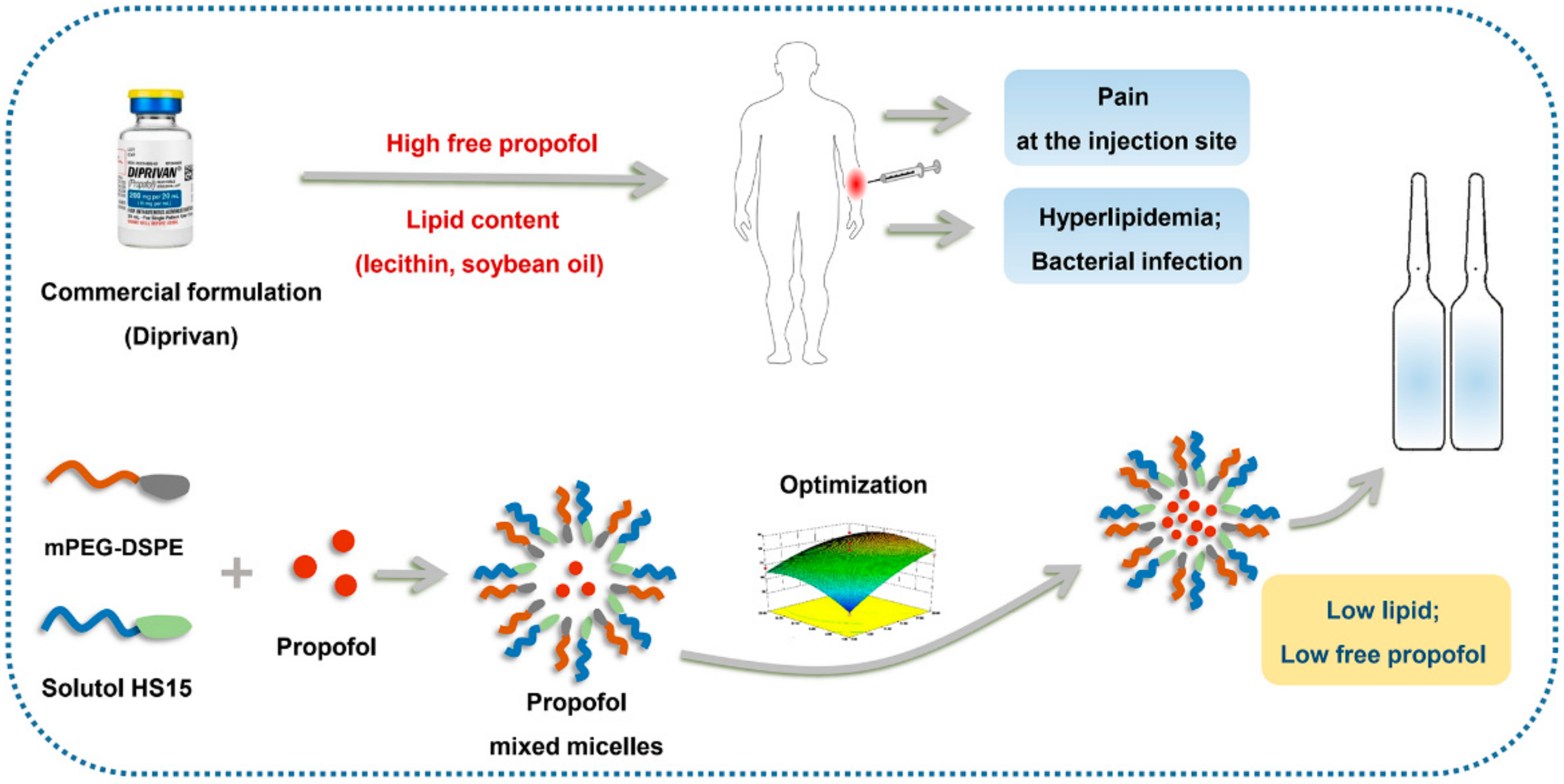
Physicochemical Characterization and Pharmacological Evaluation of Novel Propofol Micelles with Low-Lipid and Low-Free Propofol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Propofol-Mixed Micelles
2.4. Determination of Encapsulation Efficiency and Loading Content
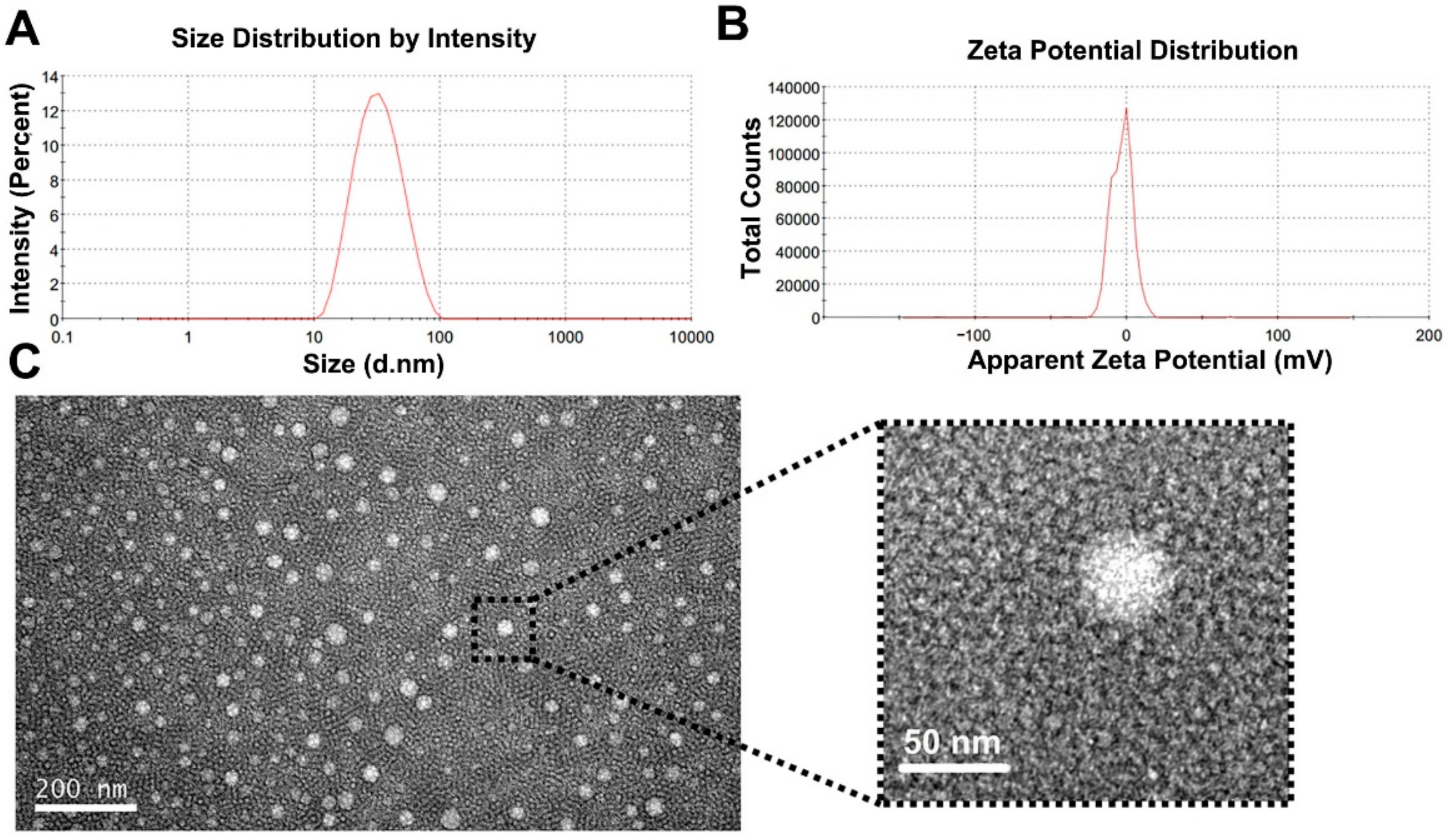
2.5. Particle Size and Zeta Potential
2.6. Morphology
2.7. pH and Osmolarity
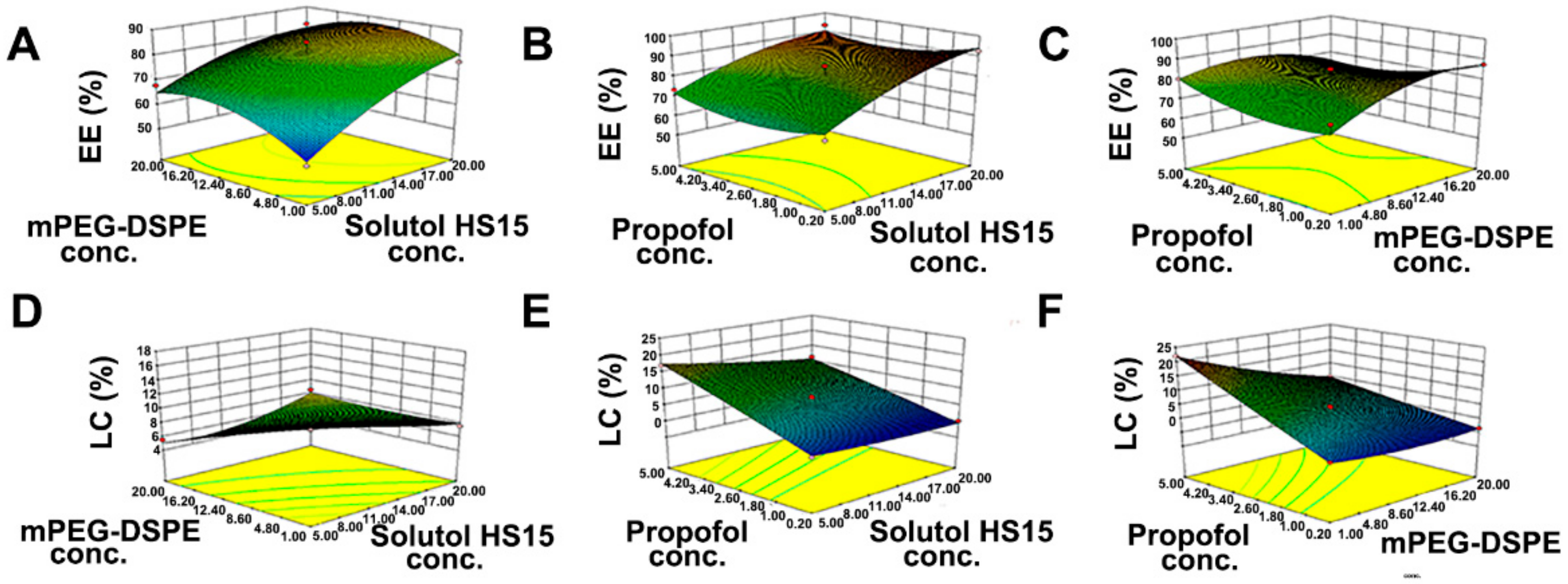
2.8. Formulation Optimization
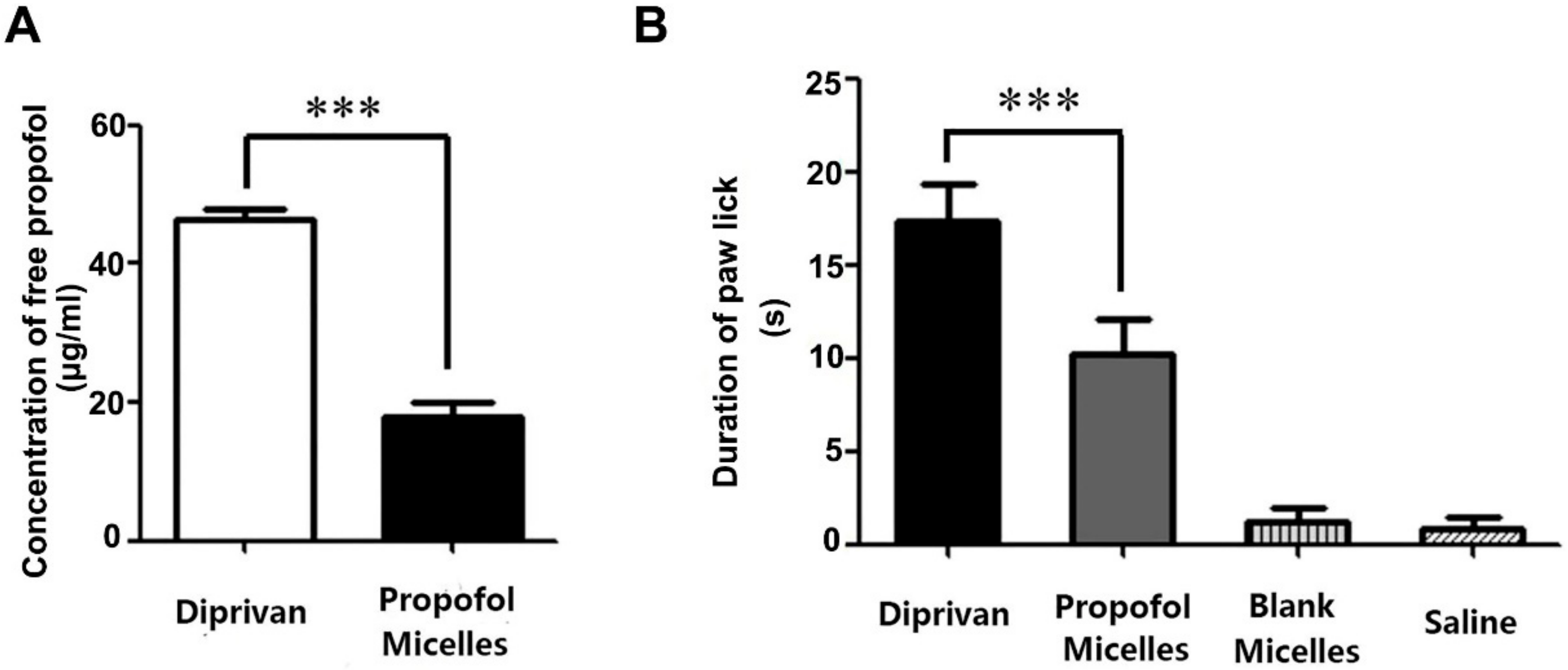
2.9. Concentration of Free Propofol
2.10. Pain on Injection
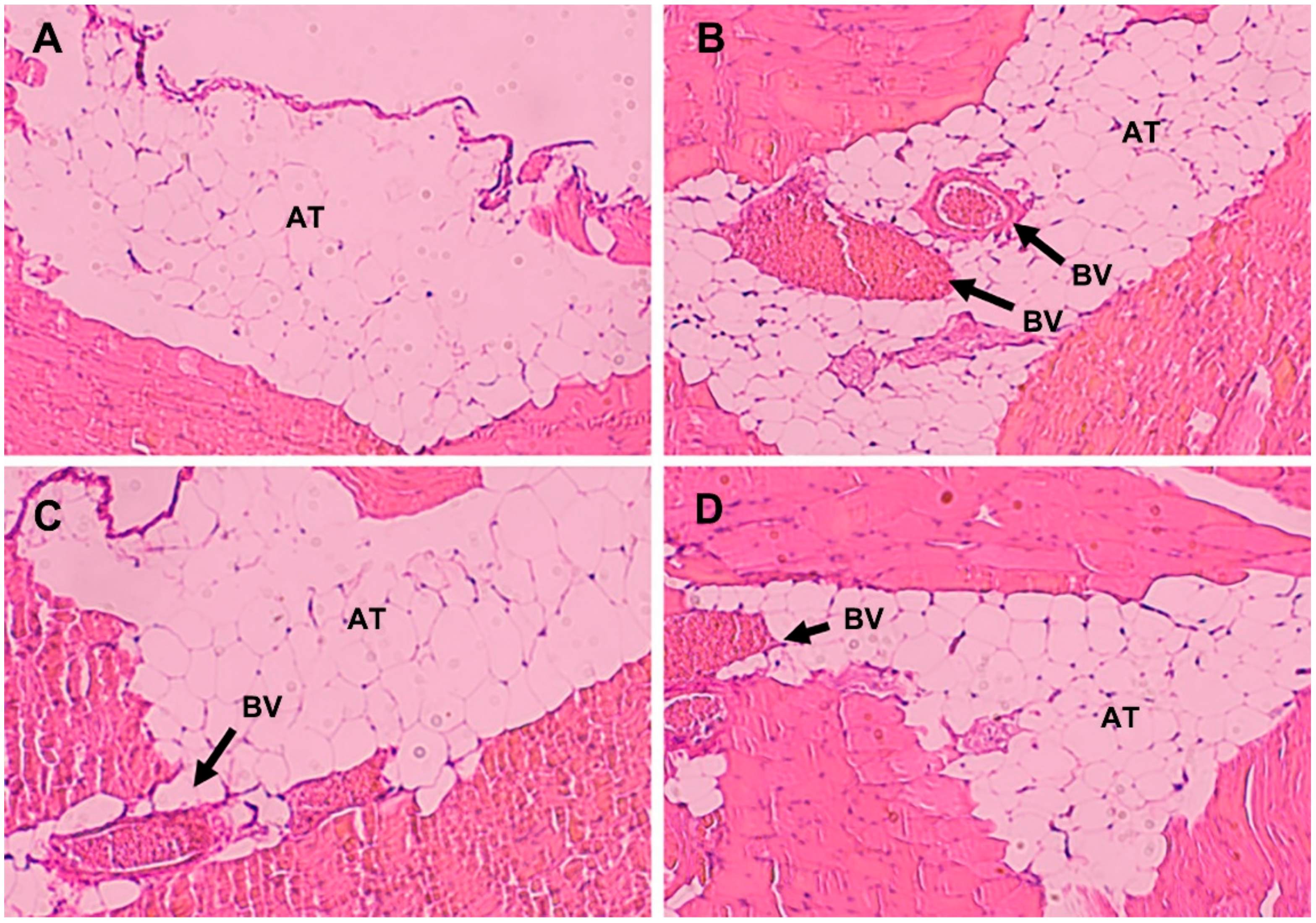
2.11. Histological Evaluation
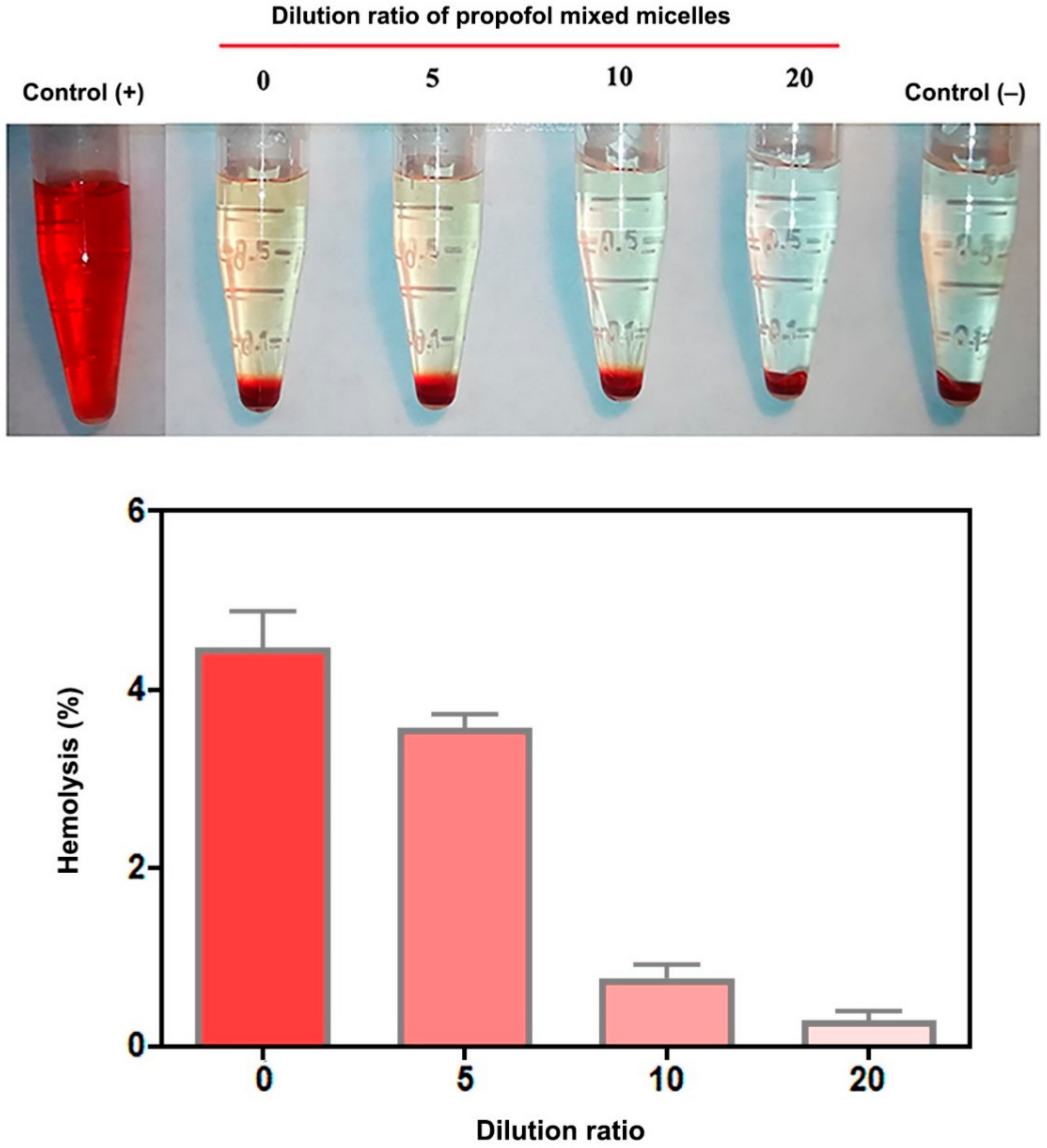
2.12. Hemolysis Test
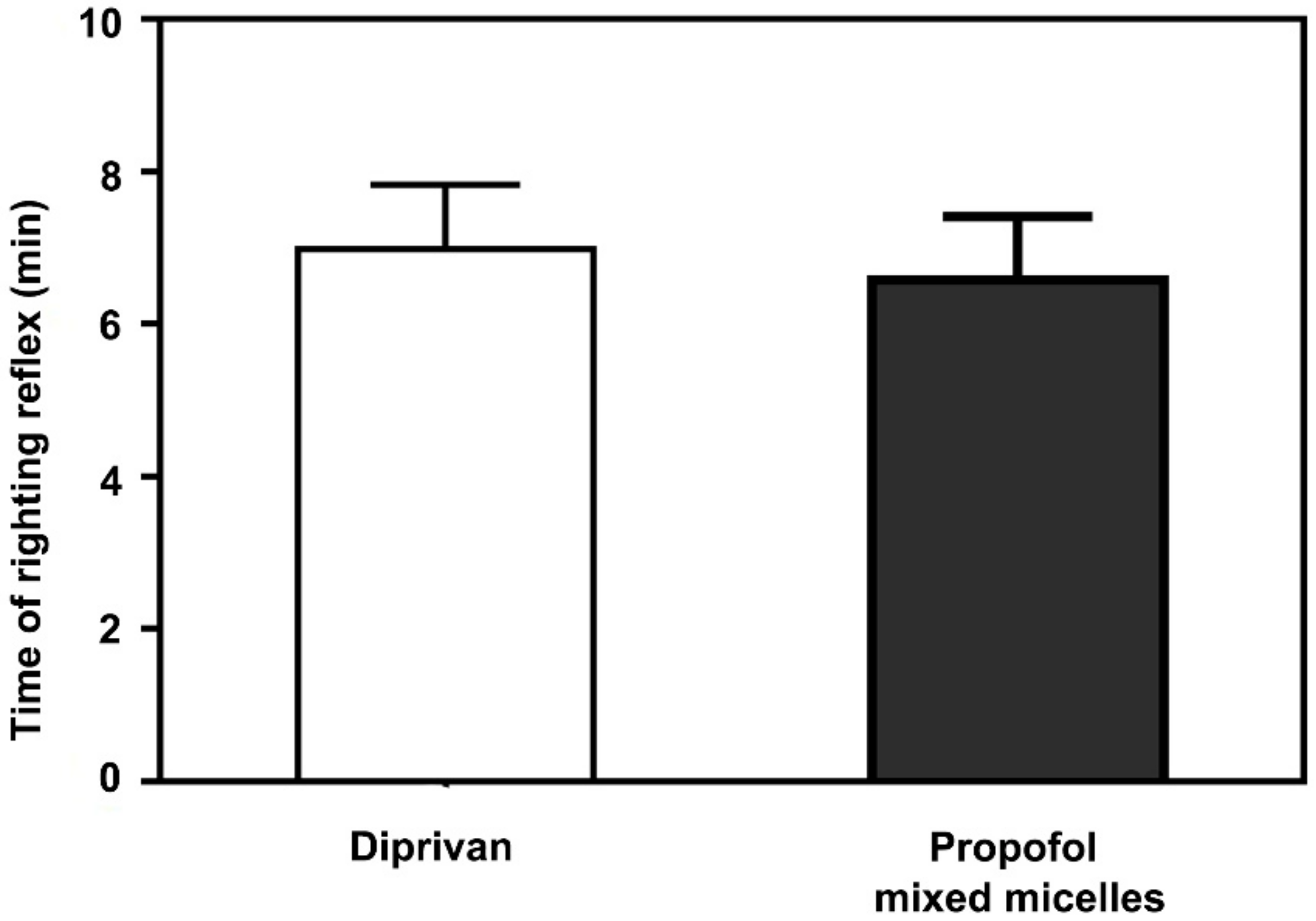
2.13. Sleep/Recovery Studies
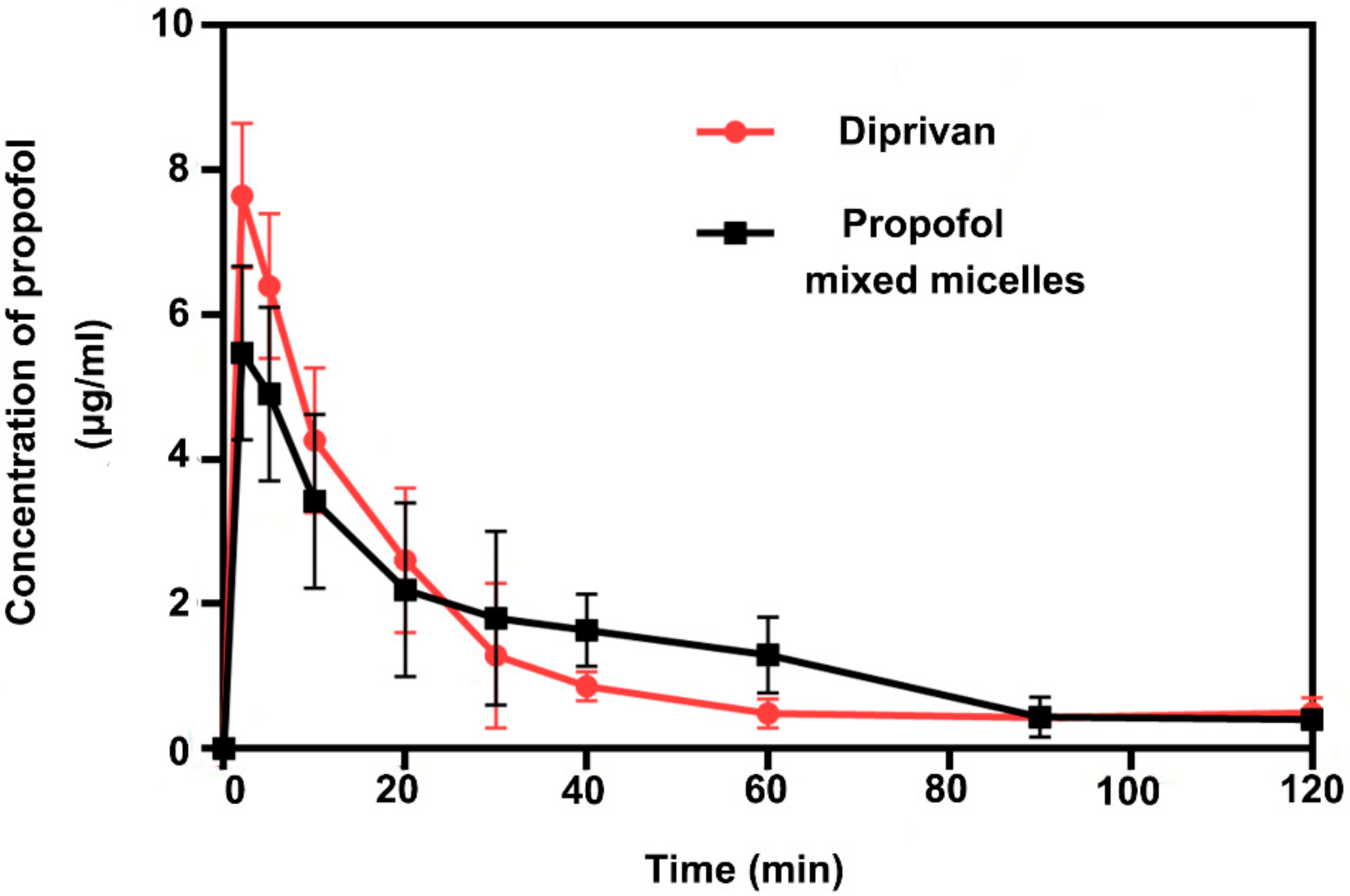
2.14. Pharmacokinetic Assessment
2.15. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Preparation Technology
3.2. Particle Size and Zeta Potential
3.3. pH and Osmolarity
3.4. Concentration of Free Propofol
3.5. Pain on Injection
3.6. Histological Evaluation
3.7. Hemolysis Test
3.8. Anesthetic Action
3.9. Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Stierer, T.; Zuckerman, R.; Sakima, N.; Parker, S.D.; Fleisher, L.A. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: A systematic review. Anesth. Analg. 2004, 98, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Latrofa, A.; Franco, M.; Lopedota, A.; Sanna, E.; Liso, G. Inclusion complexation of propofol with 2-hydroxypropyl-beta-cyclodextrin. Physicochemical, nuclear magnetic resonance spectroscopic studies, and anesthetic properties in rat. J. Pharm. Sci. 1998, 87, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Goodale, D.B. The recent development of propofol (DIPRIVAN). Intensive Care Med. 2000, 26 (Suppl. 4), S400–S404. [Google Scholar] [CrossRef] [PubMed]
- Langley, M.S.; Heel, R.C. Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs 1988, 35, 334–372. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.T.; Naguib, M. Propofol: The challenges of formulation. Anesthesiology 2005, 103, 860–876. [Google Scholar] [CrossRef] [PubMed]
- de Grood, P.M.; Ruys, A.H.; van Egmond, J.; Booij, L.H.; Crul, J.F. Propofol (‘Diprivan’) emulsion for total intravenous anaesthesia. Postgrad. Med. J. 1985, 61, 65–69. [Google Scholar] [PubMed]
- Park, J.W.; Park, E.S.; Chi, S.C.; Kil, H.Y.; Lee, K.H. The effect of lidocaine on the globule size distribution of propofol emulsions. Anesth. Analg. 2003, 97, 769–771. [Google Scholar] [CrossRef]
- Wolf, A.; Weir, P.; Segar, P.; Stone, J.; Shield, J. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet 2001, 357, 606–607. [Google Scholar] [CrossRef]
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef]
- Pergolizzi, J.J.; Gan, T.J.; Plavin, S.; Labhsetwar, S.; Taylor, R. Perspectives on the role of fospropofol in the monitored anesthesia care setting. Anesth. Res. Pr. 2011, 2011, 458920. [Google Scholar] [CrossRef] [Green Version]
- Egan, T.D.; Kern, S.E.; Johnson, K.B.; Pace, N.L. The pharmacokinetics and pharmacodynamics of propofol in a modified cyclodextrin formulation (Captisol) versus propofol in a lipid formulation (Diprivan): An electroencephalographic and hemodynamic study in a porcine model. Anesth. Analg. 2003, 97, 72–79. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, J.; Wang, L.; Ou, C.; Shu, Y.; Wu, Q.; Ma, G.; Gong, C. Gambogic acid-encapsulated polymeric micelles improved therapeutic effects on pancreatic cancer. Chin. Chem. Lett. 2019, 30, 885–888. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; He, X.; Zhang, B.; Liu, C.; Li, Q.; Zhu, Y.; Huang, W.; Zhang, W.; Qian, H.; et al. Histology and antitumor activity study of PTX-loaded micelle, a fluorescent drug delivery system prepared by PEG-TPP. Chin. Chem. Lett. 2019, 30, 1083–1088. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, X.; Gao, J.; Liu, Z.; Liu, Z.; Wang, K.; Jiang, J. Acid-resistant ROS-responsive hyperbranched polythioether micelles for ulcerative colitis therapy. Chin. Chem. Lett. 2020, 31, 3102–3106. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Zhao, X.; Liu, Z.; Liu, Z.; Wang, K.; Lia, G.; Jiang, J. Hyperbranched polymer micelles with triple-stimuli backbone-breakable iminoboronate ester linkages. Chin. Chem. Lett. 2020, 31, 1822–1826. [Google Scholar] [CrossRef]
- Yang, Z.L.; Li, X.R.; Yang, K.W.; Liu, Y. Amphotericin B-loaded poly(ethylene glycol)-poly(lactide) micelles: Preparation, freeze-drying, and in vitro release. J. Biomed. Mater. Res. A 2008, 85, 539–546. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Yang, K.; Zhou, Y.; Chen, X.; Zhang, Y.; Wang, F.; Liu, Y.; Ren, L. Self-assembled polymeric micellar nanoparticles as nanocarriers for poorly soluble anticancer drug ethaselen. Nanoscale Res. Lett. 2009, 4, 1502–1511. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, P.; Zhang, Y.; Zhou, Y.; Chen, X.; Huang, Y.; Liu, Y. Novel mixed polymeric micelles for enhancing delivery of anticancer drug and overcoming multidrug resistance in tumor cell lines simultaneously. Pharm. Res. 2010, 27, 1498–1511. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, Y.; Wang, X.; Fan, Y.; Huang, Y.; Liu, Y. Preparation and evaluation of poly(ethylene glycol)-poly(lactide) micelles as nanocarriers for oral delivery of cyclosporine A. Nanoscale Res. Lett. 2010, 5, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Avgoustakis, K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: Preparation, properties and possible applications in drug delivery. Curr. Drug Deliv. 2004, 1, 321–333. [Google Scholar] [CrossRef]
- Shuai, X.; Merdan, T.; Schaper, A.K.; Xi, F.; Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 2004, 15, 441–448. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, R.; Bromberg, L.; Temchenko, M.; Hatton, T.A.; Concheiro, A.; Alvarez-Lorenzo, C. Solubilization and stabilization of camptothecin in micellar solutions of pluronic-g-poly(acrylic acid) copolymers. J. Control. Release 2004, 97, 537–549. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Omelyanenko, V.G.; Papisov, M.I.; Bogdanov, A.J.; Trubetskoy, V.S.; Herron, J.N.; Gentry, C.A. Poly(ethylene glycol) on the liposome surface: On the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta 1994, 1195, 11–20. [Google Scholar] [CrossRef]
- Murgia, S.; Fadda, P.; Colafemmina, G.; Angelico, R.; Corrado, L.; Lazzari, P.; Monduzzi, M.; Palazzo, G. Characterization of the Solutol(R) HS15/water phase diagram and the impact of the Delta9-tetrahydrocannabinol solubilization. J. Colloid Interface Sci. 2013, 390, 129–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, Y.; Fan, Y.; Wang, X.; Huang, Y.; Liu, Y. Cyclosporin A-loaded poly(ethylene glycol)-b-poly(D,L-lactic acid) micelles: Preparation, in vitro and in vivo characterization and transport mechanism across the intestinal barrier. Mol. Pharm. 2010, 7, 1169–1182. [Google Scholar] [CrossRef]
- Darandale, S.S.; Shevalkar, G.B.; Vavia, P.R. Effect of Lipid Composition in Propofol Formulations: Decisive Component in Reducing the Free Propofol Content and Improving Pharmacodynamic Profiles. Aaps. Pharmscitech. 2017, 18, 441–450. [Google Scholar] [CrossRef]
- Sudo, R.T.; Bonfa, L.; Trachez, M.M.; Debom, R.; Rizzi, M.D.; Zapata-Sudo, G. Anesthetic profile of a non-lipid propofol nanoemulsion. Rev. Bras. Anestesiol. 2010, 60, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Klement, W.; Arndt, J.O. Pain on i.v. injection of some anaesthetic agents is evoked by the unphysiological osmolality or pH of their formulations. Br. J. Anaesth. 1991, 66, 189–195. [Google Scholar] [CrossRef]
- Klement, W.; Arndt, J.O. Pain on injection of propofol: Effects of concentration and diluent. Br. J. Anaesth. 1991, 67, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Yamakage, M.; Iwasaki, S.; Satoh, J.; Namiki, A. Changes in concentrations of free propofol by modification of the solution. Anesth. Analg. 2005, 101, 385–388. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Li, X.; Wang, X.; Li, Y.; Liu, Y.; Li, M. In vitro and in vivo evaluation of a simple microemulsion formulation for propofol. Int. J. Pharm. 2012, 425, 53–61. [Google Scholar] [CrossRef] [PubMed]
| Time (Min) | Water with 0.4% Phosphoric Acid (%) | Acetonitrile (%) |
|---|---|---|
| 0 | 50 | 50 |
| 6 | 30 | 70 |
| 13 | 30 | 70 |
| 14 | 50 | 50 |
| 16 | 50 | 50 |
| Independent Variable/Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: | 5 | 12.5 | 20 |
| X2: | 1 | 10.5 | 20 |
| X3: | 0.2 | 2.6 | 5.0 |
| Dependent variable/response | Constraints | ||
| Y1: | Maximize | ||
| Y2: | Maximize | ||
| Formulation Run | Factor 1 X1 | Factor 2 X2 | Factor 3 X3 | Response 1 Y1 | Response 2 Y2 |
|---|---|---|---|---|---|
| 1 | 5 | 1 | 2.6 | 51.66 | 17.94 |
| 2 | 20 | 1 | 2.6 | 77.32 | 8.52 |
| 3 | 5 | 20 | 2.6 | 67.88 | 6.5 |
| 4 | 20 | 20 | 2.6 | 82.55 | 5.24 |
| 5 | 5 | 10.5 | 0.2 | 66.73 | 1.05 |
| 6 | 20 | 10.5 | 0.2 | 92.45 | 0.07 |
| 7 | 5 | 10.5 | 5.0 | 73.2 | 17.96 |
| 8 | 20 | 10.5 | 5.0 | 93.38 | 12.26 |
| 9 | 12.5 | 1 | 0.2 | 75.46 | 2.34 |
| 10 | 12.5 | 20 | 0.2 | 87.32 | 0.63 |
| 11 | 12.5 | 1 | 5.0 | 80.21 | 22.54 |
| 12 | 12.5 | 20 | 5.0 | 71.52 | 8.88 |
| 13 | 12.5 | 10.5 | 2.6 | 85.15 | 8.62 |
| 14 | 12.5 | 10.5 | 2.6 | 78.44 | 7.98 |
| 15 | 12.5 | 10.5 | 2.6 | 79.52 | 8.08 |
| 16 | 12.5 | 10.5 | 2.6 | 80.43 | 7.18 |
| 17 | 12.5 | 10.5 | 2.6 | 79.10 | 8.04 |
| Source | Y1 | Remarks | Y2 | Remarks | ||
|---|---|---|---|---|---|---|
| F Value | p-Value Prob > F | F Value | p-Value Prob > F | |||
| Model | 9.95 | 0.0031 | Significant | 206.69 | <0.0001 | Significant |
| X1 | 55.62 | 0.0001 | 334.44 | <0.0001 | ||
| X2 | 9.95 | 0.0031 | 111.32 | <0.0001 | ||
| X3 | 4.53 | 0.0707 | 1223.39 | <0.0001 | ||
| X1 × 2 | 0.10 | 0.7615 | 49.19 | 0.0002 | ||
| X1 × 3 | 0.46 | 0.5198 | 105.50 | <0.0001 | ||
| X2 × 3 | 1.81 | 0.2208 | 16.46 | 0.0048 | ||
| X12 | 3.89 | 0.0890 | 14.15 | 0.0071 | ||
| X22 | 6.32 | 0.0402 | 1.15 | 0.3195 | ||
| X32 | 11.46 | 0.0117 | 5.24 | 0.0559 | ||
| Lack of Fit | 5.91 | 0.0453 | Not significant | 10.67 | 0.0223 | Not Significant |
| Response | Predicted Value | Actual Value | Deviation |
|---|---|---|---|
| EE (%) | 84.82 | 81.73 ± 0.65 | 3.64% |
| LC (%) | 19.24 | 18.46 ± 0.82 | 4.95% |
| Parameter | Unit | Diprivan | Propofol-Mixed Micelles |
|---|---|---|---|
| t1/2α | min | 10 | 6 |
| t1/2β | min | 69 | 384 |
| Tmax | min | 2 | 2 |
| Cmax | μg/L | 8 | 5 |
| AUC (0–120 min) | μg·min/L | 158 | 174 |
| CL | L/min/kg | 48 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Sun, T.; Xie, Z.; Sun, K.; Jiang, C. Physicochemical Characterization and Pharmacological Evaluation of Novel Propofol Micelles with Low-Lipid and Low-Free Propofol. Pharmaceutics 2022, 14, 414. https://doi.org/10.3390/pharmaceutics14020414
Chu Y, Sun T, Xie Z, Sun K, Jiang C. Physicochemical Characterization and Pharmacological Evaluation of Novel Propofol Micelles with Low-Lipid and Low-Free Propofol. Pharmaceutics. 2022; 14(2):414. https://doi.org/10.3390/pharmaceutics14020414
Chicago/Turabian StyleChu, Yongchao, Tao Sun, Zichen Xie, Keyu Sun, and Chen Jiang. 2022. "Physicochemical Characterization and Pharmacological Evaluation of Novel Propofol Micelles with Low-Lipid and Low-Free Propofol" Pharmaceutics 14, no. 2: 414. https://doi.org/10.3390/pharmaceutics14020414





