Nanomedicine as a Promising Tool to Overcome Immune Escape in Breast Cancer
Abstract
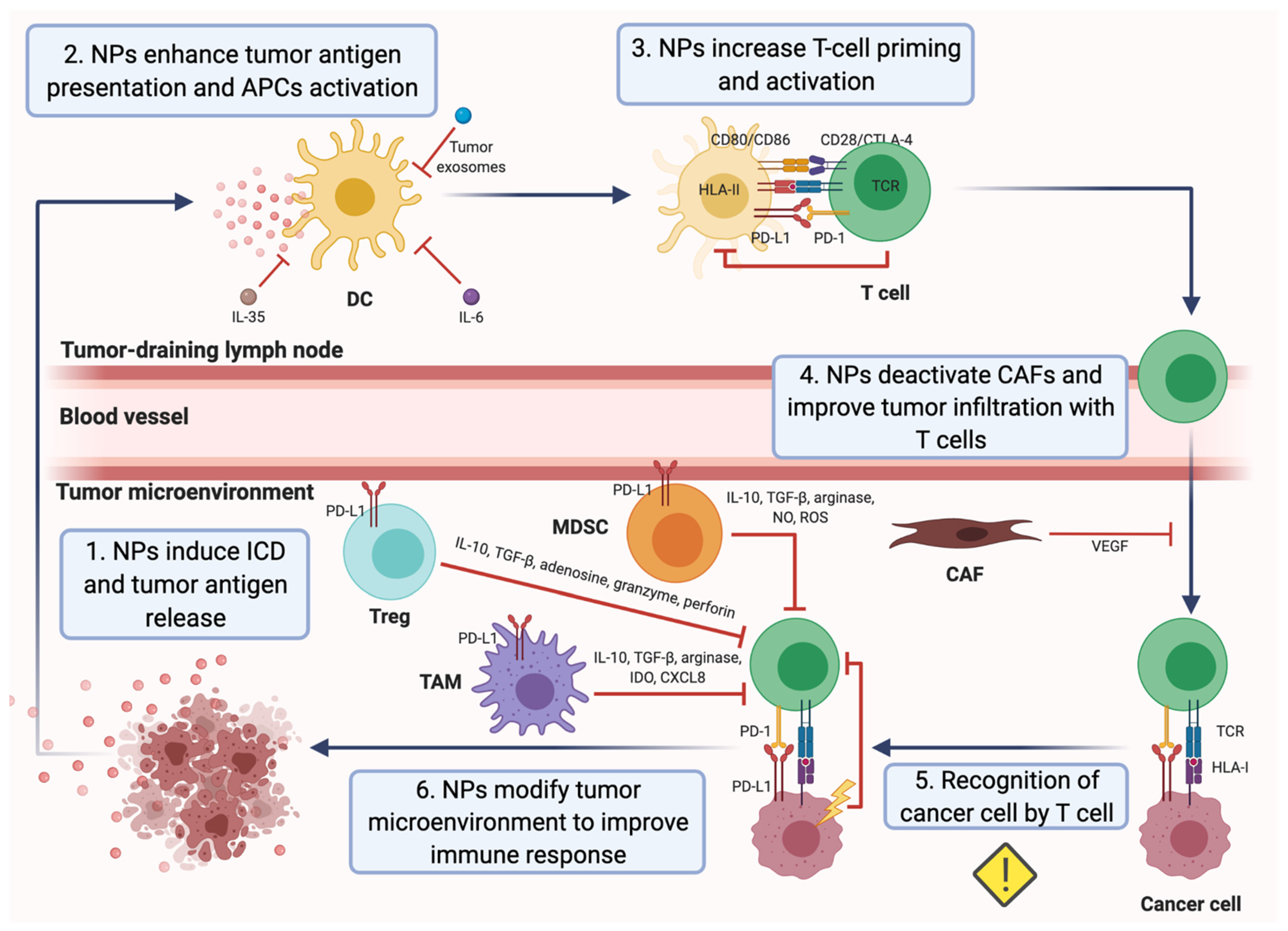
:1. Introduction
2. Mechanisms of Cancer Immune Escape
2.1. Tumor-Intrinsic Mechanisms of Immune Evasion
2.1.1. Alterations in Tumor HLA-I Expression
2.1.2. Overexpression of PD-L1
2.1.3. Production of Immunosuppressive Cytokines
2.2. Immunosuppressive Cells in Tumor Microenvironment
2.3. Impairment of Cytotoxic T-Cell Immunity
3. Nanomedicine as a Tool to Overcome Mechanisms of Cancer Immune Escape: A Promising Strategy to Treat Breast Cancer
3.1. Applications of Nanomedicine to Target Immunosuppressive Tumor Metabolism and Immunosupressive Cytokines in the TME
3.2. Applications of Nanomedicine to Target Immunosuppressive Cells within the TME
3.2.1. Nanomedicine-Based Approaches for Targeting Neutrophils
3.2.2. Nanomedicine-Based Approaches for Targeting NK Cells
3.2.3. Nanomedicine-Based Approaches for Targeting Macrophages
3.2.4. Nanomedicine-Based Approaches for Targeting CAFs
3.2.5. Nanomedicine-Based Approaches for Targeting MDSCs
3.2.6. Nanomedicine-Based Approaches for Targeting Tregs
3.3. Applications of Nanomedicine to Enhance DC Antigen Presentation and Activity
3.3.1. Nanotherapies for Inducting the ICD of Cancer Cells
3.3.2. Peptide-Based Nanovaccines
3.3.3. Gene-Based Nanovaccines
3.4. Nanomedicine-Based Approaches for Promoting Antitumor T-Cell Response
3.4.1. Nanotherapies for Promoting CTL Activation
3.4.2. Nanotherapies for Inhibiting IDO-1
3.4.3. Nanotherapies for Blocking PD-1/PD-L1
3.4.4. Nanotherapies for Promoting Antitumor Th1-Type Response
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Gatti-Mays, M.E.; Kalinsky, K.; Korde, L.A.; Sharon, E.; Amiri-Kordestani, L.; Bear, H.; McArthur, H.L.; Frank, E.; Perlmutter, J.; et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol. 2019, 5, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Seliger, B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care 2018, 13, 16–21. [Google Scholar] [CrossRef]
- Monnot, G.C.; Romero, P. Rationale for immunological approaches to breast cancer therapy. Breast 2018, 37, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Couzin-Frankel, J. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [Green Version]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current perspectives in cancer immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef] [Green Version]
- Criscitiello, C.; Curigliano, G. Immunotherapeutics for breast cancer. Curr. Opin. Oncol. 2013, 25, 602–608. [Google Scholar] [CrossRef]
- Emens, L.A. Breast cancer immunobiology driving immunotherapy: Vaccines and immune checkpoint blockade. Expert Rev. Anticancer Ther. 2012, 12, 1597–1611. [Google Scholar] [CrossRef] [Green Version]
- Lee Ventola, C. Cancer immunotherapy, part 3: Challenges and future trends. Pharm. Therp. 2017, 42, 514–521. [Google Scholar] [CrossRef]
- Sugie, T. Immunotherapy for metastatic breast cancer. Chin. Clin. Oncol. 2018, 7, 7. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Domchek, S.M.; Clark, A.S. Immunotherapy for breast cancer: What are we missing? Clin. Cancer Res. 2017, 23, 2640–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Aaltomaa, S.; Lipponen, P.; Eskelinen, M.; Kosma, V.M.; Marin, S.; Alhava, E.; Syrjänen, K. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur. J. Cancer 1992, 28, 859–864. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; De Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef]
- Retecki, K.; Seweryn, M.; Graczyk-Jarzynka, A.; Bajor, M. The Immune Landscape of Breast Cancer: Strategies for Overcoming Immunotherapy Resistance. Cancers 2021, 13, 6012. [Google Scholar] [CrossRef]
- Henriques, B.; Mendes, F.; Martins, D. Immunotherapy in Breast Cancer: When, How, and What Challenges? Biomedicines 2021, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- García-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, T.; Fernandez, M.A.; Sierra, A.; Garrido, A.; Herruzo, A.; Escobedo, A.; Fabra, A.; Garrido, F. High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum. Immunol. 1996, 50, 127–134. [Google Scholar] [CrossRef]
- Garrido, F.; Cabrera, T.; Aptsiauri, N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer 2010, 127, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.A.; Rodriguez, T.; Zinchenko, S.; Maleno, I.; Ruiz-Cabello, F.; Concha, Á.; Olea, N.; Garrido, F.; Aptsiauri, N. HLA class I alterations in breast carcinoma are associated with a high frequency of the loss of heterozygosity at chromosomes 6 and 15. Immunogenetics 2018, 70, 647–659. [Google Scholar] [CrossRef]
- Seliger, B.; Maeurer, M.J.; Ferrone, S. Antigen-processing machinery breakdown and tumor growth. Immunol. Today 2000, 21, 455–464. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Colpaert, C.; Mamessier, E.; Parizel, M.; Dirix, L.; Viens, P.; Birnbaum, D.; Van Laere, S. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015, 6, 13506–13519. [Google Scholar] [CrossRef] [Green Version]
- Soliman, H.; Khalil, F.; Antonia, S. PD-L1 Expression Is Increased in a Subset of Basal Type Breast Cancer Cells. PLoS ONE 2014, 9, e88557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinzon-Charry, A.; Ho, C.S.K.; Maxwell, T.; McGuckin, M.A.; Schmidt, C.; Furnival, C.; Pyke, C.M.; López, J.A. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br. J. Cancer 2007, 97, 1251–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorsch, S.M.; Memoli, V.A.; Stukel, T.A.; Gold, L.I.; Arrick, B.A. Immunohistochemical Staining for Transforming Growth Factor β1 Associates with Disease Progression in Human Breast Cancer. Cancer Res. 1992, 52, 6949–6952. [Google Scholar] [PubMed]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Heikkila, P.; Von Smitten, K.; Vakkila, J.; Leidenius, M. Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch. 2011, 459, 391–398. [Google Scholar] [CrossRef]
- Rubinstein, N.; Alvarez, M.; Zwirner, N.W.; Toscano, M.A.; Ilarregui, J.M.; Bravo, A.; Mordoh, J.; Fainboim, L.; Podhajcer, O.L.; Rabinovich, G.A. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection: A potential mechanism of tumor-immune privilege. Cancer Cell 2004, 5, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Heikkila, P.S.; Vaara, A.T.; von Smitten, K.A.J.; Vakkila, J.M.; Leidenius, M.H.K. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer 2009, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liyanage, U.K.; Moore, T.T.; Joo, H.-G.; Tanaka, Y.; Herrmann, V.; Doherty, G.; Drebin, J.A.; Strasberg, S.M.; Eberlein, T.J.; Goedegebuure, P.S.; et al. Prevalence of Regulatory T Cells Is Increased in Peripheral Blood and Tumor Microenvironment of Patients with Pancreas or Breast Adenocarcinoma. J. Immunol. 2002, 169, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Steurer, M.; Gastl, G.; Gunsilius, E.; Grubeck-Loebenstein, B. Increase of Regulatory T Cells in the Peripheral Blood of Cancer Patients. Clin. Cancer Res. 2003, 9, 606–612. [Google Scholar]
- Gunaydin, G.; Kesikli, S.A.; Guc, D. Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset. Oncoimmunology 2015, 4, e1034918. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Treilleux, I.; Blay, J.Y.; Bendriss-Vermare, N.; Ray-Coquard, I.; Bachelot, T.; Guastolla, J.P.; Bremond, A.; Goddard, S.; Pin, J.J.; Bartfaelemy-Dubois, C.; et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin. Cancer Res. 2004, 10, 7466–7474. [Google Scholar] [CrossRef] [Green Version]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4+ T Cells Regulate Pulmonary Metastasis of Mammary Carcinomas by Enhancing Protumor Properties of Macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Bronte, V.; Murray, P.J. Understanding local macrophage phenotypes in disease: Modulating macrophage function to treat cancer. Nat. Med. 2015, 21, 117–119. [Google Scholar] [CrossRef]
- Laoui, D.; Movahedi, K.; van Overmeire, E.; van den Bossche, J.; Schouppe, E.; Mommer, C.; Nikolaou, A.; Morias, Y.; de Baetselier, P.; van Ginderachter, J.A. Tumor-associated macrophages in breast cancer: Distinct subsets, distinct functions. Int. J. Dev. Biol. 2011, 55, 861–867. [Google Scholar] [CrossRef]
- Narita, D.; Seclaman, E.; Anghel, A.; Ilina, R.; Cireap, N.; Negru, S.; Sirbu, I.O.; Ursoniu, S.; Marian, C. Altered levels of plasma chemokines in breast cancer and their association with clinical and pathological characteristics. Neoplasma 2016, 63, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Tringler, B.; Zhuo, S.; Pilkington, G.; Torkko, K.C.; Singh, M.; Lucia, M.S.; Heinz, D.E.; Papkoff, J.; Shroyer, K.R. B7-H4 is highly expressed in ductal and lobular breast cancer. Clin. Cancer Res. 2005, 11, 1842–1848. [Google Scholar] [CrossRef] [Green Version]
- Khong, H.T.; Restifo, N.P. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 2002, 3, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Marincola, F.M.; Jaffee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of Human Solid Tumors from T–Cell Recognition: Molecular Mechanisms and Functional Significance. Adv. Immunol. 1999, 74, 181–273. [Google Scholar] [CrossRef]
- Down-regulation of CD28, TCR-zeta (zeta) and up-regulation of FAS in peripheral cytotoxic T-cells of primary breast cancer patients. Anticancer. Res. 2008, 28, 779–784.
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular permeability enhancement in solid tumor: Various factors, mechanisms involved and its implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Liu, Q.; Song, W.; Zhang, X.; Tiruthani, K.; Hu, H.; Das, M.; Goodwin, T.J.; Liu, R.; et al. Local Blockade of Interleukin 10 and C-X-C Motif Chemokine Ligand 12 with Nano-Delivery Promotes Antitumor Response in Murine Cancers. ACS Nano 2018, 2, 9830–9841. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Liu, L.; He, H.; Chen, Z.; Han, Z.; Luo, Z.; Wu, Z.; Zheng, M.; Ma, Y.; Cai, L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhao, Y.Y.; Shen, J.; Sun, X.; Liu, Y.; Liu, H.; Wang, Y.; Wang, J. Nanoenabled Modulation of Acidic Tumor Microenvironment Reverses Anergy of Infiltrating T Cells and Potentiates Anti-PD-1 Therapy. Nano Lett. 2019, 19, 2774–2783. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials 2019, 190–191, 38–50. [Google Scholar] [CrossRef]
- Ramesh, A.; Brouillard, A.; Kumar, S.; Nandi, D.; Kulkarni, A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials 2020, 227, 119559. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Mwakwari, S.C.; Austin, L.A.; Kieffer, M.J.; Oyelere, A.K.; El-Sayed, M.A. Small Molecule-Gold Nanorod Conjugates Selectively Target and Induce Macrophage Cytotoxicity towards Breast Cancer Cells. Small 2012, 8, 2819–2822. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.; Ran, W.; Meng, J.; Zhai, Y.; Zhang, P.; Yin, Q.; Yu, H.; Zhang, Z.; Li, Y. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials 2017, 144, 60–72. [Google Scholar] [CrossRef]
- Song, C.; Phuengkham, H.; Kim, Y.S.; Dinh, V.V.; Lee, I.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Um, S.H.; Lee, H.; et al. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat. Commun. 2019, 10, 3745. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Um, S.H.; Lim, Y.T. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv. Mater. 2018, 30, 1706719. [Google Scholar] [CrossRef]
- Navashenaq, J.G.; Zamani, P.; Nikpoor, A.R.; Tavakkol-Afshari, J.; Jaafari, M.R. Doxil chemotherapy plus liposomal P5 immunotherapy decreased myeloid-derived suppressor cells in murine model of breast cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102150. [Google Scholar] [CrossRef]
- Yuan, S.J.; Xu, Y.H.; Wang, C.; An, H.C.; Xu, H.Z.; Li, K.; Komatsu, N.; Zhao, L.; Chen, X. Doxorubicin-polyglycerol-nanodiamond conjugate is a cytostatic agent that evades chemoresistance and reverses cancer-induced immunosuppression in triple-negative breast cancer. J. Nanobiotechnol. 2019, 17, 110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Liu, S.; Shi, S.; Chen, Y.; Xu, F.; Wei, X.; Xu, Y. Solubilization and delivery of Ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J. Control. Release 2020, 320, 168–178. [Google Scholar] [CrossRef]
- Chen, H.; Luan, X.; Paholak, H.J.; Burnett, J.P.; Stevers, N.O.; Sansanaphongpricha, K.; He, M.; Chang, A.E.; Li, Q.; Sun, D. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine 2019, 15, 77–92. [Google Scholar] [CrossRef]
- Kopecka, J.; Porto, S.; Lusa, S.; Gazzano, E.; Salzano, G.; Pinzòn-Daza, M.L.; Giordano, A.; Desiderio, V.; Ghigo, D.; De Rosa, G.; et al. Zoledronic acid-encapsulating self-assembling nanoparticles and doxorubicin: A combinatorial approach to overcome simultaneously chemoresistance and immunoresistance in breast tumors. Oncotarget 2016, 7, 20753–20772. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Stephan, S.B.; Ene, C.I.; Smith, T.T.; Holland, E.C.; Stephan, M.T. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T cell therapy in solid malignancies. Cancer Res. 2018, 78, 3718–3730. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Du, Y.; Liang, X.; Yu, C.; Fang, J.; Lu, W.; Guo, X.; Tian, J.; Jin, Y.; Zheng, J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef]
- Lu, Q.; Qi, S.; Li, P.; Yang, L.; Yang, S.; Wang, Y.; Cheng, Y.; Song, Y.; Wang, S.; Tan, F.; et al. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J. Mater. Chem. B 2019, 7, 2499–2511. [Google Scholar] [CrossRef]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Ma, Y.; Wang, S.; Li, Y. Tumor Microenvironment-Activatable Prodrug Vesicles for Nanoenabled Cancer Chemoimmunotherapy Combining Immunogenic Cell Death Induction and CD47 Blockade. Adv. Mater. 2019, 31, 1805888. [Google Scholar] [CrossRef]
- Chen, H.; Cong, X.; Wu, C.; Wu, X.; Wang, J.; Mao, K.; Li, J.; Zhu, G.; Liu, F.; Meng, X.; et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9+ T cells. Sci. Adv. 2020, 6, 4690. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.W.; Chen, J.L.; Zhu, J.Y.; Rong, L.; Li, B.; Lei, Q.; Fan, J.X.; Zou, M.Z.; Li, C.; Cheng, S.X.; et al. Highly Integrated Nano-Platform for Breaking the Barrier between Chemotherapy and Immunotherapy. Nano Lett. 2016, 16, 4341–4347. [Google Scholar] [CrossRef]
- Ni, J.; Song, J.; Wang, B.; Hua, H.; Zhu, H.; Guo, X.; Xiong, S.; Zhao, Y. Dendritic cell vaccine for the effective immunotherapy of breast cancer. Biomed. Pharmacother. 2020, 126, 110046. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Wang, L.; Sun, Y.; Li, J.J.; Hill, B.; Yang, F.; Li, Y.; Lam, K.S. Unique Photochemo-Immuno-Nanoplatform against Orthotopic Xenograft Oral Cancer and Metastatic Syngeneic Breast Cancer. Nano Lett. 2018, 18, 7092–7103. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Long, Y.; Guo, R.; Liu, X.; Tang, X.; Rao, J.; Yin, S.; Zhang, Z.; Li, M.; He, Q. Multifunctional polymeric micelle-based chemo-immunotherapy with immune checkpoint blockade for efficient treatment of orthotopic and metastatic breast cancer. Acta Pharm. Sin. B 2019, 9, 819–831. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Lim, Y.T. A Designer Scaffold with Immune Nanoconverters for Reverting Immunosuppression and Enhancing Immune Checkpoint Blockade Therapy. Adv. Mater. 2019, 31, 1903242. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Ma, S.; Song, W.; Xu, Y.; Si, X.; Lv, S.; Zhang, Y.; Tang, Z.; Chen, X. Rationally Designed Polymer Conjugate for Tumor-Specific Amplification of Oxidative Stress and Boosting Antitumor Immunity. Nano Lett. 2020, 20, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Marrache, S.; Choi, J.H.; Tundup, S.; Zaver, D.; Harn, D.A.; Dhar, S. Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr. Biol. 2013, 5, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef]
- Zhou, P.; Qin, J.; Zhou, C.; Wan, G.; Liu, Y.; Zhang, M.; Yang, X.; Zhang, N.; Wang, Y. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials 2019, 195, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, U.; Wiltschke, C.; Jasinska, J.; Kundi, M.; Zurbriggen, R.; Garner-Spitzer, E.; Bartsch, R.; Steger, G.; Pehamberger, H.; Scheiner, O.; et al. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: A phase i study. Breast Cancer Res. Treat. 2010, 119, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.M.; Vartabedian, V.F.; Kim, M.C.; He, S.; Kang, S.M.; Selvaraj, P. Influenza virus-like particles engineered by protein transfer with tumor-associated antigens induces protective antitumor immunity. Biotechnol. Bioeng. 2015, 112, 1102–1110. [Google Scholar] [CrossRef] [Green Version]
- Arab, A.; Behravan, J.; Razazan, A.; Gholizadeh, Z.; Nikpoor, A.R.; Barati, N.; Mosaffa, F.; Badiee, A.; Jaafari, M.R. A nano-liposome vaccine carrying E75, a HER-2/neu-derived peptide, exhibits significant antitumour activity in mice. J. Drug Target. 2018, 26, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Navashenaq, J.G.; Teymouri, M.; Karimi, M.; Mashreghi, M.; Jaafari, M.R. Combination therapy with liposomal doxorubicin and liposomal vaccine containing E75, an HER-2/neu-derived peptide, reduces myeloid-derived suppressor cells and improved tumor therapy. Life Sci. 2020, 252, 117646. [Google Scholar] [CrossRef] [PubMed]
- Razazan, A.; Behravan, J.; Arab, A.; Barati, N.; Arabi, L.; Gholizadeh, Z.; Hatamipour, M.; Nikpoor, A.R.; Momtazi-Borojeni, A.A.; Mosaffa, F.; et al. Conjugated nanoliposome with the HER2/ neu-derived peptide GP2 as an effective vaccine against breast cancer in mice xenograft model. PLoS ONE 2017, 12, e0185099. [Google Scholar] [CrossRef]
- Zupančič, E.; Curato, C.; Kim, J.S.; Yeini, E.; Porat, Z.; Viana, A.S.; Globerson-Levin, A.; Waks, T.; Eshhar, Z.; Moreira, J.N.; et al. Nanoparticulate vaccine inhibits tumor growth via improved T cell recruitment into melanoma and huHER2 breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Kokate, R.A.; Chaudhary, P.; Sun, X.; Thamake, S.I.; Maji, S.; Chib, R.; Vishwanatha, J.K.; Jones, H.P. Rationalizing the use of functionalized poly-lactic-co-glycolic acid nanoparticles for dendritic cell-based targeted anticancer therapy. Nanomedicine 2016, 11, 479–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, D.F.; Saenz, R.; Bharati, I.S.; Seible, D.; Zhang, L.; Esener, S.; Messmer, B.; Larsson, M.; Messmer, D. Enhanced anti-tumor immune responses and delay of tumor development in human epidermal growth factor receptor 2 mice immunized with an immunostimulatory peptide in poly(d,l-lactic-co-glycolic) acid nanoparticles. Breast Cancer Res. 2015, 17, 48. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Feng, Z.; Wang, C.; Su, Q.; Song, H.; Zhang, C.; Huang, P.; Liang, X.J.; Dong, A.; Kong, D.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2020, 230, 119649. [Google Scholar] [CrossRef]
- Palladini, A.; Thrane, S.; Janitzek, C.M.; Pihl, J.; Clemmensen, S.B.; de Jongh, W.A.; Clausen, T.M.; Nicoletti, G.; Landuzzi, L.; Penichet, M.L.; et al. Virus-like particle display of HER2 induces potent anti-cancer responses. Oncoimmunology 2018, 7, e1408749. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Shukla, S.; Wang, C.; Masarapu, H.; Steinmetz, N.F. Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine. J. Am. Chem. Soc. 2019, 141, 6509–6518. [Google Scholar] [CrossRef]
- Bolli, E.; O’Rourke, J.P.; Conti, L.; Lanzardo, S.; Rolih, V.; Christen, J.M.; Barutello, G.; Forni, M.; Pericle, F.; Cavallo, F. A Virus-Like-Particle immunotherapy targeting Epitope-Specific anti-xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo. Oncoimmunology 2018, 7, e1408746. [Google Scholar] [CrossRef] [Green Version]
- Alipour Talesh, G.; Ebrahimi, Z.; Badiee, A.; Mansourian, M.; Attar, H.; Arabi, L.; Jalali, S.A.; Jaafari, M.R. Poly (I: C)-DOTAP cationic nanoliposome containing multi-epitope HER2-derived peptide promotes vaccine-elicited anti-tumor immunity in a murine model. Immunol. Lett. 2016, 176, 57–64. [Google Scholar] [CrossRef]
- Shariat, S.; Badiee, A.; Jalali, S.A.; Mansourian, M.; Yazdani, M.; Mortazavi, S.A.; Jaafari, M.R. P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014, 355, 54–60. [Google Scholar] [CrossRef]
- Zamani, P.; Navashenaq, J.G.; Nikpoor, A.R.; Hatamipour, M.; Oskuee, R.K.; Badiee, A.; Jaafari, M.R. MPL nano-liposomal vaccine containing P5 HER2/neu-derived peptide pulsed PADRE as an effective vaccine in a mice TUBO model of breast cancer. J. Control. Release 2019, 303, 223–236. [Google Scholar] [CrossRef]
- Zamani, P.; Teymouri, M.; Nikpoor, A.R.; Navashenaq, J.G.; Gholizadeh, Z.; Darban, S.A.; Jaafari, M.R. Nanoliposomal vaccine containing long multi-epitope peptide E75-AE36 pulsed PADRE-induced effective immune response in mice TUBO model of breast cancer. Eur. J. Cancer 2020, 129, 80–96. [Google Scholar] [CrossRef]
- Barati, N.; Nikpoor, A.R.; Razazan, A.; Mosaffa, F.; Badiee, A.; Arab, A.; Gholizadeh, Z.; Behravan, J.; Jaafari, M.R. Nanoliposomes carrying HER2/neu-derived peptide AE36 with CpG-ODN exhibit therapeutic and prophylactic activities in a mice TUBO model of breast cancer. Immunol. Lett. 2017, 190, 108–117. [Google Scholar] [CrossRef]
- Yamano, T.; Kaneda, Y.; Hiramatsu, S.H.; Huang, S.; Tran, A.N.; Giuliano, A.E.; Hoon, D.S.B. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007, 14, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lv, D.; Liu, S.; Gong, J.; Wang, D.; Xiong, M.; Chen, X.; Xiang, R.; Tan, X. Alginic Acid-Coated Chitosan Nanoparticles Loaded with Legumain DNA Vaccine: Effect against Breast Cancer in Mice. PLoS ONE 2013, 8, e60190. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer. Mol. Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Jadidi-Niaragh, F.; Atyabi, F.; Rastegari, A.; Kheshtchin, N.; Arab, S.; Hassannia, H.; Ajami, M.; Mirsanei, Z.; Habibi, S.; Masoumi, F.; et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J. Control. Release 2017, 246, 46–59. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A.M. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol. 2015, 5, 692. [Google Scholar] [CrossRef] [Green Version]
- Gao, A.; Chen, B.; Gao, J.; Zhou, F.; Saeed, M.; Hou, B.; Li, Y.; Yu, H. Sheddable Prodrug Vesicles Combating Adaptive Immune Resistance for Improved Photodynamic Immunotherapy of Cancer. Nano Lett. 2020, 20, 353–362. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Li, W.; Yang, Q.; Tan, L.; Jia, Y.; Qu, Y.; Qian, Z. Photosensitizer Micelles Together with IDO Inhibitor Enhance Cancer Photothermal Therapy and Immunotherapy. Adv. Sci. 2018, 5, 1700891. [Google Scholar] [CrossRef]
- Liu, R.; An, Y.; Jia, W.; Wang, Y.; Wu, Y.; Zhen, Y.; Cao, J.; Gao, H. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J. Control. Release 2020, 321, 589–601. [Google Scholar] [CrossRef]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-Amplified Drug Delivery with Light-Inducible Nanocargoes to Enhance Cancer Immunotherapy. Adv. Mater. 2019, 31, 1902960. [Google Scholar] [CrossRef]
- Feng, B.; Zhou, F.; Hou, B.; Wang, D.; Wang, T.; Fu, Y.; Ma, Y.; Yu, H.; Li, Y. Binary Cooperative Prodrug Nanoparticles Improve Immunotherapy by Synergistically Modulating Immune Tumor Microenvironment. Adv. Mater. 2018, 30, 1803001. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.P.; Wang, X.; Ahmed, A.; Jiang, W.; Ji, Y.; Meng, H.; Nel, A.E. Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway. ACS Nano 2018, 12, 11041–11061. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Sun, J.; Xu, J.; Moharil, P.; Chen, J.; Xu, J.; Zhu, J.; Li, J.; Huang, Y.; Xu, P.; et al. Dual functional immunostimulatory polymeric prodrug carrier with pendent indoximod for enhanced cancer immunochemotherapy. Acta Biomater. 2019, 90, 300–313. [Google Scholar] [CrossRef]
- Sun, J.J.; Chen, Y.C.; Huang, Y.X.; Zhao, W.C.; Liu, Y.H.; Venkataramanan, R.; Lu, B.F.; Li, S. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol. Sin. 2017, 38, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xia, R.; Huang, Y.; Zhao, W.; Li, J.; Zhang, X.; Wang, P.; Venkataramanan, R.; Fan, J.; Xie, W.; et al. An immunostimulatory dual-functional nanocarrier that improves cancer immunochemotherapy. Nat. Commun. 2016, 7, 13443. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, X.; Zhao, S.; Liao, X.; Younis, M.R.; Wang, S.; Zhang, C.; Lu, G. JQ1-Loaded Polydopamine Nanoplatform Inhibits c-MYC/Programmed Cell Death Ligand 1 to Enhance Photothermal Therapy for Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2019, 11, 46626–46636. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Z.; Lv, H.; Li, F.; Sun, S.; Li, J.; Lee, C. Immune Checkpoint Blockade Mediated by a Small-Molecule Nanoinhibitor Targeting the PD-1/PD-L1 Pathway Synergizes with Photodynamic Therapy to Elicit Antitumor Immunity and Antimetastatic Effects on Breast Cancer. Small 2019, 15, 1903881. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; He, X.; Yang, Z.; Yang, X.; Xiao, W.; Liu, R.; Xie, R.; Qin, L.; Gao, H. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials 2019, 217, 119309. [Google Scholar] [CrossRef]
- Lang, T.; Liu, Y.; Zheng, Z.; Ran, W.; Zhai, Y.; Yin, Q.; Zhang, P.; Li, Y. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 2019, 31, 1806202. [Google Scholar] [CrossRef]
- Mi, Y.; Smith, C.C.; Yang, F.; Qi, Y.; Roche, K.C.; Serody, J.S.; Vincent, B.G.; Wang, A.Z. A Dual Immunotherapy Nanoparticle Improves T-Cell Activation and Cancer Immunotherapy. Adv. Mater. 2018, 30, 1706098. [Google Scholar] [CrossRef]
- Li, G.; Gao, Y.; Gong, C.; Han, Z.; Qiang, L.; Tai, Z.; Tian, J.; Gao, S. Dual-Blockade Immune Checkpoint for Breast Cancer Treatment Based on a Tumor-Penetrating Peptide Assembling Nanoparticle. ACS Appl. Mater. Interfaces 2019, 11, 39513–39524. [Google Scholar] [CrossRef]
- Faghfuri, E.; Yazdi, M.H.; Mahdavi, M.; Sepehrizadeh, Z.; Faramarzi, M.A.; Mavandadnejad, F.; Shahverdi, A.R. Dose-Response Relationship Study of Selenium Nanoparticles as an Immunostimulatory Agent in Cancer-bearing Mice. Arch. Med. Res. 2015, 46, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Varastehmoradi, B.; Faramarzi, M.A.; Shahverdi, A.R. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: An in vivo study. Biol. Trace Elem. Res. 2012, 149, 22–28. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A.R. The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneim. Forsch. Drug Res. 2012, 62, 525–531. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J. Pharm. Sci. 2013, 21, 33. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, M.H.; Mahdavi, M.; Faghfuri, E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Hassan, Z.M.; Gholami, M.; Shahverdi, A.R. Th1 immune response induction by biogenic selenium nanoparticles in mice with breast cancer: Preliminary vaccine model. Iran. J. Biotechnol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Manna, S.; Kumar, S.; Chattopadhyay, S.; Saha, B.; Roy, S. Immunostimulatory effect of chitosan conjugated green copper oxide nanoparticles in tumor immunotherapy. Cytokine 2020, 127, 154958. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.Y.X.; Jain, P.; Susnjar, A.; Rhudy, J.; Folci, M.; Ballerini, A.; Gilbert, A.; Singh, S.; Bruno, G.; Filgueira, C.S.; et al. Nanofluidic drug-eluting seed for sustained intratumoral immunotherapy in triple negative breast cancer. J. Control. Release 2018, 285, 23–34. [Google Scholar] [CrossRef]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Wen, A.M.; Sheen, M.R.; Fields, J.; Rojanasopondist, P.; Steinmetz, N.F.; Fiering, S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016, 11, 295–303. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef]
- Miao, L.; Wang, Y.; Lin, C.M.; Xiong, Y.; Chen, N.; Zhang, L.; Kim, W.Y.; Huang, L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J. Control. Release 2015, 217, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, D.; Trad, M.; Hanke, N.T.; Larmonier, C.B.; Janikashvili, N.; Bonnotte, B.; Katsanis, E.; Larmonier, N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014, 74, 104–118. [Google Scholar] [CrossRef] [Green Version]
- Patton, D.T.; Garden, O.A.; Pearce, W.P.; Clough, L.E.; Monk, C.R.; Leung, E.; Rowan, W.C.; Sancho, S.; Walker, L.S.K.; Vanhaesebroeck, B.; et al. Cutting Edge: The Phosphoinositide 3-Kinase p110δ Is Critical for the Function of CD4 + CD25 + Foxp3 + Regulatory T Cells. J. Immunol. 2006, 177, 6598–6602. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Steinmetz, N.F. Emerging nanotechnologies for cancer immunotherapy. Exp. Biol. Med. 2016, 241, 1116–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.Y.; Selvan, S.T.; Yang, Y.; Kim, M.J.; Yi, D.K.; Kwon, I.C.; Kim, K. Engineering nanoparticle strategies for effective cancer immunotherapy. Biomaterials 2018, 178, 597–607. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 2015, 220, 141–148. [Google Scholar] [CrossRef]
- Hashemzadeh, N.; Dolatkhah, M.; Adibkia, K.; Aghanejad, A.; Barzegar-Jalali, M.; Omidi, Y.; Barar, J. Recent advances in breast cancer immunotherapy: The promising impact of nanomedicines. Life Sci. 2021, 271, 119110. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–79. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.D.; Nam, G.H.; Kwak, G.; Yang, Y.; Kwon, I.C. Harnessing designed nanoparticles: Current strategies and future perspectives in cancer immunotherapy. Nano Today 2017, 17, 23–37. [Google Scholar] [CrossRef]
- Schmittnaegel, M.; Rigamonti, N.; Kadioglu, E.; Cassará, A.; Rmili, C.W.; Kiialainen, A.; Kienast, Y.; Mueller, H.J.; Ooi, C.H.; Laoui, D.; et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 2017, 9, eaak9670. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, K.; Datta, M.; Hato, T.; Kitahara, S.; Chen, I.X.; Matsui, A.; Kikuchi, H.; Mamessier, E.; Aoki, S.; Ramjiawan, R.R.; et al. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology 2020, 71, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef] [Green Version]
- Shrimali, R.K.; Yu, Z.; Theoret, M.R.; Chinnasamy, D.; Restifo, N.P.; Rosenberg, S.A. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010, 70, 6171–6180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Min, Y.; Rodgers, Z.; Zhang, L.; Wang, A.Z. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1456. [Google Scholar] [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef]
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. miRNA nanotherapeutics for cancer. Drug Discov. Today 2017, 22, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Tyner, K.; Lee, S.; Wolfgang, M.; Li, M.; Zou, P. Physiologically Based Pharmacokinetic (PBPK) Modeling of Pharmaceutical Nanoparticles. AAPS J. 2016, 19, 26–42. [Google Scholar] [CrossRef]
- Sadauskas, E.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Wallin, H. Protracted elimination of gold nanoparticles from mouse liver. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 162–169. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, 2006484. [Google Scholar] [CrossRef]


| Mechanism of Immune Escape as Therapeutic Target | Strategy to Overcome Immunosuppression in the TME | Applications of Nanomedicine to Ameliorate Cancer Immune Escape |
|---|---|---|
| Intrinsic immunosuppression of tumor cells | Inhibition of immunosuppressive cytokines | Nanodelivery of IL-10 protein trap [55] |
| Specific nanodelivery of drugs in the acidic tumor area | Manganese dioxide nanoshells that release Ce6 and Dox under tumor acidic pH [56] Core-shell gold nanocage NPs that release @manganese dioxide under tumor acidic pH [57] | |
| Neutralization of tumor acidity | Nanodelivery of siRNA by cationic lipid-assisted NPs to knockdown lactate dehydrogenase A in tumor cells [58] | |
| Immunosuppressive NK cells within the TME | Promotion of antitumor activity of NK cells | Extracellular vesicles derived from human NK cells pre-exposed to IL-15 [59] |
| Immunosuppressive macrophages within the TME | Repolarization of M2-like TAMs to the M1 phenotype | Nanodelivery of CSF1R and MAPK inhibitors into TAMs [60] Iron oxide NPs (ferumoxytol) targeting TAMs [61] |
| Enhancement of antitumor macrophage activity | Delivery of macrolides into TAMs by colloidal gold nanorods [62] | |
| Immunosuppressive CAFs within the TME | Inactivation of CAFs | Puerarin nanoemulsion [63] Injectable hydrogel carrying losartan [64] |
| Immunosuppressive MDSCs within the TME | Depletion of MDSCs in the TME | Syringeable immunomodulatory multidomain nanogel containing clodronate, GEM and R837 [65] |
| Implantable synthetic immune niche containing GEM and a cancer vaccine [66] | ||
| Liposomal nano-formulation of HER2/neu-derived P5 peptide and PEGylated liposomal Dox [67] | ||
| Dox-polyglycerol-nanodiamond conjugate [68] | ||
| Immunosuppressive Tregs within the TME | Depletion of Tregs | Ursolic acid liposomes [69] |
| Iron-oxide NPs [70] | ||
| Zoledronic acid containing-NPs [71] | ||
| NPs carrying an immunostimulant-invariant natural killer T-cell agonist and a selective inhibitor of the PI3K p110δ isoform [72] | ||
| Impairment of DCs’ activity and antigen presentation | Enhancement of tumor recognition by the induction of ICD of cancer cells | Zn-pyrophosphate shell NPs containing pyrolipid PS [73] |
| Tumor-targeted polypyrrole NP with camptothecin and a near-infrared dye [74] | ||
| Polydopamine nanomedicine that delivers a fluorescent agent and the TLR7/8 agonist R848 [75] | ||
| TME-activatable vesicles carrying OXA prodrug and a PEGylated PS [76] | ||
| Acidity-responsive nanocarrier that releases siCD47 into tumor cells and CCL25 protein in the tumor stroma [77] | ||
| Highly integrated mesoporous silica NPs carrying Dox [78] | ||
| Cancer cell membrane-coated calcium carbonate NPs containing low-dose Dox and Ce6 [79] | ||
| NPs with a superior photothermal conversion efficacy carrying a PS agent and R837 [80] | ||
| Poly(lactic-co-glycolic) acid-NPs that release a photothermal agent together with R837 [81] | ||
| Impairment of DCs’ activity and antigen presentation | Enhancement of tumor recognition by the induction of ICD of cancer cells | Low-molecular-weight heparin-d-α-tocopheryl succinate micelles carrying Dox and R837 [82] Immune nanoconverters carrying R848 and Dox [83] Coated with prodrug hyaluronic acid-Dox nanocores carrying R848 [84] Tumor-specific enhanced oxidative stress polymer conjugate to release QM and generate CA [85] Gold NP-based coat that delivers CpG-ODN and zinc phthalocyanine PS [86] Light-responsive chitosan-coated hollow CuS NPs assembling CpG-ODN [87] |
| Potentiation of DC maturation | Polymeric cooper chelator RPTDH, pH-sensitive NPs carrying R848 [88] | |
| Enhancement of tumor antigen presentation | Peptide-based nanovaccines [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] Gene-based nanovaccines [106,107,108,109] | |
| Impairment of antitumor T-cell response | Potentiation of T-cell activation | Synthetic multivalent antibodies retargeted exosomes (SMART-Exos) expressing on the surface anti-human CD3 and anti-human HER2 antibodies [110] |
| DC-derived exosomes [111] | ||
| Impairment of T-cell inactivation in the TME | IDO-1 inhibition [112,113,114,115,116,117,118,119,120] | |
| PD-1/PD-L1 blockade [121,122,123,124,125,126] | ||
| Promotion of the Th1 response | Selenium NPs as nanovaccines [127,128,129,130,131] | |
| Chitosan-coated green synthesized copper oxide NPs with tumor lysate antigen [132] | ||
| Nanofluidic-based drug eluting seed carrying aOX40 and CD40 monoclonal antibodies [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Ocón, A.; Blaya-Cánovas, J.L.; López-Tejada, A.; Blancas, I.; Sánchez-Martín, R.M.; Garrido, M.J.; Griñán-Lisón, C.; Calahorra, J.; Cara, F.E.; Ruiz-Cabello, F.; et al. Nanomedicine as a Promising Tool to Overcome Immune Escape in Breast Cancer. Pharmaceutics 2022, 14, 505. https://doi.org/10.3390/pharmaceutics14030505
Navarro-Ocón A, Blaya-Cánovas JL, López-Tejada A, Blancas I, Sánchez-Martín RM, Garrido MJ, Griñán-Lisón C, Calahorra J, Cara FE, Ruiz-Cabello F, et al. Nanomedicine as a Promising Tool to Overcome Immune Escape in Breast Cancer. Pharmaceutics. 2022; 14(3):505. https://doi.org/10.3390/pharmaceutics14030505
Chicago/Turabian StyleNavarro-Ocón, Alba, Jose L. Blaya-Cánovas, Araceli López-Tejada, Isabel Blancas, Rosario M. Sánchez-Martín, María J. Garrido, Carmen Griñán-Lisón, Jesús Calahorra, Francisca E. Cara, Francisco Ruiz-Cabello, and et al. 2022. "Nanomedicine as a Promising Tool to Overcome Immune Escape in Breast Cancer" Pharmaceutics 14, no. 3: 505. https://doi.org/10.3390/pharmaceutics14030505
APA StyleNavarro-Ocón, A., Blaya-Cánovas, J. L., López-Tejada, A., Blancas, I., Sánchez-Martín, R. M., Garrido, M. J., Griñán-Lisón, C., Calahorra, J., Cara, F. E., Ruiz-Cabello, F., Marchal, J. A., Aptsiauri, N., & Granados-Principal, S. (2022). Nanomedicine as a Promising Tool to Overcome Immune Escape in Breast Cancer. Pharmaceutics, 14(3), 505. https://doi.org/10.3390/pharmaceutics14030505








