Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lys-PCLA Bioconjugates
2.3. Characterization
2.4. Sol-Gel Phase Transition Behavior
2.5. Rheology Measurement
2.6. In Vitro Cytotoxicity and Imaging Cell Viability
2.7. In Situ Gelation In Vivo
2.8. In Vivo Cell Recruitment by Hydrogels
2.9. Statistical Analysis
3. Results and Discussion
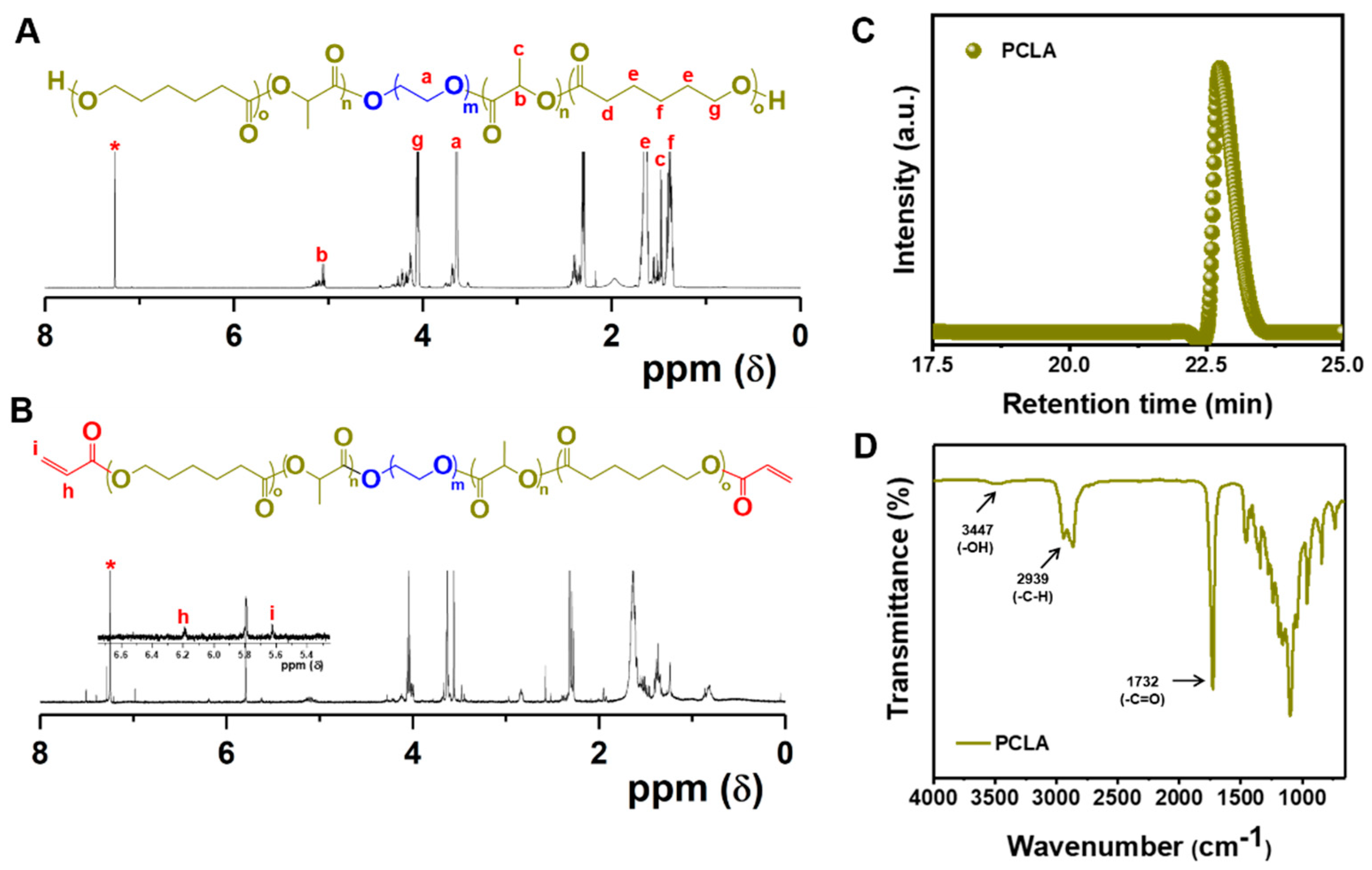
3.1. Synthesis and Characterization of Lys-PCLA Bioconjugate
3.2. Sol-to-Gel Phase Diagram
3.3. Rheological Properties of Hydrogels
3.4. In Vitro Biocompatibility of Hydrogels
3.5. In Vivo In Situ Gelation
3.6. In Vivo Recruitment of Host Cells by Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Adu-Berchie, K.; Mooney, D.J. Biomaterials as Local Niches for Immunomodulation. Acc. Chem. Res. 2020, 53, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Peppas, N.A. Hydrogels and scaffolds for immunomodulation. Adv. Mater. 2014, 26, 6530–6541. [Google Scholar] [CrossRef]
- Chirila, T.V. Oxygen Permeability of Silk Fibroin Hydrogels and Their Use as Materials for Contact Lenses: A Purposeful Analysis. Gels 2021, 7, 58. [Google Scholar] [CrossRef]
- Lee, M.K.; Rich, M.H.; Baek, K.; Lee, J.; Kong, H. Bioinspired Tuning of Hydrogel Permeability-Rigidity Dependency for 3D Cell Culture. Sci. Rep. 2015, 5, 8948. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Shao, Y.; Zhou, X.; Liu, Q.; Zhu, Z.; Zhou, B.; Dong, Y.; Stephanopoulos, N.; Gui, S.; Yan, H.; et al. Highly Permeable DNA Supramolecular Hydrogel Promotes Neurogenesis and Functional Recovery after Completely Transected Spinal Cord Injury. Adv. Mater. 2021, 33, 2102428. [Google Scholar] [CrossRef]
- Norioka, C.; Inamoto, Y.; Hajime, C.; Kawamura, A.; Miyata, T. A universal method to easily design tough and stretchable hydrogels. NPG Asia Mater. 2021, 13, 34. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [Green Version]
- Blöhbaum, J.; Paulus, I.; Pöppler, A.-C.; Tessmar, J.; Groll, J. Influence of charged groups on the cross-linking efficiency and release of guest molecules from thiol–ene cross-linked poly(2-oxazoline) hydrogels. J. Mater. Chem. B 2019, 7, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Yu, W.-j.; Yu, Y.; Li, R.-y.; Gao, Q.; Ren, K.-f.; Ji, J. A Bioinspired Hydrogel-Elastomer Hybrid Surface for Enhanced Mechanical Properties and Lubrication. ACS Appl. Mater. Interfaces 2021, 13, 50461–50469. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Bin, X.; Gao, X.; Qin, M.; Wang, W.; Bin, C.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing γ-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Villela Castrejón, J.; González-Ortiz, A. Rational design and engineering of carbon nano-onions reinforced natural protein nanocomposite hydrogels for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103696. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; González-Ortiz, A.; Lopez Romo, I.; Barrera, E.V. Development of Functionalized Carbon Nano-Onions Reinforced Zein Protein Hydrogel Interfaces for Controlled Drug Release. Pharmaceutics 2019, 11, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [Green Version]
- Giang Phan, V.H.; Duong, H.T.T.; Thambi, T.; Nguyen, T.L.; Turabee, M.H.; Yin, Y.; Kim, S.H.; Kim, J.; Jeong, J.H.; Lee, D.S. Modularly engineered injectable hybrid hydrogels based on protein-polymer network as potent immunologic adjuvant in vivo. Biomaterials 2019, 195, 100–110. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Kim, S.H.; Nguyen, T.L.; Phan, V.H.G.; Kim, J.; Jeong, J.H.; Lee, D.S. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 2020, 230, 119599. [Google Scholar] [CrossRef]
- Ziegler, C.E.; Graf, M.; Nagaoka, M.; Lehr, H.; Goepferich, A.M. In Situ Forming iEDDA Hydrogels with Tunable Gelation Time Release High-Molecular Weight Proteins in a Controlled Manner over an Extended Time. Biomacromolecules 2021, 22, 3223–3236. [Google Scholar] [CrossRef]
- Shih, H.; Lin, C.-C. Photoclick Hydrogels Prepared from Functionalized Cyclodextrin and Poly(ethylene glycol) for Drug Delivery and in Situ Cell Encapsulation. Biomacromolecules 2015, 16, 1915–1923. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.T.; Phan, V.H.G.; Lee, D.S.; Thambi, T.; Huynh, D.P. Bioresorbable pH- and temperature-responsive injectable hydrogels-incorporating electrosprayed particles for the sustained release of insulin. Polym. Degrad. Stab. 2019, 162, 36–46. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Wu, Y.; Gao, J. Locally Injectable Hydrogels for Tumor Immunotherapy. Gels 2021, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Gale, E.C.; Alcántara-Hernández, M.; Luo, W.; Axpe, E.; Verma, R.; Yin, Q.; Yu, A.C.; Lopez Hernandez, H.; Maikawa, C.L.; et al. Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity. ACS Cent. Sci. 2020, 6, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Im, S.; Kim, W.J. Injectable Immunological Based on Polymerized Phenyl Boronic Acid and Mannan for Cancer Immunotherapy. J. Control. Release 2022, 345, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Song, H.; Qin, Y.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Engineering Dendritic-Cell-Based Vaccines and PD-1 Blockade in Self-Assembled Peptide Nanofibrous Hydrogel to Amplify Antitumor T-Cell Immunity. Nano Lett. 2018, 18, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Jia, L.; Pang, M.; Yang, X.; Yang, Y.; Kamel Elyzayati, S.; Liao, Y.; Wang, H.; Zhu, Y.; Wang, Q. Injectable Adhesive Hydrogel as Photothermal-Derived Antigen Reservoir for Enhanced Anti-Tumor Immunity. Adv. Funct. Mater. 2021, 31, 2010587. [Google Scholar] [CrossRef]
- Wang, H.; Mooney, D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018, 17, 761–772. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Li, X.; He, T.; Gong, S.; Zhu, S.; Zhang, M.; Wu, Q.; Gong, C. A spontaneous multifunctional hydrogel vaccine amplifies the innate immune response to launch a powerful antitumor adaptive immune response. Theranostics 2021, 11, 6936–6949. [Google Scholar] [CrossRef]
- Weiden, J.; Schluck, M.; Ioannidis, M.; van Dinther, E.A.W.; Rezaeeyazdi, M.; Omar, F.; Steuten, J.; Voerman, D.; Tel, J.; Diken, M.; et al. Robust Antigen-Specific T Cell Activation within Injectable 3D Synthetic Nanovaccine Depots. ACS Biomater. Sci. Eng. 2021, 7, 5622–5632. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, L.; Joo, K.-I.; Hu, B.; Fang, J.; Wang, P. In Situ Modulation of Dendritic Cells by Injectable Thermosensitive Hydrogels for Cancer Vaccines in Mice. Biomacromolecules 2014, 15, 3836–3845. [Google Scholar] [CrossRef] [Green Version]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef] [Green Version]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Z.; Kong, Q.; Zhang, R.; Yang, X. Enhancing the antitumor activity of tea polyphenols encapsulated in biodegradable nanogels by macromolecular self-assembly. RSC Adv. 2019, 9, 10004–10016. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Jin, D.; Qu, X.; Liu, H.; Chen, X.; Yin, M.; Liu, C. A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2019, 192, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell Wall Hydrolases in Bacteria: Insight on the Diversity of Cell Wall Amidases, Glycosidases and Peptidases Toward Peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.J.; Thambi, T.; Lee, D.S. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J. Mater. Chem. B 2015, 3, 8892–8901. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Thambi, T.; Duong, H.T.T.; Lee, D.S. Poly(amino carbonate urethane)-based biodegradable, temperature and pH-sensitive injectable hydrogels for sustained human growth hormone delivery. Sci. Rep. 2016, 6, 29978. [Google Scholar] [CrossRef] [Green Version]
- Dávila, J.L.; Freitas, M.S.; Inforçatti Neto, P.; Silveira, Z.C.; Silva, J.V.L.; d’Ávila, M.A. Fabrication of PCL/β-TCP scaffolds by 3D mini-screw extrusion printing. J. Appl. Polym. Sci. 2016, 133, 43031. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2021, 31, 2008123. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, V.H.G.; Le, T.M.D.; Janarthanan, G.; Ngo, P.-K.T.; Lee, D.S.; Thambi, T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J. Ind. Eng. Chem. 2021, 96, 345–355. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, S.H.; Giang Phan, V.H.; Thambi, T.; Lee, D.S. Therapeutic effects of boronate ester cross-linked injectable hydrogels for the treatment of hepatocellular carcinoma. Biomater. Sci. 2021, 9, 7275–7286. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Lee, E.; Maeng, J.H.; Thambi, T.; Kim, B.S.; Lee, D.; Lee, D.S. Pancreatic cancer therapy using an injectable nanobiohybrid hydrogel. RSC Adv. 2016, 6, 41644–41655. [Google Scholar] [CrossRef]
- Bocedi, A.; Cattani, G.; Martelli, C.; Cozzolino, F.; Castagnola, M.; Pucci, P.; Ricci, G. The extreme hyper-reactivity of Cys94 in lysozyme avoids its amorphous aggregation. Sci. Rep. 2018, 8, 16050. [Google Scholar] [CrossRef] [PubMed]
- Zavodszky, M.; Chen, C.W.; Huang, J.K.; Zolkiewski, M.; Wen, L.; Krishnamoorthi, R. Disulfide bond effects on protein stability: Designed variants of Cucurbita maxima trypsin inhibitor-V. Protein. Sci. 2001, 10, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Thambi, T.; Giang Phan, V.H.; Lee, D.S. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydr. Polym. 2020, 233, 115832. [Google Scholar] [CrossRef] [PubMed]
- Asai, D.; Fukuda, T.; Morokuma, K.; Funamoto, D.; Yamaguchi, Y.; Mori, T.; Katayama, Y.; Shibayama, K.; Nakashima, H. Injectable Polypeptide Hydrogel Depot System for Assessment of the Immune Response–Inducing Efficacy of Sustained Antigen Release Alone. Macromol. Biosci. 2019, 19, 1900167. [Google Scholar] [CrossRef]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [Green Version]










| Copolymer Design | 1H NMR Spectra a | GPC b | ||
|---|---|---|---|---|
| PCLA-PEG-PCLA | Mn (g mol−1) | CL/LA (mol−1) | Mn (g mol−1) | PDI |
| 2170-1500-2170 | 2.37 | 1912-1500-1912 | 1.38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, V.H.G.; Murugesan, M.; Manivasagan, P.; Nguyen, T.L.; Phan, T.-H.; Luu, C.H.; Ho, D.-K.; Li, Y.; Kim, J.; Lee, D.S.; et al. Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment. Pharmaceutics 2022, 14, 709. https://doi.org/10.3390/pharmaceutics14040709
Phan VHG, Murugesan M, Manivasagan P, Nguyen TL, Phan T-H, Luu CH, Ho D-K, Li Y, Kim J, Lee DS, et al. Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment. Pharmaceutics. 2022; 14(4):709. https://doi.org/10.3390/pharmaceutics14040709
Chicago/Turabian StylePhan, V.H. Giang, Mohanapriya Murugesan, Panchanathan Manivasagan, Thanh Loc Nguyen, Thuy-Hien Phan, Cuong Hung Luu, Duy-Khiet Ho, Yi Li, Jaeyun Kim, Doo Sung Lee, and et al. 2022. "Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment" Pharmaceutics 14, no. 4: 709. https://doi.org/10.3390/pharmaceutics14040709








