Modelling the Compaction Step of a Platform Direct Compression Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation Design
2.2. Formulation Characterisation
2.3. Tablet Production
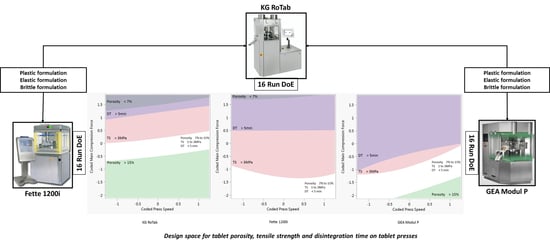
2.4. Design of Experiment
2.5. Measurement of Tablet Properties
2.6. Data Analysis
3. Results
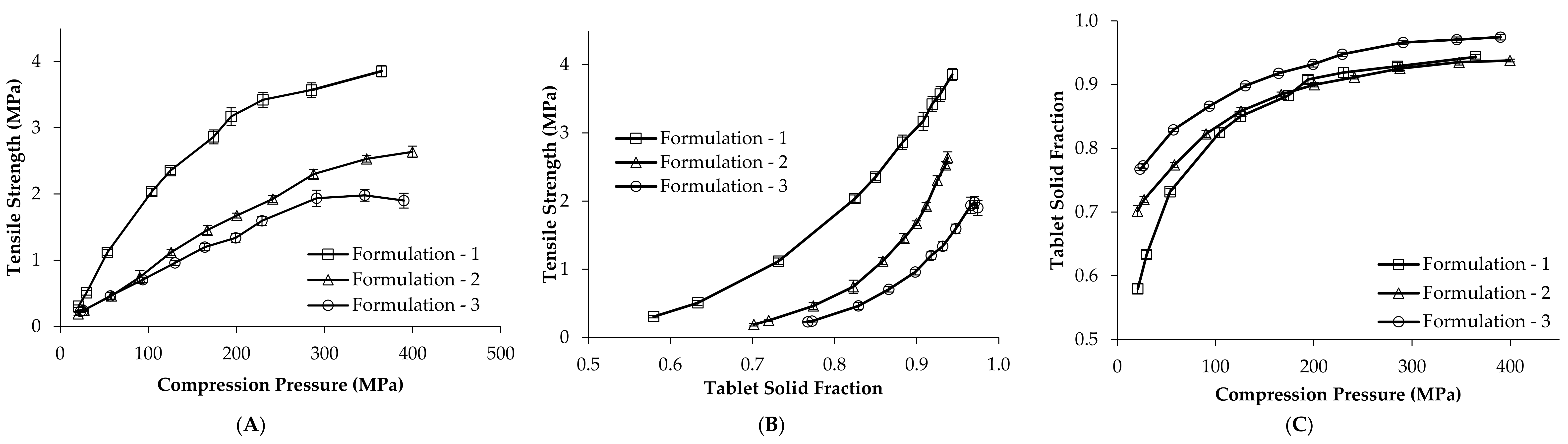
3.1. Deformation Behaviour of Placebo Blends
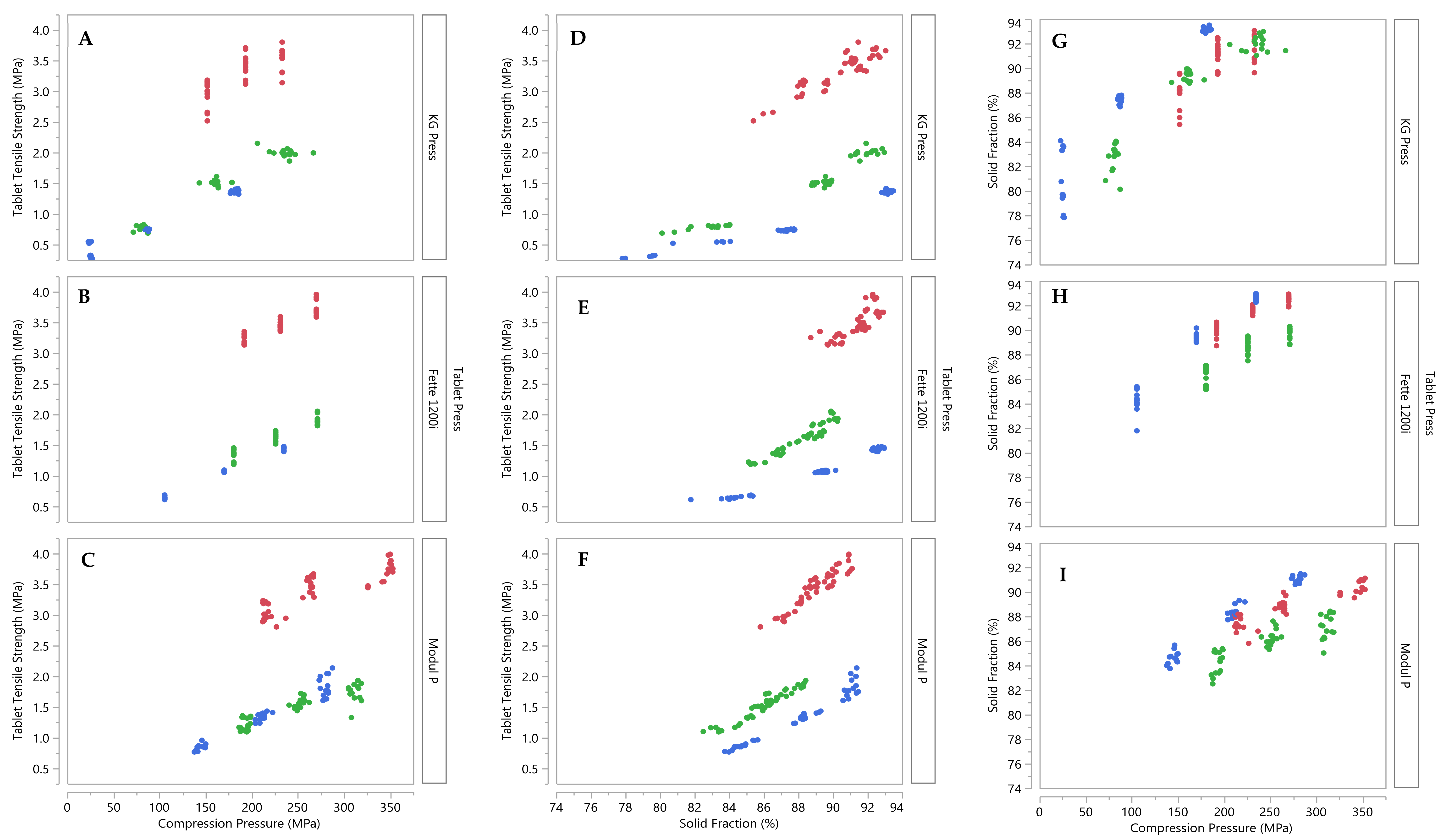
3.2. Development of Tablet Tensile Strength, Porosity and Disintegration Time Models
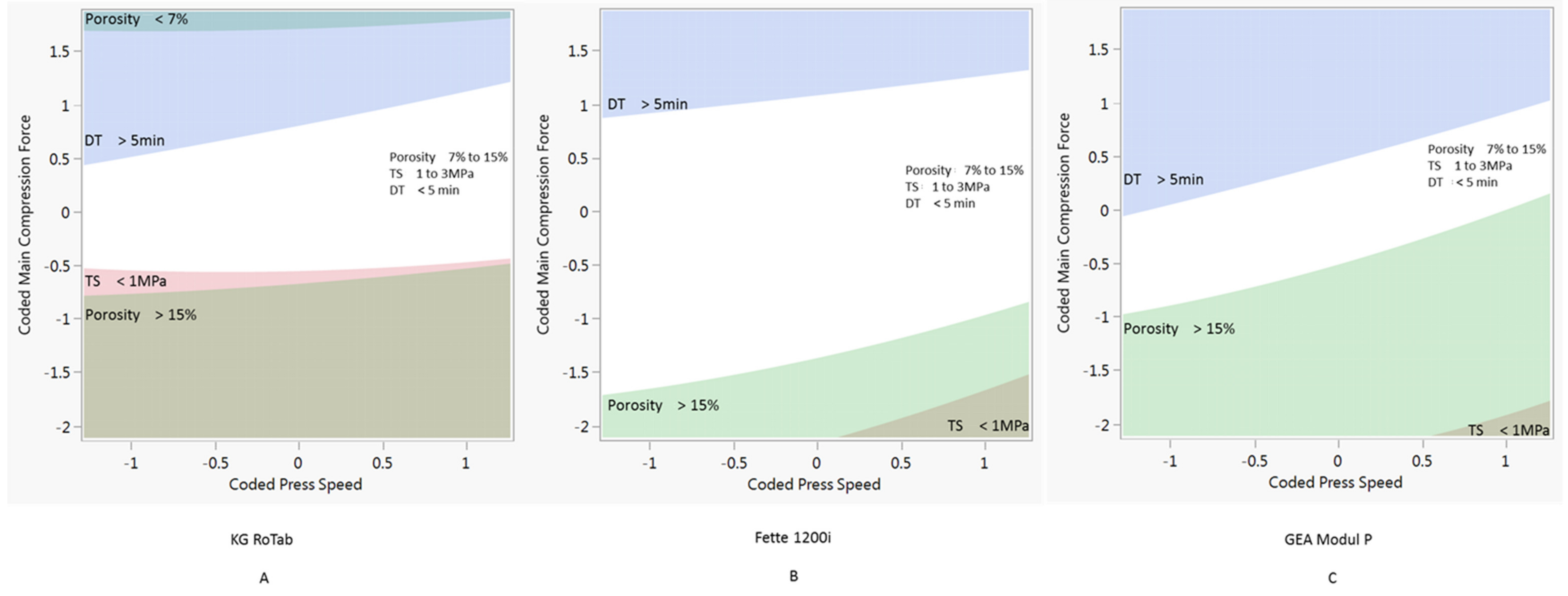
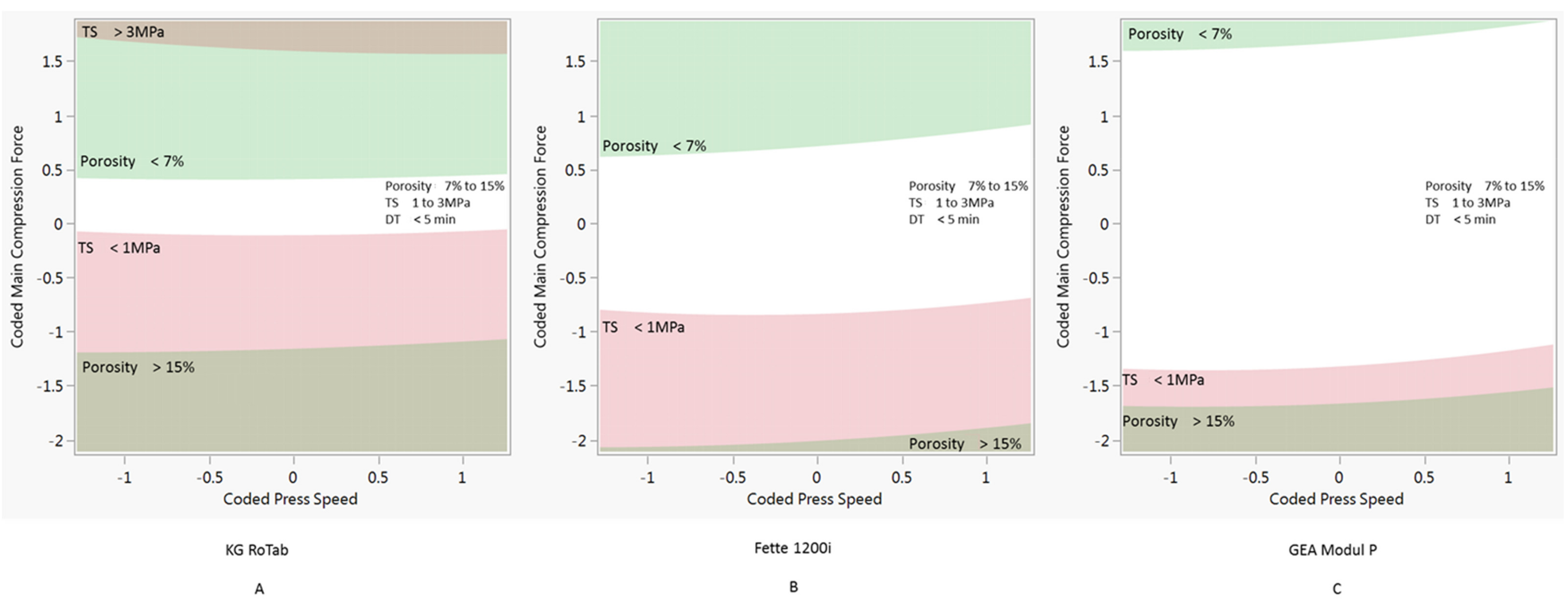
3.3. Design Space
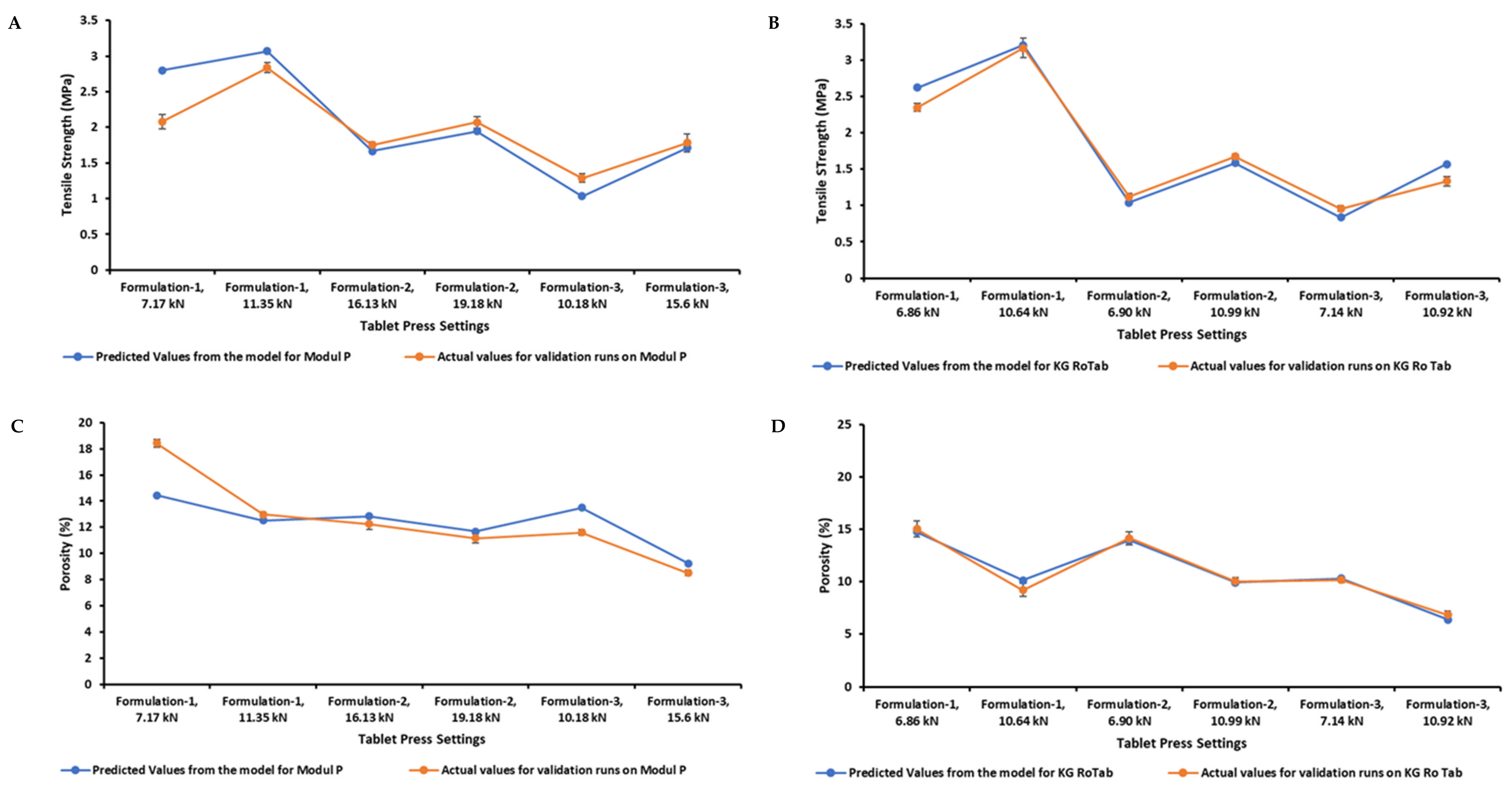
3.4. Tensile Strength and Porosity Model Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, N.L.; Schomberg, A.K.; Finke, J.H.; Abraham, T.G.; Kwade, A.; Herrmann, C. Process Modeling and Simulation of Tableting—An Agent-Based Simulation Methodology for Direct Compression. Pharmaceutics 2021, 13, 996. [Google Scholar] [CrossRef]
- Peddapatla, R.V.G.; Sheridan, G.; Slevin, C.; Swaminathan, S.; Browning, I.; Reilly, C.O.; Worku, Z.A.; Egan, D.; Sheehan, S.; Crean, A.M. Process Model Approach to Predict Tablet Weight Variability for Direct Compression Formulations at Pilot and Production Scale. Pharmaceutics 2021, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Scicolone, J.; Snee, R.D.; Kumar, A.; Bertels, J.; Cappuyns, P.; Van Assche, I.; Cuitiño, A.M.; Muzzio, F. Prediction of tablet weight variability in continuous manufacturing. Int. J. Pharm. 2020, 575, 118727. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; De Beer, T.; Vervaet, C.; Remon, J.P. Reduction of tablet weight variability by optimizing paddle speed in the forced feeder of a high-speed rotary tablet press. Drug Dev. Ind. Pharm. 2015, 41, 530–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry; INDs for Phase 2 and Phase 3 Studies Chemistry, Manufacturing, and Controls Information. May 2003. Available online: https://www.fda.gov/media/70822/download (accessed on 22 December 2021).
- Sanderson, S.; Uzemeri, M. The Innovation Imperative: Strategies for Managing Product Models and Families; Irwin: Chicago, IL, USA, 1997. [Google Scholar]
- Gronemeyer, P.; Ditz, R.; Strube, J. Trends in Upstream and Downstream Process Development for Antibody Manufacturing. Bioengineering 2014, 1, 188–212. [Google Scholar] [CrossRef] [Green Version]
- Sen, M.; García Muñoz, S. Development and implementation of a hybrid scale up model for a batch high shear wet granulation operation. AIChE J. 2021, 67, e17183. [Google Scholar] [CrossRef]
- Van den Ban, S.; Pitt, K.G.; Whiteman, M. Application of a tablet film coating model to define a process-imposed transition boundary for robust film coating. Pharm. Dev. Technol. 2018, 23, 176–182. [Google Scholar] [CrossRef]
- Toson, P.; Lopes, D.G.; Paus, R.; Kumar, A.; Geens, J.; Stibale, S.; Quodbach, J.; Kleinebudde, P.; Hsiao, W.-K.; Khinast, J. Model-based approach to the design of pharmaceutical roller-compaction processes. Int. J. Pharm-X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Dall Agnol, R. The Manufacturing Classification System McS Working Group. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression physics in the formulation development of tablets. Crit. Rev. Ther. Drug Carrier Syst. 2006, 23, 1–65. [Google Scholar] [CrossRef]
- Zimmermann, M.; Schaldach, G.; Thommes, M.; Bauer-Brandl, A. Comparison of the Performance of Single Punch and Rotary Tablet Presses from Different Vendors. TechnoPharm 2018, 8, 96–103. [Google Scholar]
- Narang, A.S.; Rao, V.M.; Guo, H.; Lu, J.; Desai, D.S. Effect of force feeder on tablet strength during compression. Int. J. Pharm. 2010, 401, 7–15. [Google Scholar] [CrossRef]
- Leuenberger, H.; Ineichen, L. Percolation theory and physics of compression. Eur. J. Pharm. Biopharm. 1997, 44, 269–272. [Google Scholar] [CrossRef]
- Wang, J.; Wen, H.; Desai, D. Lubrication in tablet formulations. Eur. J. Pharm. Biopharm. 2010, 75, 1–15. [Google Scholar] [CrossRef]
- Pitt, K.; Pitt, K.G.; Heasley, M.G. Determination of the tensile strength of elongated tablets. Powder Technol. 2016, 238, 169–175. [Google Scholar] [CrossRef]
- Aburub, A.; Mishra, D.; Buckner, I. Use of Compaction Energetics for Understanding Particle Deformation Mechanism. Pharm. Dev. Technol. 2007, 124, 405–414. [Google Scholar] [CrossRef]
- Brady, J.E.; Dürig, T.; Shang, S.S. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms; Yihong, Q., Yisheng, C., Geoff, G.Z.Z., Lirong, L., William, P., Eds.; Academic: Cambridge, MA, USA, 2009; pp. 187–217. ISBN 9780444532428. [Google Scholar]
- Sun, C.; Grant, D.J.W. Influence of Elastic Deformation of Particles on Heckel Analysis. Pharm. Dev. Technol. 2001, 6, 193–200. [Google Scholar] [CrossRef]
- Joiris, E.; Di Martino, P.; Berneron, C.; Guyot-Hermann, A.; Guyot, J. Compression Behavior of Orthorhombic Paracetamol. Pharm. Res. 1998, 15, 1122–1130. [Google Scholar] [CrossRef]
- Tye, C.K.; Sun, C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Heckel, R.W. Density-Pressure Relationships in Powder Compaction. Trans. Met. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Wünsch, I.; Friesen, I.; Puckhaber, D.; Schlegel, T.; Finke, J.H. Scaling Tableting Processes from Compaction Simulator to Rotary Presses—Mind the Sub-Processes. Pharmaceutics 2020, 12, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Placebo Blend Composition (% w/w) | ||||
|---|---|---|---|---|
| Components | Function | Formulation 1 Plastic | Formulation 2 Elastic | Formulation 3 Brittle |
| Microcrystalline cellulose | Diluent | 87.3 | 38.5 | 9.7 |
| Lactose | Diluent | 9.7 | 38.5 | 87.3 |
| Starch | Elastic component | 0 | 20 | 0 |
| Crospovidone | Disintegrant | 1 | 1 | 1 |
| Colloidal silicon dioxide | Flow aid | 1 | 1 | 1 |
| Magnesium stearate | Lubricant | 1 | 1 | 1 |
| Placebo Blend Characterisation | ||||
| Yield pressure (Py) (MPa)—Gamlen | 40 | 49 | 76 | |
| Yield pressure (Py) (MPa)—KG RoTab | 74 | 141 | 130 | |
| Elastic recovery after compaction (mm) | 3.80 ± 0.01 | 3.98 ± 0.01 | 3.98 ± 0.01 | |
| Elastic recovery after 24 h (mm) | 3.81 ± 0.01 | 4.01 ± 0.01 | 3.98 ± 0.01 | |
| Tablet Press | Level | Pre-compression Force (kN) | Compression Force (kN) | Tablet Press Speed (Tablets per Hour) | Dwell Time (ms) |
|---|---|---|---|---|---|
| KG RoTab | −1 | 1 | 8.83 (Form. 1) | 9600 | 66 |
| 4.81 (Form. 2) | |||||
| 1.50 (Form. 3) | |||||
| 0 | 2.5 | 11.16 (Form. 1) | 14,400 | 44 | |
| 9.40 (Form. 2) | |||||
| 5.14 (Form. 3) | |||||
| 1 | 5 | 13.50 (Form. 1) | 19,200 | 33 | |
| 13.98 (Form. 2) | |||||
| 10.56 (Form. 3) | |||||
| Fette 1200i | −1 | 1 | 11.12 (Form. 1) | 28,000 | 33 |
| 10.45 (Form. 2) | |||||
| 6.12 (Form. 3) | |||||
| 0 | 2.5 | 13.39 (Form. 1) | 86,000 | 11 | |
| 13.08 (Form. 2) | |||||
| 9.86 (Form. 3) | |||||
| 1 | 5 | 15.65 (Form. 1) | 130,000 | 7 | |
| 15.71 (Form. 2) | |||||
| 13.60 (Form. 3) | |||||
| Modul P | −1 | 1 | 12.40 (Form. 1) | 46,000 | 21 |
| 11.41 (Form. 2) | |||||
| 8.29 (Form. 3) | |||||
| 0 | 2.5 | 15.19 (Form. 1) | 93,000 | 11 | |
| 14.27 (Form. 2) | |||||
| 12.20 (Form. 3) | |||||
| 1 | 5 | 17.97 (Form. 1) | 140,000 | 7 | |
| 18.03 (Form. 2) | |||||
| 16.10 (Form. 3) |
| Statistical Parameter | Model | ||
|---|---|---|---|
| Tensile Strength | Porosity | Disintegration Time | |
| R2 | 0.9918 | 0.9756 | 0.9628 |
| Adjusted R2 | 0.9901 | 0.9706 | 0.9554 |
| Root mean square error | 0.0603 | 0.0461 | 1.0124 |
| Observations (or sum weights) | 430 | 430 | 429 |
| Model Terms | Tensile Strength Model | Porosity Model | Disintegration Time Model |
|---|---|---|---|
| Tablet press | <0.0001 | <0.0001 | <0.0001 |
| Formulation | <0.0001 | <0.0001 | <0.0001 |
| Coded main compression force | <0.0001 | <0.0001 | <0.0001 |
| Coded press speed | <0.0001 | <0.0001 | <0.0001 |
| Pre-compression force | <0.0001 | <0.0001 | NS |
| Coded press speed * Coded press speed | <0.0001 | NS | NS |
| Pre-compression force * Pre-compression force | <0.0001 | <0.0001 | NS |
| Tablet press * Formulation | <0.0001 | <0.0001 | <0.0001 |
| Tablet press * Coded main compression force | <0.0001 | <0.0001 | <0.0001 |
| Tablet press * Coded press speed | <0.0001 | NS | <0.0001 |
| Tablet press * Pre-compression force | <0.0001 | 0.0023 | NS |
| Formulation * Main compression force | <0.0001 | <0.0001 | <0.0001 |
| Formulation * Coded press speed | NS | <0.0001 | <0.0001 |
| Formulation * Pre-compression force | NS | 0.014 | NS |
| Coded main compression force * Coded press speed | 0.0006 | NS | NS |
| Coded main compression force * Pre-compression force | 0.0008 | 0.0005 | NS |
| Coded press speed * Pre-compression force | 0.0465 | NS | <0.0001 |
| Tablet press * Formulation * Coded main compression force | <0.0001 | NS | <0.0001 |
| Tablet press * Formulation * Coded press speed | 0.0012 | NS | NS |
| Tablet press * Formulation * Pre-compression force | NS | 0.0457 | NS |
| Tablet press * Coded main compression force * Coded press speed | NS | NS | 0.0023 |
| Tablet press * Coded press speed * Pre-compression force | 0.0117 | NS | NS |
| Formulation * Coded main compression force *Coded press speed | NS | NS | 0.0091 |
| Formulation * Coded main compression force * Pre-compression force | <0.0001 | 0.0056 | NS |
| Tablet press * Formulation * Coded main compression force * Coded press speed | NS | NS | 0.0058 |
| Tablet press * Formulation * Coded main compression force * Pre-compression force | <0.0001 | 0.0029 | <0.0001 |
| Tablet press * Coded main compression force * Coded press speed pre-compression force | 0.0265 | NS | 0.0014 |
| Formulation * Pre-compression force * Coded main compression force * Coded press speed | 0.0349 | NS | NS |
| Tablet press * Formulation * Pre-compression force (kN) * Main compression force * Press speed | NS | NS | 0.0323 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peddapatla, R.V.G.; Slevin, C.; Sheridan, G.; Beirne, C.; Swaminathan, S.; Browning, I.; O’Reilly, C.; Worku, Z.A.; Egan, D.; Sheehan, S.; et al. Modelling the Compaction Step of a Platform Direct Compression Process. Pharmaceutics 2022, 14, 695. https://doi.org/10.3390/pharmaceutics14040695
Peddapatla RVG, Slevin C, Sheridan G, Beirne C, Swaminathan S, Browning I, O’Reilly C, Worku ZA, Egan D, Sheehan S, et al. Modelling the Compaction Step of a Platform Direct Compression Process. Pharmaceutics. 2022; 14(4):695. https://doi.org/10.3390/pharmaceutics14040695
Chicago/Turabian StylePeddapatla, Raghu V. G., Conor Slevin, Gerard Sheridan, Caoimhe Beirne, Shrikant Swaminathan, Ivan Browning, Clare O’Reilly, Zelalem A. Worku, David Egan, Stephen Sheehan, and et al. 2022. "Modelling the Compaction Step of a Platform Direct Compression Process" Pharmaceutics 14, no. 4: 695. https://doi.org/10.3390/pharmaceutics14040695
APA StylePeddapatla, R. V. G., Slevin, C., Sheridan, G., Beirne, C., Swaminathan, S., Browning, I., O’Reilly, C., Worku, Z. A., Egan, D., Sheehan, S., & Crean, A. M. (2022). Modelling the Compaction Step of a Platform Direct Compression Process. Pharmaceutics, 14(4), 695. https://doi.org/10.3390/pharmaceutics14040695