Vascular Cell Adhesion Molecule |
| VEGF | Endothelial growth factor |
| VLA | Very Late Antigen |
References
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-Z.; Lin, Y.-H.; Su, L.-J.; Wu, M.-S.; Jeng, H.-Y.; Chang, H.-C.; Huang, Y.-H.; Ling, T.-Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.; Chailakhjan, R.; Lalykina, K. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Perez, R.L.; Zou, B.; Grosu, A.-L.; Huber, P.E.; Nicolay, N.H. The therapeutic potential of mesenchymal stromal cells in the treatment of chemotherapy-induced tissue damage. Stem Cell Rev. Rep. 2019, 15, 356–373. [Google Scholar] [CrossRef]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Álvarez-Viejo, M.; Menéndez-Menéndez, Y.; Otero-Hernández, J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J. Stem Cells 2015, 7, 470. [Google Scholar] [CrossRef]
- Fitter, S.; Gronthos, S.; Ooi, S.S.; Zannettino, A.C. The mesenchymal precursor cell marker antibody STRO-1 binds to cell surface heat shock cognate 70. Stem Cells 2017, 35, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Devireddy, L.R.; Boxer, L.; Myers, M.J.; Skasko, M.; Screven, R. Questions and challenges in the development of mesenchymal stromal/stem cell-based therapies in veterinary medicine. Tissue Eng. Part B Rev. 2017, 23, 462–470. [Google Scholar] [CrossRef]
- Schachtele, S.; Clouser, C.; Aho, J. Markers and Methods to Verify Mesenchymal Stem Cell Identity Potency, and Quality. Available online: https://resources.rndsystems.com/images/site/wp-msc-13763.pdf (accessed on 29 January 2020).
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Puerta, G.J.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. Role of mesenchymal stromal cells as therapeutic agents: Potential mechanisms of action and implications in their clinical use. J. Clin. Med. 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, P.; Clares, B.; Hmadcha, A.; Ruiz, A.; Soria, B. Development of a cell-based medicinal product: Regulatory structures in the European Union. Br. Med. Bull. 2013, 105, 85–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secretariat, C.C.S.; Schneider, C.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.; Paphitou, A.; Haunerova, I.; Maimets, T.; Trouvin, J. Challenges with advanced therapy medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar]
- Van Wilder, P. Advanced therapy medicinal products and exemptions to the Regulation 1394/2007: How confident can we be? An exploratory analysis. Front. Pharmacol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Turner, L. US stem cell clinics, patient safety, and the FDA. Trends Mol. Med. 2015, 21, 271–273. [Google Scholar] [CrossRef]
- López-Beas, J.; Guadix, J.A.; Clares, B.; Soriano-Ruiz, J.L.; Zugaza, J.L.; Gálvez-Martín, P. An overview of international regulatory frameworks for mesenchymal stromal cell-based medicinal products: From laboratory to patient. Med. Res. Rev. 2020, 40, 1315–1334. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise review: MSC adhesion cascade—insights into homing and transendothelial migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [Green Version]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J. Mesenchymal stromal cell therapeutic delivery: Translational challenges to clinical application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplant. 2017, 26, 585–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, C.-W.; Park, Y.-B.; Kim, S.H.; Lee, H.-J. Intra-articular mesenchymal stem cells in osteoarthritis of the knee: A systematic review of clinical outcomes and evidence of cartilage repair. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 277–288.e272. [Google Scholar] [CrossRef]
- Satué, M.; Schüler, C.; Ginner, N.; Erben, R.G. Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helal, M.A.; Shaheen, N.E.; Abu Zahra, F.A. Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats. Immunopharmacol. Immunotoxicol. 2016, 38, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Cooney, D.S.; Wimmers, E.G.; Ibrahim, Z.; Grahammer, J.; Christensen, J.M.; Brat, G.A.; Wu, L.W.; Sarhane, K.A.; Lopez, J.; Wallner, C. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, L.; Zhang, Y.; Sun, Y.; Li, G. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 2015, 24, 2643–2655. [Google Scholar] [CrossRef] [Green Version]
- Yakubovich, D.C.; Sheyn, D.; Bez, M.; Schary, Y.; Yalon, E.; Sirhan, A.; Amira, M.; Yaya, A.; De Mel, S.; Da, X. Systemic administration of mesenchymal stem cells combined with parathyroid hormone therapy synergistically regenerates multiple rib fractures. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Coppin, L.; Sokal, E.; Stéphenne, X. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: Current status and future perspectives. Cells 2019, 8, 1160. [Google Scholar] [CrossRef] [Green Version]
- Stamnitz, S.; Klimczak, A. Mesenchymal stem cells, bioactive factors, and scaffolds in bone repair: From research perspectives to clinical practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Amorim, I.; Magalhães, R.; João, F.; Almeida, D.; Amado, S.; Prada, J.; Pires, I. Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo. Stem Cells Int. 2021, 2021, 6613029. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, Z.; Qian, K.; Wang, J.; Zhu, H. Harnessing the properties of biomaterial to enhance the immunomodulation of mesenchymal stem cells. Tissue Eng. Part B Rev. 2019, 25, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobacchi, C.; Erreni, M.; Strina, D.; Palagano, E.; Villa, A.; Menale, C. 3D bone biomimetic scaffolds for basic and translational studies with mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem cell homing: From physiology to therapeutics. Stem Cells 2020, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Teo, G.S.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, J.M.; Carman, C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 2012, 30, 2472–2486. [Google Scholar] [CrossRef] [Green Version]
- Sackstein, R.; Merzaban, J.S.; Cain, D.W.; Dagia, N.M.; Spencer, J.A.; Lin, C.P.; Wohlgemuth, R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008, 14, 181–187. [Google Scholar] [CrossRef]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J. Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Display a Novel Interaction between P-Selectin and Galectin-1. Scand. J. Immunol. 2014, 80, 12–21. [Google Scholar] [CrossRef]
- Bailey, A.M.; Lawrence, M.B.; Shang, H.; Katz, A.J.; Peirce, S.M. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput. Biol. 2009, 5, e1000294. [Google Scholar] [CrossRef]
- Langer, H.F.; Stellos, K.; Steingen, C.; Froihofer, A.; Schönberger, T.; Krämer, B.; Bigalke, B.; May, A.E.; Seizer, P.; Müller, I. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J. Mol. Cell. Cardiol. 2009, 47, 315–325. [Google Scholar] [CrossRef]
- Gao, H.; Priebe, W.; Glod, J.; Banerjee, D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 2009, 27, 857–865. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, F.; He, D.; Zhang, L.; Shen, J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019, 109, 1233–1239. [Google Scholar] [CrossRef]
- Belema-Bedada, F.; Uchida, S.; Martire, A.; Kostin, S.; Braun, T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell 2008, 2, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Schenk, S.; Mal, N.; Finan, A.; Zhang, M.; Kiedrowski, M.; Popovic, Z.; McCarthy, P.M.; Penn, M.S. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 2007, 25, 245–251. [Google Scholar] [CrossRef]
- Steingen, C.; Brenig, F.; Baumgartner, L.; Schmidt, J.; Schmidt, A.; Bloch, W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell. Cardiol. 2008, 44, 1072–1084. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Ryu, J.M.; Han, H.J. Autotaxin-LPA Axis Regulates h MSC Migration by Adherent Junction Disruption and Cytoskeletal Rearrangement Via LPAR1/3-Dependent PKC/GSK3 β/β-C atenin and PKC/R ho GTP ase Pathways. Stem Cells 2015, 33, 819–832. [Google Scholar] [CrossRef]
- Joel, M.D.M.; Yuan, J.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. MSC: Immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am. J. Transl. Res. 2019, 11, 3890. [Google Scholar]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef]
- Will, H.; Atkinson, S.J.; Butler, G.S.; Smith, B.; Murphy, G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation: Regulation by TIMP-2 and TIMP-3. J. Biol. Chem. 1996, 271, 17119–17123. [Google Scholar] [CrossRef] [Green Version]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Bayo, J.; Real, A.; Fiore, E.J.; Malvicini, M.; Sganga, L.; Bolontrade, M.; Andriani, O.; Bizama, C.; Fresno, C.; Podhajcer, O. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget 2017, 8, 80235. [Google Scholar] [CrossRef]
- Hou, Y.; Ryu, C.H.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.S. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol. Int. 2014, 38, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, H.; Watanabe, M.; Kaji, N.; Okamoto, Y.; Tokeshi, M.; Miyamoto, Y.; Noguchi, H.; Baba, Y.; Hayashi, S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials 2012, 33, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Honczarenko, M.; Le, Y.; Swierkowski, M.; Ghiran, I.; Glodek, A.M.; Silberstein, L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006, 24, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.A.; Abreu, S.C.; Cruz, F.F.; Teixeira, A.C.; Lopes-Pacheco, M.; Bandeira, E.; Olsen, P.C.; Diaz, B.L.; Takyia, C.M.; Freitas, I.P. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir. Res. 2014, 15, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tano, N.; Narita, T.; Kaneko, M.; Ikebe, C.; Coppen, S.R.; Campbell, N.G.; Shiraishi, M.; Shintani, Y.; Suzuki, K. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol. Ther. 2014, 22, 1864–1871. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Fang, T.; Qi, Y.; Yin, X.; Di, T.; Feng, G.; Lei, Z.; Zhang, Y.; Huang, Z. Combined use of mesenchymal stromal cell sheet transplantation and local injection of SDF-1 for bone repair in a rat nonunion model. Cell Transplant. 2016, 25, 1801–1817. [Google Scholar] [CrossRef]
- Corradetti, B.; Taraballi, F.; Martinez, J.O.; Minardi, S.; Basu, N.; Bauza, G.; Evangelopoulos, M.; Powell, S.; Corbo, C.; Tasciotti, E. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- De Cássia Noronha, N.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 1–21. [Google Scholar]
- Magne, B.; Dedier, M.; Nivet, M.; Coulomb, B.; Banzet, S.; Lataillade, J.-J.; Trouillas, M. IL-1β–primed mesenchymal stromal cells improve epidermal substitute engraftment and wound healing via matrix metalloproteinases and transforming growth factor-β1. J. Investig. Dermatol. 2020, 140, 688–698.e21. [Google Scholar] [CrossRef]
- Bader, A.M.; Klose, K.; Bieback, K.; Korinth, D.; Schneider, M.; Seifert, M.; Choi, Y.-H.; Kurtz, A.; Falk, V.; Stamm, C. Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS ONE 2015, 10, e0138477. [Google Scholar] [CrossRef]
- Beegle, J.; Lakatos, K.; Kalomoiris, S.; Stewart, H.; Isseroff, R.R.; Nolta, J.A.; Fierro, F.A. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015, 33, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, M.W.; Yoo, K.H.; Lee, T.-H.; Kim, H.J.; Jang, I.K.; Chun, Y.H.; Kim, H.J.; Park, S.J.; Lee, S.H. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS ONE 2014, 9, e83363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 2020, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal stromal/stem cells: A new era in the cell-based targeted gene therapy of cancer. Front. Immunol. 2017, 8, 1770. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Cha, B.-H.; Jung, M.; Kim, A.S.; Bull, D.A.; Won, Y.-W. Cell surface engineering and application in cell delivery to heart diseases. J. Biol. Eng. 2018, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.-J.; Lee, P.-T.; Chen, C.-L.; Hsu, C.-Y.; Huang, W.-C.; Huang, C.-W.; Fang, H.-C. CD44 fucosylation on mesenchymal stem cell enhances homing and macrophage polarization in ischemic kidney injury. Exp. Cell Res. 2017, 350, 91–102. [Google Scholar] [CrossRef]
- Sarkar, D.; Zhao, W.; Gupta, A.; Loh, W.L.; Karnik, R.; Karp, J.M. Cell surface engineering of mesenchymal stem cells. In Mesenchymal Stem Cell Assays and Applications; Springer: Totowa, NJ, USA, 2011; pp. 505–523. [Google Scholar]
- Park, J.S.; Suryaprakash, S.; Lao, Y.-H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- English, K.; Ryan, J.; Tobin, L.; Murphy, M.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25Highforkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Pourgholaminejad, A.; Aghdami, N.; Baharvand, H.; Moazzeni, S.M. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine 2016, 85, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-γ produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front. Immunol. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Tinco, R.; Bertani, G.; Pisciotta, A.; Bertoni, L.; Pignatti, E.; Maccaferri, M.; Bertacchini, J.; Sena, P.; Vallarola, A.; Tupler, R. Role of PD-L1 in licensing immunoregulatory function of dental pulp mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, E.Y.; Kim, H.S.; Park, E.J.; Lee, H.J.; Lee, T.Y.; Kim, K.S.; Bae, S.-C.; Hong, J.T.; Kim, Y. Effect of Human Mesenchymal Stem Cells on Xenogeneic T and B Cells Isolated from Lupus-Prone MRL. Faslpr Mice. Stem Cells Int. 2020, 2020, 5617192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the Therapeutic Potential of Stem Cells and their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [Green Version]
- Kronsteiner, B.; Wolbank, S.; Peterbauer, A.; Hackl, C.; Redl, H.; van Griensven, M.; Gabriel, C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011, 20, 2115–2126. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Götherström, C.; Seidel, C.; Sundberg, B.; Sundin, M.; Rosendahl, K.; Tammik, C.; Ringden, O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand. J. Immunol. 2004, 60, 307–315. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Gong, J.; Liu, Y. Indoleamine 2, 3-dioxygenase regulation of immune response. Mol. Med. Rep. 2018, 17, 4867–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, W.; Zhang, J.; Yuan, Z.; Ren, G.; Zhang, L.; Chen, X.; Rabson, A.B.; Roberts, A.I.; Wang, Y.; Shi, Y. Mesenchymal Stem Cells Employ IDO to Regulate Immunity in Tumor Microenvironment. Cancer Res. 2014, 84, 1576–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Sheng, Z.; Sun, Y.; Wang, Y.; Xu, M.; Zhang, Z.; Li, H.; Shao, L.; Zhang, Y.; Yu, J. Human leukocyte antigen-G upregulates immunoglobulin-like transcripts and corrects dysfunction of immune cells in immune thrombocytopenia. Haematologica 2021, 106, 770. [Google Scholar] [PubMed] [Green Version]
- Contini, P.; Murdaca, G.; Puppo, F.; Negrini, S. HLA-G Expressing Immune Cells in Immune Mediated Diseases. Front. Immunol. 2020, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Reesink, H.L.; Sutton, R.M.; Shurer, C.R.; Peterson, R.P.; Tan, J.S.; Su, J.; Paszek, M.J.; Nixon, A.J. Galectin-1 and galectin-3 expression in equine mesenchymal stromal cells (MSCs), synovial fibroblasts and chondrocytes, and the effect of inflammation on MSC motility. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, Y.; Sato, K.; Mashima, K.; Nakano, H.; Ikeda, T.; Umino, K.; Morita, K.; Izawa, J.; Takayama, N.; Hayakawa, H. Mesenchymal Stromal Cells Inhibit Aerobic Glycolysis in Activated T Cells by Negatively Regulating Hexokinase II Activity Through PD-1/PD-L1 Interaction. Transplant. Cell. Ther. 2021, 27, 231.e1–231.e8. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Shi, S. Interplay between mesenchymal stem cells and lymphocytes: Implications for immunotherapy and tissue regeneration. J. Dent. Res. 2012, 91, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, N.; Li, X.; Zhou, S.; Liu, R.; Shi, D.; Lu, L.; Sun, L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell. Immunol. 2012, 274, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hu, C.; Chen, J.; Cen, P.; Wang, J.; Li, L. Interaction between mesenchymal stem cells and B-cells. Int. J. Mol. Sci. 2016, 17, 650. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Chen, X.; Liu, Q.; Zhang, X.; Huang, K.; Liu, L.; Li, H.; Zhou, M.; Huang, F.; Fan, Z. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 2015, 29, 636–646. [Google Scholar] [CrossRef] [Green Version]
- Day, R.B.; Bhattacharya, D.; Nagasawa, T.; Link, D.C. Granulocyte colony-stimulating factor reprograms bone marrow stromal cells to actively suppress B lymphopoiesis in mice. Blood J. Am. Soc. Hematol. 2015, 125, 3114–3117. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood J. Am. Soc. Hematol. 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+ CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Maria Spaggiari, G.; Moretta, L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol. Cell Biol. 2013, 91, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Govahi, A.; Fallah, M.; Rezvanfar, M.A.; Asghari, M.H.; Abdollahi, M. The mechanisms of cellular crosstalk between mesenchymal stem cells and natural killer cells: Therapeutic implications. J. Cell. Physiol. 2021, 236, 2413–2429. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Du, L. The role of secreted factors in stem cells-mediated immune regulation. Cell. Immunol. 2018, 326, 24–32. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood J. Am. Soc. Hematol. 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Jiang, X.-X.; Zhang, Y.; Liu, B.; Zhang, S.-X.; Wu, Y.; Yu, X.-D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [Green Version]
- Dokic, J.; Tomic, S.; Colic, M. Cross-talk between mesenchymal stem/stromal cells and dendritic cells. Curr. Stem Cell Res. Ther. 2016, 11, 51–65. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2-dependent mechanism. Sci. Rep. 2016, 6, 1–17. [Google Scholar]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zhao, X.; Wang, Y.; Zhang, X.; Chen, X.; Xu, C.; Yuan, Z.-r.; Roberts, A.I.; Zhang, L.; Zheng, B. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 2012, 11, 812–824. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Jakob, M.; Hemeda, H.; Bruderek, K.; Janeschik, S.; Bootz, F.; Lang, S. Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J. Leukoc. Biol. 2010, 88, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Jakob, M.; Bruderek, K.; Bootz, F.; Giebel, B.; Radtke, S.; Mauel, K.; Jäger, M.; Flohé, S.B.; Lang, S. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS ONE 2014, 9, e106903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial activity of mesenchymal stem cells: Current status and new perspectives of antimicrobial peptide-based therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kügler, A.; De Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Beken, S.V.; Wlaschek, M.; Kluth, M.A. Suppression of neutrophil-mediated tissue damage—a novel skill of mesenchymal stem cells. Stem Cells 2016, 34, 2393–2406. [Google Scholar] [CrossRef]
- Li, C.; Cheung, M.K.; Han, S.; Zhang, Z.; Chen, L.; Chen, J.; Zeng, H.; Qiu, J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 1–18. [Google Scholar] [CrossRef]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar]
- Weiss, D.J.; English, K.; Krasnodembskaya, A.; Isaza-Correa, J.M.; Hawthorne, I.J.; Mahon, B.P. The necrobiology of mesenchymal stromal cells affects therapeutic efficacy. Front. Immunol. 2019, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Burke, A.; Raman, S.; Ryan, A.; Ritter, T.; Barry, F.; Murphy, M. Mesenchymal stem cell therapy for osteoarthritis: How apoptotic cells modulate inflammation. Osteoarthr. Cartil. 2018, 26, S297. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-L.; Leu, S.; Sung, H.-C.; Zhen, Y.-Y.; Cho, C.-L.; Chen, A.; Tsai, T.-H.; Chung, S.-Y.; Chai, H.-T.; Sun, C.-K. Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in rat sepsis syndrome induced by cecal puncture and ligation. J. Transl. Med. 2012, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.S.; Galleu, A.; von Bonin, M.; Bornhäuser, M.; Dazzi, F. Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: Implications for the monitoring of mesenchymal stromal cell activity. Haematologica 2019, 104, e438. [Google Scholar] [CrossRef] [Green Version]
- Rastaldo, R.; Vitale, E.; Giachino, C. Dual role of autophagy in regulation of mesenchymal stem cell senescence. Front. Cell Dev. Biol. 2020, 8, 276. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Wu, D.; Li, L. Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia-or ischemia-induced injury. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Cen, S.; Wang, P.; Xie, Z.; Liu, Z.; Deng, W.; Su, H.; Wu, X.; Wang, S.; Li, J. Autophagy improves the immunosuppression of CD4+ T cells by mesenchymal stem cells through transforming growth factor-β1. Stem Cells Transl. Med. 2016, 5, 1496–1505. [Google Scholar] [CrossRef] [Green Version]
- Lv, B.; Hua, T.; Li, F.; Han, J.; Fang, J.; Xu, L.; Sun, C.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am. J. Transl. Res. 2017, 9, 2492. [Google Scholar]
- Park, H.J.; Shin, J.Y.; Kim, H.N.; Oh, S.H.; Lee, P.H. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a parkinsonian model. Neurobiol. Aging 2014, 35, 1920–1928. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 1465. [Google Scholar] [CrossRef] [PubMed]
- Renner, P.; Eggenhofer, E.; Rosenauer, A.; Popp, F.; Steinmann, J.; Slowik, P.; Geissler, E.; Piso, P.; Schlitt, H.; Dahlke, M. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant. Proc. 2009, 41, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Stem-Cell Drug Fails Crucial Trials. Nature Reports Stem Cells 2009. Available online: https://www.nature.com/articles/stemcells.2009.121#citeas (accessed on 5 January 2022). [CrossRef] [Green Version]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-x.; Qiu, X.-T.; Li, C.-C. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Han, Z.; Jing, Y.; Yang, X.; Zhang, S.; Sun, K.; Hao, C.; Meng, Y.; Yu, F.; Liu, X. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013, 4, e844. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.-C.; Shyu, W.-C.; Chiang, M.-F.; Lin, S.-Z.; Chang, Y.-C.; Wang, H.-J.; Su, C.-Y.; Li, H. Enhancement of neuroplasticity through upregulation of β1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol. Dis. 2007, 27, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, X.; Zhang, L.; Li, W.; Wu, H.; Yuan, X.; Mao, F.; Wang, M.; Zhu, W.; Qian, H. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014, 5, e1295. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, B.; McGuigan, D.H.; Koivula, F.N.M.; Elttayef, A.; Dale, T.P.; Yang, Y.; Kelly, C.; Forsyth, N.R. The Secretome of Mesenchymal Stem Cells Prevents Islet Beta Cell Apoptosis via an IL-10-Dependent Mechanism. Open Stem Cell J. 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-T.; Lin, C.-H.; Chiang, B.-L.; Jui, H.-Y.; Wu, K.K.-Y.; Lee, C.-M. Prostaglandin E2 potentiates mesenchymal stem cell–induced IL-10+ IFN-γ+ CD4+ regulatory T cells to control transplant arteriosclerosis. J. Immunol. 2013, 190, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, K.; Osugi, M.; Kawai, T.; Wakayama, Y.; Sakaguchi, K.; Nakamura, S.; Katagiri, W. Secretomes of mesenchymal stem cells induce early bone regeneration by accelerating migration of stem cells. J. Oral Maxillofac. Surg. Med. Pathol. 2018, 30, 445–451. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.-Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Rad, F.; Ghorbani, M.; Roushandeh, A.M.; Roudkenar, M.H. Mesenchymal stem cell-based therapy for autoimmune diseases: Emerging roles of extracellular vesicles. Mol. Biol. Rep. 2019, 46, 1533–1549. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Lai, P.; Weng, J.; Guo, L.; Chen, X.; Du, X. Novel insights into MSC-EVs therapy for immune diseases. Biomark. Res. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [Green Version]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Pavlyukov, M.S.; Yu, H.; Bastola, S.; Minata, M.; Shender, V.O.; Lee, Y.; Zhang, S.; Wang, J.; Komarova, S.; Wang, J. Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell 2018, 34, 119–135.e10. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.; Poon, I.K. Apoptotic cell-derived extracellular vesicles: More than just debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Lien, G.-S.; Liu, J.-F.; Chien, M.-H.; Hsu, W.-T.; Chang, T.-H.; Ku, C.-C.; Ji, A.T.-Q.; Tan, P.; Hsieh, T.-L.; Lee, L.-M. The ability to suppress macrophage-mediated inflammation in orbital fat stem cells is controlled by miR-671-5p. Stem Cell Res. Ther. 2014, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wu, M.; You, M.; Chen, Y.; Luo, M.; Chen, Q. The therapeutic applications of mesenchymal stromal cells from human perinatal tissues in autoimmune diseases. Stem Cell Res. Ther. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Sadeghi, S.; Soudi, S.; Shafiee, A.; Hashemi, S.M. Mesenchymal stem cell therapies for COVID-19: Current status and mechanism of action. Life Sci. 2020, 118493. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Čemerin, M.; Matišić, V.; Molnar, V.; Strbad, M.; Girandon, L.; Zenić, L.; Knežević, M.; Minger, S.; Polančec, D. Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment. Pharmaceutics 2021, 13, 1481. [Google Scholar] [CrossRef] [PubMed]
| Positive Markers | Biological Meaning |
| CD73 | Production of extracellular adenosine |
| CD90 | Cell-to-cell and cell-to-extracellular matrix interactions |
| CD105 | Vascular hemostasis |
| Negative Markers | Cell Exclusion |
| CD11b | Monocytes |
| CD14 | Macrophages |
| CD19 and CD79 | B Cells |
| CD34 | Hematopoietic and Endothelial Cells |
| CD45 | Leucocytes |
| HLA- DR | Antigen-presenting cells and Lymphocytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvites, R.; Branquinho, M.; Sousa, A.C.; Lopes, B.; Sousa, P.; Maurício, A.C. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity—Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics 2022, 14, 381. https://doi.org/10.3390/pharmaceutics14020381
Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity—Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics. 2022; 14(2):381. https://doi.org/10.3390/pharmaceutics14020381
Chicago/Turabian StyleAlvites, Rui, Mariana Branquinho, Ana C. Sousa, Bruna Lopes, Patrícia Sousa, and Ana Colette Maurício. 2022. "Mesenchymal Stem/Stromal Cells and Their Paracrine Activity—Immunomodulation Mechanisms and How to Influence the Therapeutic Potential" Pharmaceutics 14, no. 2: 381. https://doi.org/10.3390/pharmaceutics14020381
APA StyleAlvites, R., Branquinho, M., Sousa, A. C., Lopes, B., Sousa, P., & Maurício, A. C. (2022). Mesenchymal Stem/Stromal Cells and Their Paracrine Activity—Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics, 14(2), 381. https://doi.org/10.3390/pharmaceutics14020381







