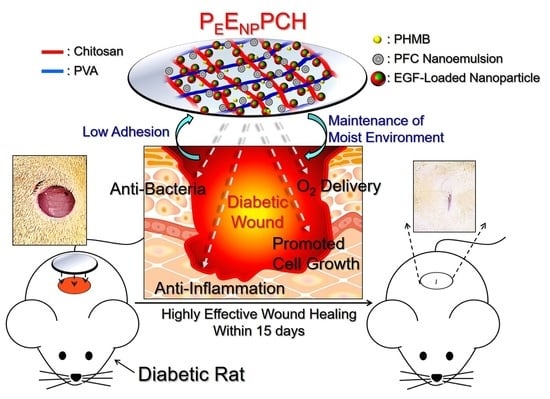
Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Evaluation of PEs and ENPs
2.2. Fabrication of the PEENPPCH
2.3. Evaluation of the Mechanical and Thermal Properties of the PEENPPCH
2.4. Assessment of the Hydration Capability of the PEENPPCH
2.5. Analysis of In Vitro Degradation of the PEENPPCH
2.6. Analyses of the Kinetics of PHMB and EGF Release from the PEENPPCH
2.7. Bacterial Cultivation
2.8. Cell Culture
2.9. Assessment of the Oxygen Delivery Capacity of the PEENPPCH
2.10. Assessment of the Antibacterial Effect and Cytotoxicity of the PEENPPCH In Vitro
2.11. Evaluation of the Anti-Inflammatory Effect of the PEENPPCH In Vitro
2.12. Evaluation of Effect of the PEENPPCH on Cell Growth
2.13. Animal Study
2.14. Histological Study
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the PEs, ENPs, and PEENPPCH
3.2. Mechanical Property, Thermal Property, and Degradation of the PEENPPCH
3.3. Hydration and Drug Release Kinetics of the PEENPPCH
3.4. Oxygen Delivery by the PEENPPCH
3.5. Antibacterial and Cytotoxic Effects of the PEENPPCH In Vitro
3.6. Effect of the PEENPPCH on Promoting Cell Growth
3.7. Anti-Inflammatory Effect of the PEENPPCH
3.8. Diabetic Wound Healing Effect of the PEENPPCH In Vivo
3.9. Histological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Gurney, J.K.; Stanley, J.; York, S.; Rosenbaum, D.; Sarfati, D. Risk of Lower Limb Amputation in a National Prevalent Cohort of Patients with Diabetes. Diabetologia 2018, 61, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortington, L.V.; Geertzen, J.H.; van Netten, J.J.; Postema, K.; Rommers, G.M.; Dijkstra, P.U. Short and Long Term Mortality Rates After a Lower Limb Amputation. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 124–131. [Google Scholar] [CrossRef] [Green Version]
- López-Valverde, M.E.; Aragón-Sánchez, J.; López-de-Andrés, A.; Guerrero-Cedeño, V.; Tejedor-Méndez, R.; Víquez-Molina, G.; Jiménez-García, R. Perioperative and Long-Term All-Cause Mortality in Patients with Diabetes Who Underwent a Lower Extremity Amputation. Diabetes Res. Clin. Pract. 2018, 141, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef]
- Gottrup, F. Oxygen in Wound Healing and Infection. World J. Surg. 2004, 28, 312–315. [Google Scholar] [CrossRef]
- Chen, C.E.; Ko, J.Y.; Fong, C.Y.; Juhn, R.J. Treatment of Diabetic Foot Infection with Hyperbaric Oxygen Therapy. Foot Ankle Surg. 2010, 16, 91–95. [Google Scholar] [CrossRef]
- Kunkemoeller, B.; Kyriakides, T.R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef]
- Huang, H.; Du, W.; Brekken, R.A. Extracellular Matrix Induction of Intracellular Reactive Oxygen Species. Antioxid. Redox Signal. 2017, 27, 774–784. [Google Scholar] [CrossRef]
- Kimmel, H.M.; Grant, A.; Ditata, J. The Presence of Oxygen in Wound Healing. Wounds 2016, 28, 264–270. [Google Scholar]
- Hunt, T.K.; Pai, M.P. The Effect of Varying Ambient Oxygen Tensions on Wound Metabolism and Collagen Synthesis. Surg. Gynecol. Obstet. 1972, 135, 561–567. [Google Scholar] [PubMed]
- Agarwal, V.; Aroor, S.; Gupta, N.; Gupta, A.; Agarwal, N.; Kaur, N. New Technique of Applying Topical Oxygen Therapy as a Cost-Effective Procedure. Indian J. Surg. 2015, 77 (Suppl. S3), 1456–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyboer, M., 3rd; Sharma, D.; Santiago, W.; McCulloch, N. Hyperbaric Oxygen Therapy: Side Effects Defined and Quantified. Adv. Wound Care 2017, 6, 210–224. [Google Scholar] [CrossRef] [Green Version]
- Gordillo, G.M.; Sen, C.K. Evidence-Based Recommendations for the Use of Topical Oxygen Therapy in the Treatment of Lower Extremity Wounds. Int. J. Low Extrem. Wounds 2009, 8, 105–111. [Google Scholar] [CrossRef]
- Lowe, K.C. Perfluorochemical Respiratory Gas Carriers: Benefits to Cell Culture System. J. Fluor. Chem. 2002, 118, 19–26. [Google Scholar] [CrossRef]
- Pilarek, M.; Glazyrina, J.; Neubauer, P. Enhanced Growth and Recombinant Protein Production of Escherichia coli by a Perfluorinated Oxygen Carrier in Miniaturized Fed-Batch Cultures. Microb. Cell Fact. 2011, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Yeh, Y.L.; Lin, K.H.; Hsu, Y.C. Using Fluorochemical as Oxygen Carrier to Enhance the Growth of Marine Microalga Nannochloropsis oculata. Bioprocess Biosyst. Eng. 2013, 36, 1071–1078. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [Green Version]
- Brandl, F.; Kastner, F.; Gschwind, R.M.; Blunk, T.; Tessmar, J.; Göpferich, A. Hydrogel-Based Drug Delivery Systems: Comparison of Drug Diffusivity and Release Kinetics. J. Control. Release 2010, 142, 221–228. [Google Scholar] [CrossRef]
- Grade, S.; Eberhard, J.; Neumeister, A.; Wagener, P.; Winkel, A.; Stiesch, M.; Barcikowski, S. Serum Albumin Reduces the Antibacterial and Cytotoxic Effects of Hydrogel-Embedded Colloidal Silver Nanoparticles. RSC Adv. 2012, 2, 7190–7196. [Google Scholar] [CrossRef]
- Jain, K.K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Fraker, C.A.; Mendez, A.J.; Inverardi, L.; Ricordi, C.; Stabler, C.L. Optimization of Perfluoro Nano-Scale Emulsions: The Importance of Particle Size for Enhanced Oxygen Transfer in Biomedical Applications. Colloids Surf. B Biointerfaces 2012, 98, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kuo, P.W.; Chen, C.J.; Sue, C.J.; Hsu, Y.F.; Pan, M.C. Indocyanine Green-Camptothecin Co-Loaded Perfluorocarbon Double-Layer Nanocomposite: A Versatile Nanotheranostics for Photochemotherapy and FDOT Diagnosis of Breast Cancer. Pharmaceutics 2021, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Liu, Y.; McGuinness, G.B.; Cahill, P.A. Characterization of Poly(vinyl alcohol)/Chitosan Hydrogels as Vascular Tissue Engineering Scaffolds. Macromol. Symp. 2008, 269, 106–110. [Google Scholar] [CrossRef]
- Rana, M.M.; Rahman, M.S.; Ullah, M.A.; Siddika, A.; Hossain, M.L.; Akhter, M.S.; Hasan, M.Z.; Asaduzzaman, S.M. Amnion and collagen-based blended hydrogel improves burn healing efficacy on a rat skin wound model in the presence of wound dressing biomembrane. Biomed. Mater. Eng. 2020, 31, 1–17. [Google Scholar] [CrossRef]
- Koosha, M.; Aalipour, H.; Sarraf Shirazi, M.J.; Jebali, A.; Chi, H.; Hamedi, S.; Wang, N.; Li, T.; Moravvej, H. Physically Crosslinked Chitosan/PVA Hydrogels Containing Honey and Allantoin with Long-Term Biocompatibility for Skin Wound Repair: An In Vitro and In Vivo Study. J. Funct. Biomater. 2021, 12, 61. [Google Scholar] [CrossRef]
- Knapp, J.L.; González-Pinzón, R.; Haggerty, R. The resazurin-resorufin system: Insights from a decade of “smart” tracer development for hydrologic applications. Water Resour. Res. 2018, 54, 6877–6889. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef]
- Hsiao, K.H.; Huang, C.M.; Lee, Y.H. Development of Rifampicin-Indocyanine Green-Loaded Perfluorocarbon Nanodroplets for Photo-Chemo-Probiotic Antimicrobial Therapy. Front. Pharmacol. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Lee, Y.H.; Tuyet, P.T. Synthesis and biological evaluation of quercetin-zinc (II) complex for anti-cancer and anti-metastasis of human bladder cancer cells. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In vivo evaluation of the wound healing properties of bio-nanofiber chitosan/polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.D.; Thomas, V.S.; Black, A.L.; Verba, T.; Lesicko, J.G.; Amini, R. Pressure-Induced Microstructural Changes in Porcine Tricuspid Valve Leaflets. Acta Biomater. 2018, 67, 248–258. [Google Scholar] [CrossRef]
- Lewandowska, K. Miscibility and Thermal Stability of Poly(vinyl alcohol)/Chitosan Mixture. Thermochim. Acta 2009, 493, 42–48. [Google Scholar] [CrossRef]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and Chemical Characterization of Poly(hexamethylene biguanide) Hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Yang, J.M.; Su, W.Y.; Leu, T.L.; Yang, M.C. Evaluation of Chitosan/PVA Blended Hydrogel Membrances. J. Membr. Sci. 2004, 236, 39–51. [Google Scholar] [CrossRef]
- Bae, J.Y.; Jung, J.I.; Seo, S.J.; Bae, B.S. Formation and Thermal-Induced Changes of Mesostructures in Fluorinated Organosilicate Films. Micropor. Mesopor. Mat. 2007, 98, 283–291. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; Mansoori Moghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Sánchez-Díaz, J.C.; Becerra, F.; Cruz-Barba, L.E.; González Álvarez, A. Swelling Characterization and Drug Delivery Kinetics of Polyacrylamide-Coitaconic acid/Chitosan Hydrogels. Express Polym. Lett. 2009, 3, 25–32. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Davydova, V.N.; Kalitnik, A.A.; Markov, P.A.; Volod’ko, A.V.; Popov, S.V.; Ermak, I.M. Cytokine-Inducing and Anti-Inflammatory Activity of Chitosan and Its Low Molecular Derivative. Appl. Biochem. Microbiol. 2016, 52, 476–482. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Lin, S.-J. Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation. Pharmaceutics 2022, 14, 537. https://doi.org/10.3390/pharmaceutics14030537
Lee Y-H, Lin S-J. Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation. Pharmaceutics. 2022; 14(3):537. https://doi.org/10.3390/pharmaceutics14030537
Chicago/Turabian StyleLee, Yu-Hsiang, and Sheng-Jhe Lin. 2022. "Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation" Pharmaceutics 14, no. 3: 537. https://doi.org/10.3390/pharmaceutics14030537
APA StyleLee, Y.-H., & Lin, S.-J. (2022). Chitosan/PVA Hetero-Composite Hydrogel Containing Antimicrobials, Perfluorocarbon Nanoemulsions, and Growth Factor-Loaded Nanoparticles as a Multifunctional Dressing for Diabetic Wound Healing: Synthesis, Characterization, and In Vitro/In Vivo Evaluation. Pharmaceutics, 14(3), 537. https://doi.org/10.3390/pharmaceutics14030537