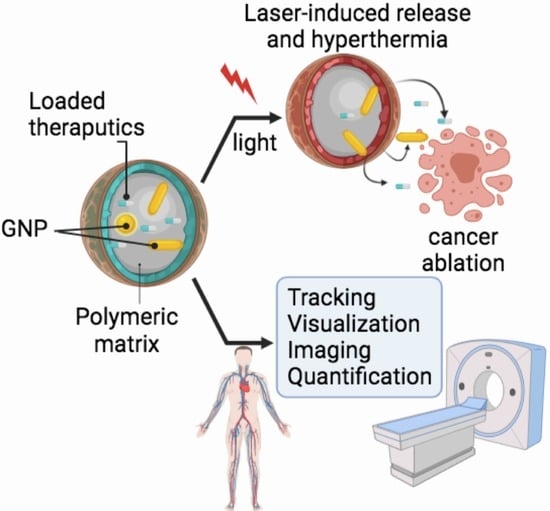
PLGA-Gold Nanocomposite: Preparation and Biomedical Applications
Abstract
:1. Introduction
- (1)
- Discuss the outstanding properties of gold nanoprobes to justify their use;
- (2)
- Discuss current available methods for gold nanoprobe’s surface functionalization and subsequent encapsulation into polymeric carriers;
- (3)
- Provide examples from the research work of our own and other groups on how labeling with gold nanoprobes allows for the precise tracking, visualization and quantification of pharmaceutical polymeric nanocarriers as well as the fabrication of laser-responsive drug delivery systems as summarized in Figure 1.
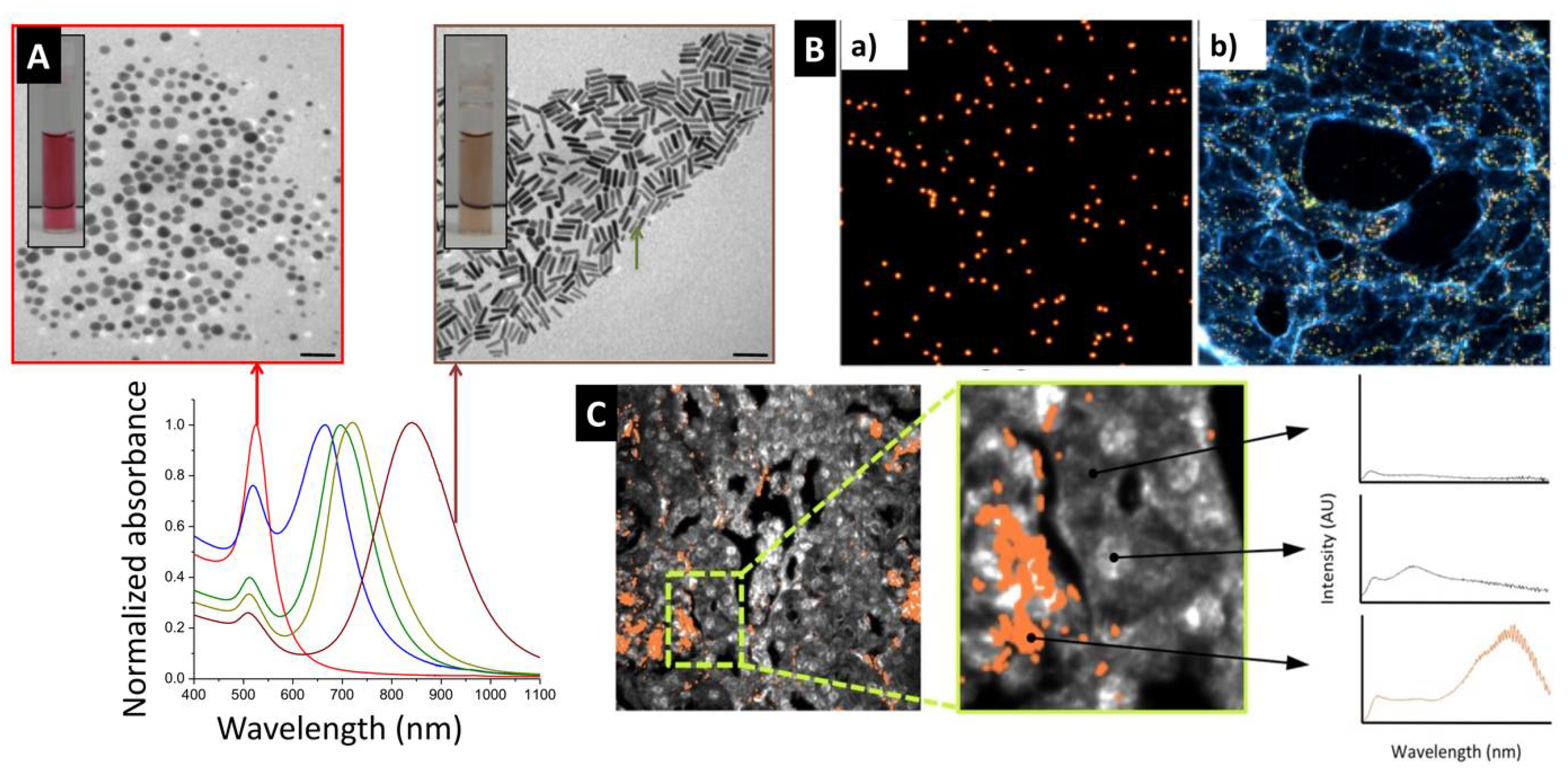
2. Brilliant Optical Extinction of Encapsulated GNP Enables Outstanding Sensing and Tracking Capabilities
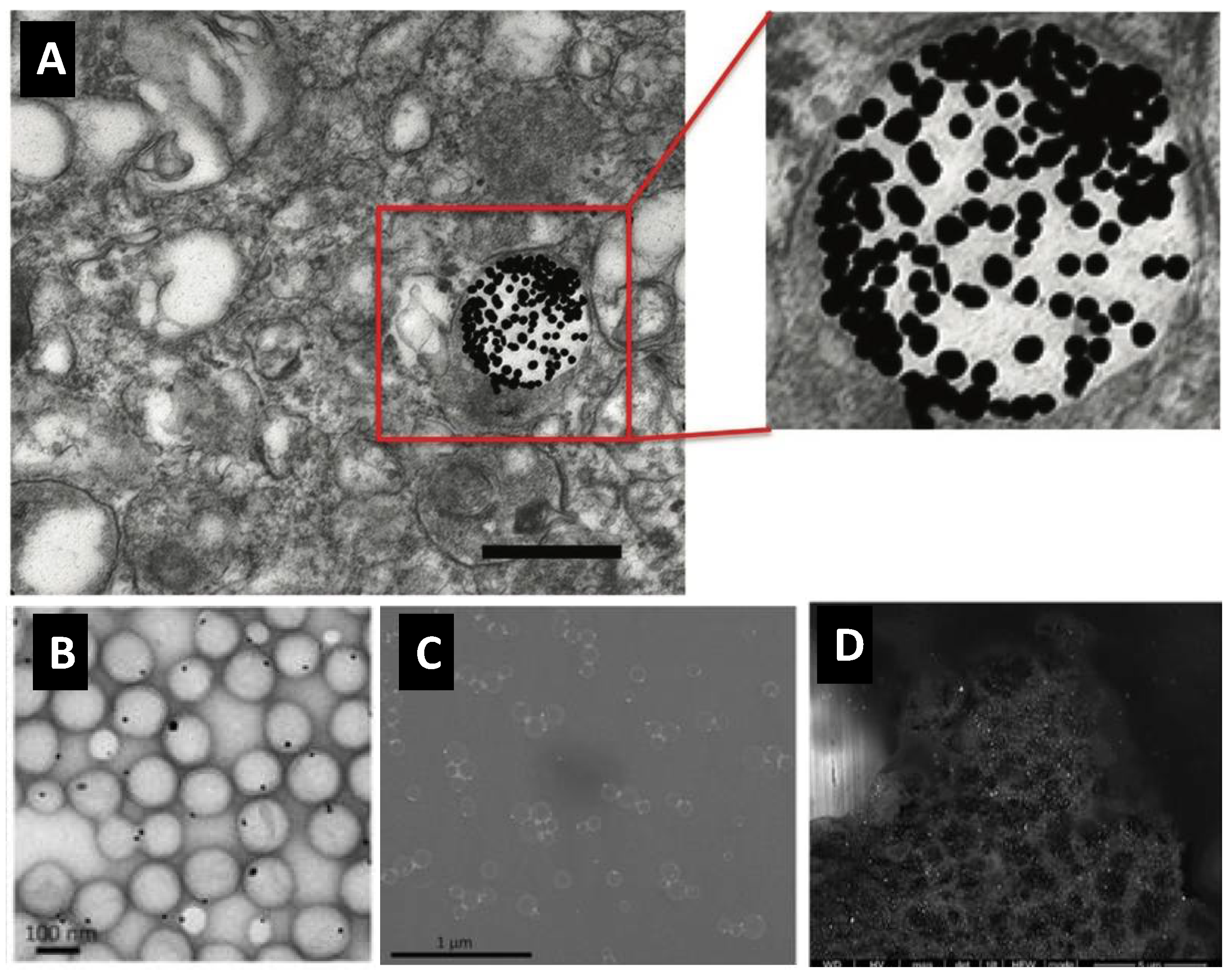
3. Gold Nanoprobes Enables Electron Microscopy-Based High Spatial Resolution Imaging
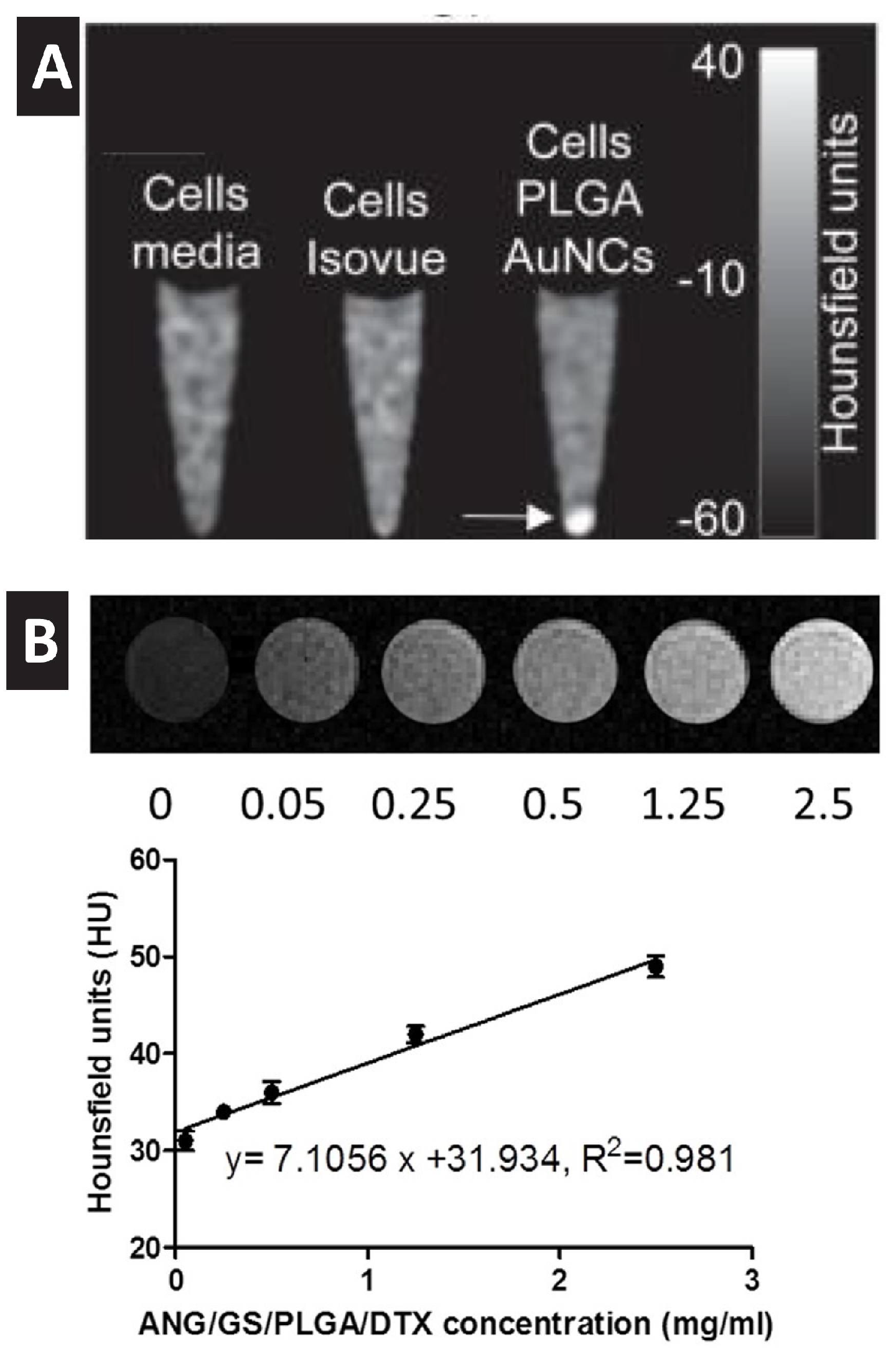
4. Gold Nanoprobes Enables Computed Tomography Imaging
5. Gold Nanoprobes Enable Mass Spectrometry-Based Quantification
6. Labeling Polymeric Nanocarriers with Gold Nanoprobes as Raman Active Tags
7. Encapsulation Approaches and Surface Functionalization of Gold Nanoparticles
8. Doping Polymeric Nanotherapeutics with NIR-Absorbing Gold Nanoparticles: Novel Drug Delivery Systems
9. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page Faulk, W.; Malcolm Taylor, G. Communication to the editors: An immunocolloid method for the electron microscope. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Montón, H.; Nogués, C.; Rossinyol, E.; Castell, O.; Roldán, M. QDs versus Alexa: Reality of promising tools for immunocytochemistry. J. Nanobiotechnol. 2009, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indoria, S.; Singh, V.; Hsieh, M.F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2011, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Alkilany, A.M.; Lohse, S.E.; Murphy, C.J. The Gold Standard: Gold Nanoparticle Libraries To Understand the Nano-Bio Interface. Acc. Chem. Res. 2013, 46, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Chen, K.C.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Stone, J.W.; Sisco, P.N.; Alkilany, A.; Kinard, B.E.; Hankins, P. Chemical sensing and imaging with metallic nanorods. Chem. Commun. 2008, 8, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Vaitukaitis, J.L. Development of the home pregnancy test. Ann. N. Y. Acad. Sci. 2004, 1038, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Huang, C.; Shi, F.-J.; Zeng, X.-Y.; Lu, T.; Ding, S.-N.; Jiao, Y.-J. Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst 2020, 145, 5345–5352. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Stoeva, S.I.; Mirkin, C.A. Bio-Bar-Code-Based DNA Detection with PCR-like Sensitivity. J. Am. Chem. Soc. 2004, 126, 5932–5933. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Perez, P.; Tsoutsi, D.; Xu, R.; Rivera Gil, P. Hyperspectral-Enhanced Dark Field Microscopy for Single and Collective Nanoparticle Characterization in Biological Environments. Materials 2018, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- SoRelle, E.D.; Liba, O.; Campbell, J.L.; Dalal, R.; Zavaleta, C.L.; de la Zerda, A. A hyperspectral method to assay the microphysiological fates of nanomaterials in histological samples. eLife 2016, 5, e16352. [Google Scholar] [CrossRef]
- Wang, Y.; Pasternak, M.; Sathiyamoorthy, K.; Kolios, M.C. Anti-HER2 PLGA-PEG polymer nanoparticle containing gold nanorods and paclitaxel for laser-activated breast cancer detection and therapy. Biomed. Opt. Express 2021, 12, 2171–2185. [Google Scholar] [CrossRef] [PubMed]
- Meir, R.; Popovtzer, R. Cell tracking using gold nanoparticles and computed tomography imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1480. [Google Scholar] [CrossRef] [PubMed]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qiang, S.; Wang, L. Gold Nanomaterials for Imaging-Guided Near-Infrared in vivo Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.; Jung, S.Y.; Lee, S.J. Gold nanoparticle contrast agents in advanced X-ray imaging technologies. Molecules 2013, 18, 5858–5890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjug. Chem. 2020, 31, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Orlov, I.; Schertel, A.; Zuber, G.; Klaholz, B.; Drillien, R.; Weiss, E.; Schultz, P.; Spehner, D. Live cell immunogold labelling of RNA polymerase II. Sci. Rep. 2015, 5, 8324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilany, A.M.; Abulateefeh, S.R.; Murphy, C.J. Facile Functionalization of Gold Nanoparticles with PLGA Polymer Brushes and Efficient Encapsulation into PLGA Nanoparticles: Toward Spatially Precise Bioimaging of Polymeric Nanoparticles. Part. Part. Syst. Charact. 2019, 36, 1800414. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Sebastian, V.; Szczupak, B.; Imbuluzqueta, E.; Llop, J.; Blanco Prieto, M.J. Visualization of hybrid gold-loaded polymeric nanoparticles in cells using scanning electron microscopy. J. Drug Deliv. Sci. Technol. 2017, 42, 315–320. [Google Scholar] [CrossRef]
- Abstiens, K.; Fleischmann, D.; Gregoritza, M.; Goepferich, A.M. Gold-Tagged Polymeric Nanoparticles with Spatially Controlled Composition for Enhanced Detectability in Biological Environments. ACS Appl. Nano Mater. 2019, 2, 917–926. [Google Scholar] [CrossRef]
- Taghavi, H.; Bakhshandeh, M.; Montazerabadi, A.; Nazari Moghadam, H.; Mazloom Shahri, S.B.; Keshtkar, M. Comparison of Gold Nanoparticles and Iodinated Contrast Media in Radiation Dose Reduction and Contrast Enhancement in Computed Tomography. Iran. J. Radiol. 2020, 17, e92446. [Google Scholar] [CrossRef] [Green Version]
- Mieszawska, A.J.; Gianella, A.; Cormode, D.P.; Zhao, Y.; Meijerink, A.; Langer, R.; Farokhzad, O.C.; Fayad, Z.A.; Mulder, W.J. Engineering of lipid-coated PLGA nanoparticles with a tunable payload of diagnostically active nanocrystals for medical imaging. Chem. Commun. 2012, 48, 5835–5837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Zhang, B.; Zheng, C.; Ji, R.; Ren, X.; Guo, F.; Sun, S.; Shi, J.; Zhang, H.; Zhang, Z.; et al. The tumor-targeting core–shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J. Control. Rel. 2015, 220, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, T.; Motiei, M.; Romman, Z.; Popovtzer, A.; Popovtzer, R. Targeted gold nanoparticles enable molecular CT imaging of cancer: An in vivo study. Int. J. Nanomed. 2011, 6, e64. [Google Scholar]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Lee, N.; Arifin, D.R.; Shats, I.; Janowski, M.; Walczak, P.; Hyeon, T.; Bulte, J.W.M. In Vivo Micro-CT Imaging of Human Mesenchymal Stem Cells Labeled with Gold-Poly-L-Lysine Nanocomplexes. Adv. Funct. Mater. 2017, 27, 1604213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allabashi, R.; Stach, W.; de la Escosura-Muñiz, A.; Liste-Calleja, L.; Merkoçi, A. ICP-MS: A powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J. Nanoparticle Res. 2008, 11, 2003. [Google Scholar] [CrossRef]
- Wang, H.; Chen, B.; He, M.; Li, X.; Chen, P.; Hu, B. Study on uptake of gold nanoparticles by single cells using droplet microfluidic chip-inductively coupled plasma mass spectrometry. Talanta 2019, 200, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-N.; Feng, L.-X.; Shi, J.-W.; Chen, H.-Q.; Wang, B.; Wang, M.; Wang, H.-F.; Feng, W.-Y. Single-Cell Isotope Dilution Analysis with LA–ICP–MS: A New Approach for Quantification of Nanoparticles in Single Cells. Anal. Chem. 2020, 92, 14339–14345. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Traub, H.; Jakubowski, N.; Drescher, D.; Baranov, V.I.; Kneipp, J. Trends in single-cell analysis by use of ICP-MS. Anal. Bioanal. Chem. 2014, 406, 6963–6977. [Google Scholar] [CrossRef] [PubMed]
- Theiner, S.; Loehr, K.; Koellensperger, G.; Mueller, L.; Jakubowski, N. Single-cell analysis by use of ICP-MS. J. Anal. At. Spectrom. 2020, 35, 1784–1813. [Google Scholar] [CrossRef]
- Meermann, B.; Nischwitz, V. ICP-MS for the analysis at the nanoscale—A tutorial review. J. Anal. At. Spectrom. 2018, 33, 1432–1468. [Google Scholar] [CrossRef]
- Ermolenko, Y.; Gorunova, O.N.; Dunina, V.V.; Petrenko, D.B.; Novikova, N.G.; Alekseeva, A.; Osipova, N.; Kochetkov, K.A.; Morozov, A.; Gelperina, S. Quantitative analysis of palladacycle-tagged PLGA nanoparticle biodistribution in rat organs by means of atomic absorption spectrometry and inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2021, 36, 2423–2430. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Toro-González, M.; Keever, T.J.; McKnight, T.E.; Davern, S.M. Poly(lactic-co-glycolic acid) Nanoparticles as Delivery Systems for the Improved Administration of Radiotherapeutic Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 10565–10570. [Google Scholar] [CrossRef]
- Lenzi, E.; de Aberasturi, D.J.; Liz-Marzan, L.M. Surface-Enhanced Raman Scattering Tags for Three-Dimensional Bioimaging and Biomarker Detection. ACS Sens. 2019, 4, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, C.; Xu, M.X.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Single-Molecule Surface-Enhanced Raman Spectroscopy. Annu. Rev. Phys. Chem. 2012, 63, 65–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Wang, Y.; Reder, N.P.; Liu, J.T.C. Multiplexed Molecular Imaging of Biomarker-Targeted SERS Nanoparticles on Fresh Tissue Specimens with Channel-Compressed Spectrometry. PLoS ONE 2016, 11, e0163473. [Google Scholar] [CrossRef] [PubMed]
- Menon, H.; Yadav, A.; Subramanian, P.; Sengar, M.; Rath, S.; Kavathiya, K.; Gota, V. Pharmacokinetic and Pharmacodynamic Properties of a Biosimilar Rituximab (Reditux®) Are Identical to the Innovator Brand MabThera®–Experience from a Tertiary Cancer Centre in Western India. Blood 2014, 124, 2246. [Google Scholar] [CrossRef]
- Zavaleta, C.L.; Garai, E.; Liu, J.T.C.; Sensarn, S.; Mandella, M.J.; Van de Sompel, D.; Friedland, S.; Van Dam, J.; Contag, C.H.; Gambhir, S.S. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc. Natl. Acad. Sci. USA 2013, 110, e2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendrell, M.; Maiti, K.K.; Dhaliwal, K.; Chang, Y.T. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013, 31, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Peng, X.-H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Strozyk, M.S.; Jimenez de Aberasturi, D.; Liz-Marzán, L.M. Composite Polymer Colloids for SERS-Based Applications. Chem. Rec. 2018, 18, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Strozyk, M.S.; de Aberasturi, D.J.; Gregory, J.V.; Brust, M.; Lahann, J.; Liz-Marzán, L.M. Spatial Analysis of Metal–PLGA Hybrid Microstructures Using 3D SERS Imaging. Adv. Funct. Mater. 2017, 27, 1701626. [Google Scholar] [CrossRef] [Green Version]
- Alkilany, A.M.; Alsotari, S.; Alkawareek, M.Y.; Abulateefeh, S.R. Facile Hydrophobication of Glutathione-Protected Gold Nanoclusters and Encapsulation into Poly(lactide-co-glycolide) Nanocarriers. Sci. Rep. 2019, 9, 11098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque-Michel, E.; Larrea, A.; Lahuerta, C.; Sebastian, V.; Imbuluzqueta, E.; Arruebo, M.; Blanco-Prieto, M.J.; Santamaría, J. A simple approach to obtain hybrid Au-loaded polymeric nanoparticles with a tunable metal load. Nanoscale 2016, 8, 6495–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Parak, W. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.A.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Stability of fluorescent labels in PLGA polymeric nanoparticles: Quantum dots versus organic dyes. Int. J. Pharm. 2015, 494, 471–478. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.; Kang, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Zhu, C.; Yin, H.; Jiang, M.; Zhang, J.; Qin, B.; Luo, Z.; Yuan, X.; Yang, J.; Li, W.; et al. Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for the Treatment of Metastatic Tumors. ACS Nano 2018, 12, 7647–7662. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, J.; Zhu, C.; Jiang, M.; Guo, X.; Li, W.; Yin, X.; Yin, H.; Qin, B.; Yuan, X.; et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J. Control. Release 2018, 278, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Dias, D.R.; Costa, E.C.; Correia, I.J. Thermo- and pH-responsive nano-in-micro particles for combinatorial drug delivery to cancer cells. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 104, 42–51. [Google Scholar] [CrossRef] [PubMed]

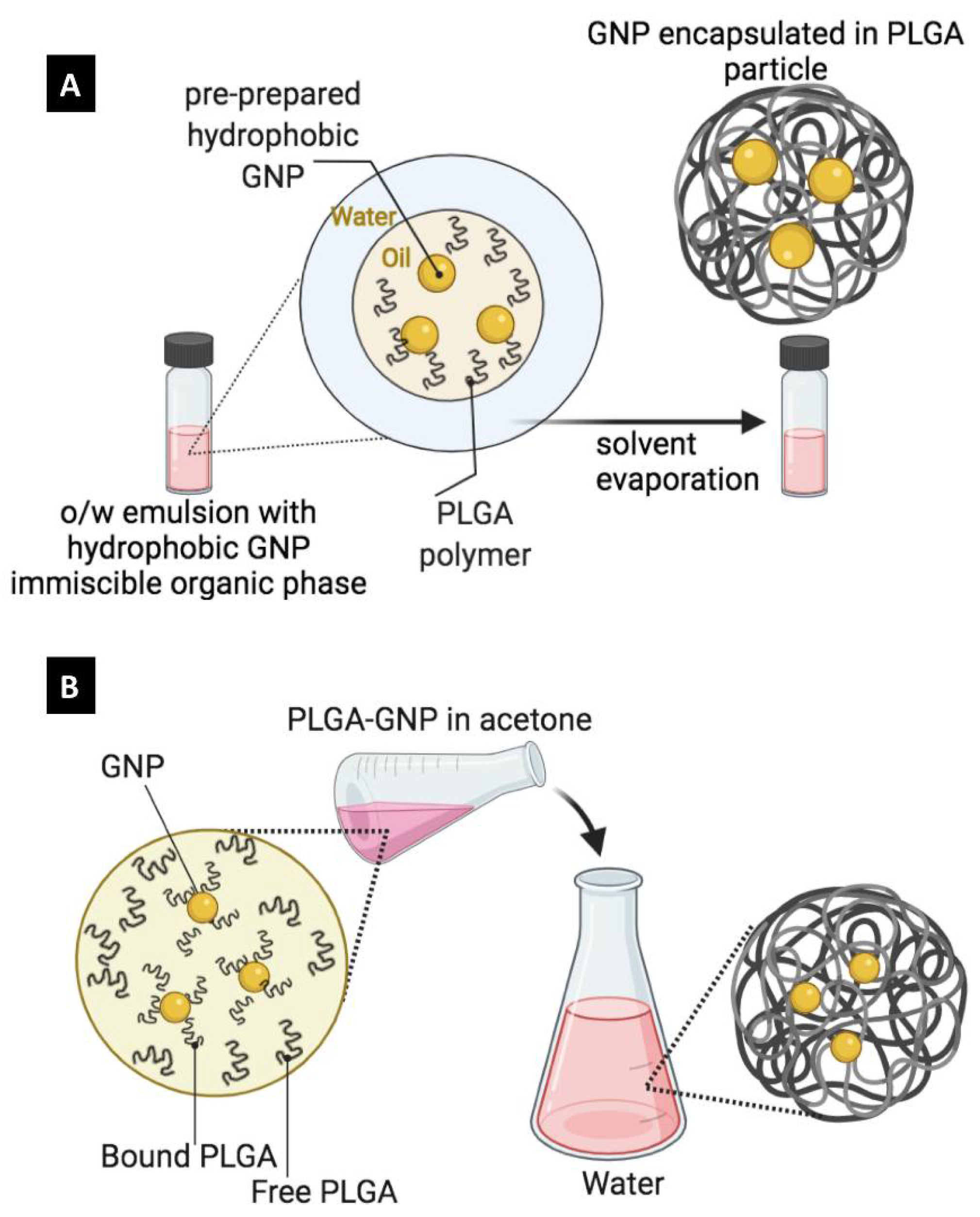

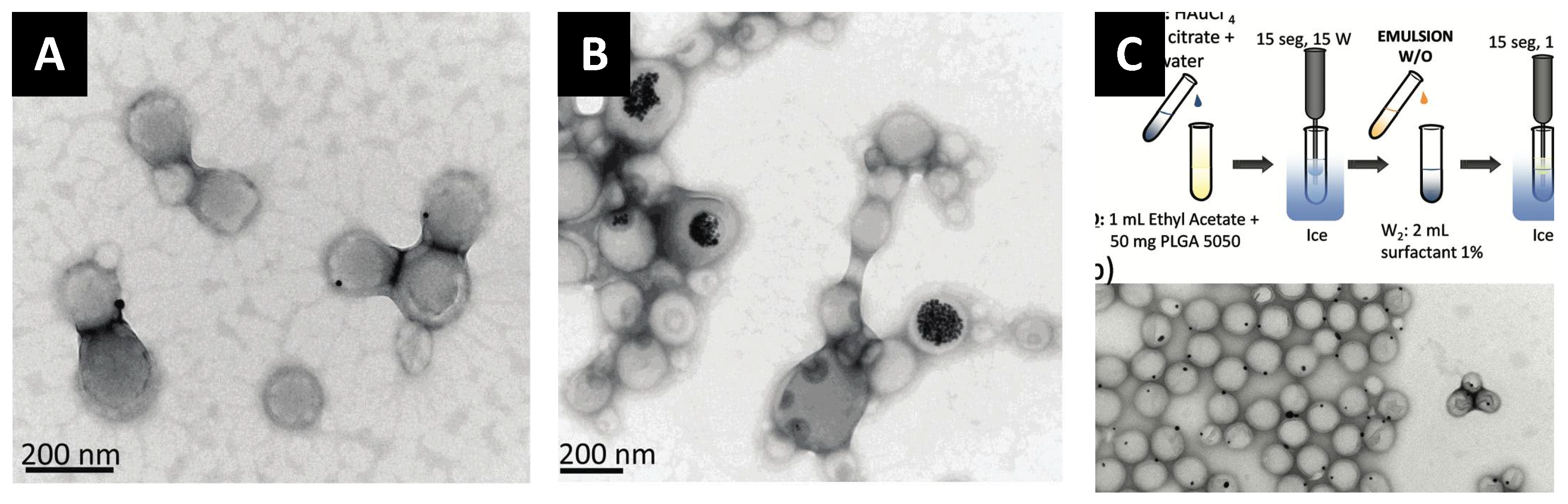

| Emulsion-Evaporation | Nanoprecipitation |
|---|---|
| Longer and more complex process | Shorter and simpler process |
| Requires mechanical emulsification to form a stable emulsion | Simple mixing |
| GNP can be hydrophilic (w/o/w emulsion) or hydrophobic (o/w) | GNP should be hydrophobic |
| Hydrophobic GNP should be dissolved in immiscible organic solvents (DCM, chloroform ethyl acetate) | Hydrophobic GNP should be dissolved in miscible organic solvents (acetone, DMSO, DMF, THF) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkilany, A.M.; Rachid, O.; Alkawareek, M.Y.; Billa, N.; Daou, A.; Murphy, C.J. PLGA-Gold Nanocomposite: Preparation and Biomedical Applications. Pharmaceutics 2022, 14, 660. https://doi.org/10.3390/pharmaceutics14030660
Alkilany AM, Rachid O, Alkawareek MY, Billa N, Daou A, Murphy CJ. PLGA-Gold Nanocomposite: Preparation and Biomedical Applications. Pharmaceutics. 2022; 14(3):660. https://doi.org/10.3390/pharmaceutics14030660
Chicago/Turabian StyleAlkilany, Alaaldin M., Ousama Rachid, Mahmoud Y. Alkawareek, Nashiru Billa, Anis Daou, and Catherine J. Murphy. 2022. "PLGA-Gold Nanocomposite: Preparation and Biomedical Applications" Pharmaceutics 14, no. 3: 660. https://doi.org/10.3390/pharmaceutics14030660
APA StyleAlkilany, A. M., Rachid, O., Alkawareek, M. Y., Billa, N., Daou, A., & Murphy, C. J. (2022). PLGA-Gold Nanocomposite: Preparation and Biomedical Applications. Pharmaceutics, 14(3), 660. https://doi.org/10.3390/pharmaceutics14030660