Nanoparticles Targeting the Molecular Pathways of Heart Remodeling and Regeneration
Abstract
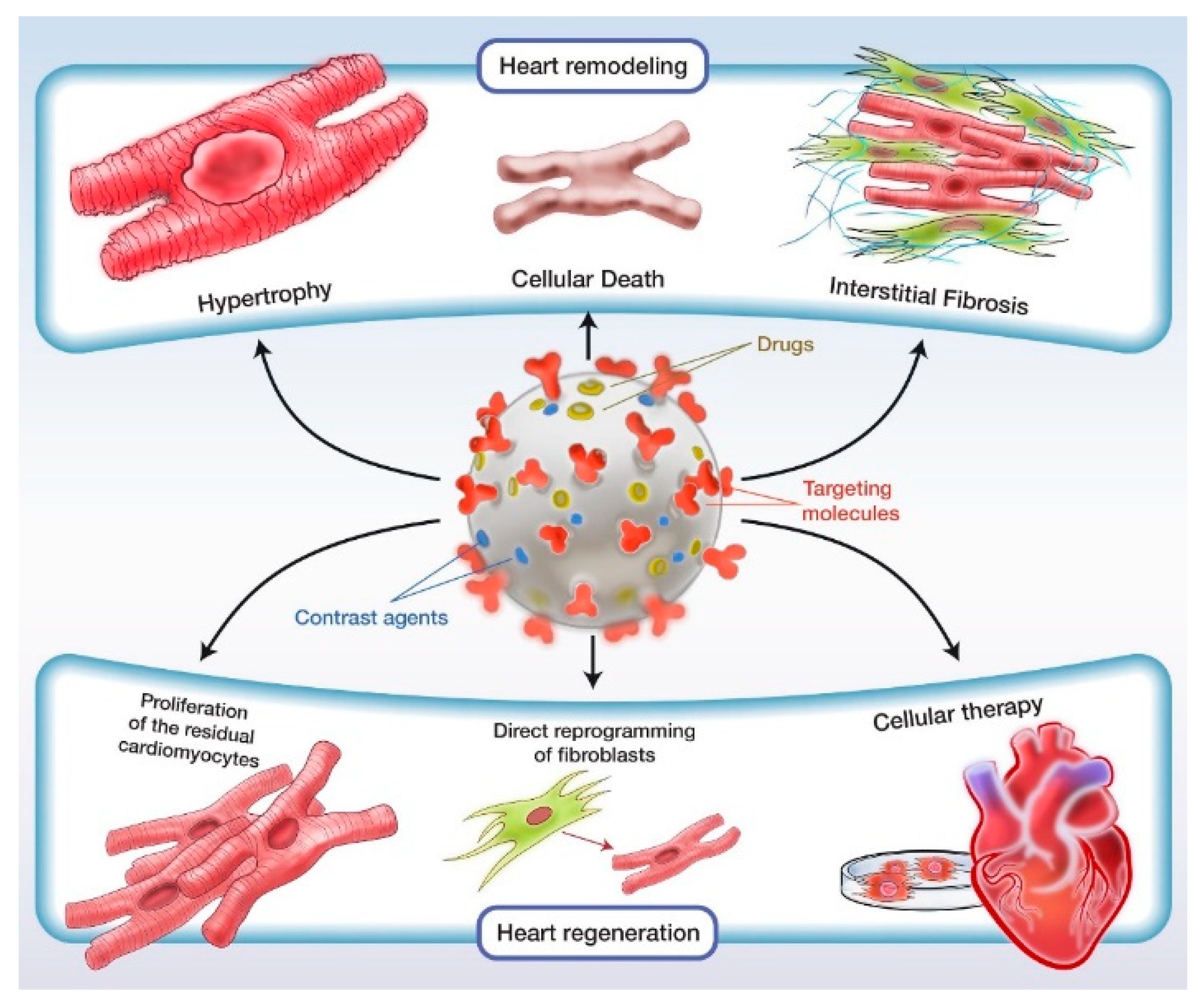
:1. Introduction
2. Heart Remodeling
2.1. Proteins of Interest
2.1.1. Vascular Endothelial Growth Factor
2.1.2. Angiotensin II
2.1.3. Apelin
2.2. Inflammation
3. Heart Regeneration
3.1. Cellular Therapy
3.2. Cell Reprogramming
3.3. Stimulation of Residual Cardiomyocytes
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease: Analysis of Data from the World Health Organization and Coronary Artery Disease Risk Factors from NCD Risk Factor Collaboration. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Lestini, B.J.; Sagnella, S.M.; Xu, Z.; Shive, M.S.; Richter, N.J.; Jayaseharan, J.; Case, A.J.; Kottke-Marchant, K.; Anderson, J.M.; Marchant, R.E. Surface Modification of Liposomes for Selective Cell Targeting in Cardiovascular Drug Delivery. J. Control. Release 2002, 78, 235–247. [Google Scholar] [CrossRef]
- Bowey, K.; Tanguay, J.F.; Tabrizian, M. Liposome Technology for Cardiovascular Disease Treatment and Diagnosis. Expert Opin. Drug Deliv. 2012, 9, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pietersz, G.; Peter, K.; Wang, X. Nanobiotechnology Approaches for Cardiovascular Diseases: Site-Specific Targeting of Drugs and Nanoparticles for Atherothrombosis. J. Nanobiotechnol. 2022, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Dehaini, D.; Zhou, J.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanoparticle Technology for Cardiovascular Disease Detection and Treatment. Nanoscale Horiz. 2019, 5, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging Macrophages with Nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Suarez, S.; Almutairi, A.; Christman, K.L. Micro- and Nanoparticles for Treating Cardiovascular Disease. Biomater. Sci. 2015, 3, 564–580. [Google Scholar] [CrossRef]
- Prajnamitra, R.P.; Chen, H.C.; Lin, C.J.; Chen, L.L.; Hsieh, P.C.H. Nanotechnology Approaches in Tackling Cardiovascular Diseases. Molecules 2019, 24, 2017. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, V.; Fang, J.C. Gastrointestinal and Liver Issues in Heart Failure. Circulation 2016, 133, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodríguez, G.B.; Patrício, T.; Degli Esposti, L.; et al. Inhalation of Peptide-Loaded Nanoparticles Improves Heart Failure. Sci. Transl. Med. 2018, 10, eaan6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Jarai, B.M.; Kolewe, E.L.; Stillman, Z.S.; Raman, N.; Fromen, C.A. Polymeric Nanoparticles. Nanoparticles Biomed. Appl. 2020, 12, 303–324. [Google Scholar] [CrossRef]
- Levchenko, T.S.; Hartner, W.C.; Torchilin, V.P. Liposomes in Diagnosis and Treatment of Cardiovascular Disorders. Methodist DeBakey Cardiovasc. J. 2012, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mydin, R.B.S.M.N.; Moshawih, S. Nanoparticles in Nanomedicine Application: Lipid-Based Nanoparticles and Their Safety Concerns. Nanotechnol. Appl. Energy Drug Food 2019, 227–232. [Google Scholar] [CrossRef]
- Ruiz-Esparza, G.U.; Flores-Arredondo, J.H.; Segura-Ibarra, V.; Torre-Amione, G.; Ferrari, M.; Blanco, E.; Serda, R.E. The Physiology of Cardiovascular Disease and Innovative Liposomal Platforms for Therapy. Int. J. Nanomed. 2013, 8, 629. [Google Scholar] [CrossRef] [Green Version]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. Charact. Biol. Nanomater. Drug Deliv. Nanosci. Nanotechnol. Drug Deliv. 2019, 47–76. [Google Scholar] [CrossRef]
- Chalikwar, S.S.; Belgamwar, V.S.; Talele, V.R.; Surana, S.J.; Patil, M.U. Formulation and Evaluation of Nimodipine-Loaded Solid Lipid Nanoparticles Delivered via Lymphatic Transport System. Colloids Surfaces. B Biointerfaces 2012, 97, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhuang, J.; Lu, Y.; Dong, X.; Zhao, W.; Wu, W. In Vivo Fate of Lipid-Based Nanoparticles. Drug Discov. Today 2017, 22, 166–172. [Google Scholar] [CrossRef]
- Aimo, A.; Gaggin, H.K.; Barison, A.; Emdin, M.; Januzzi, J.L. Imaging, Biomarker, and Clinical Predictors of Cardiac Remodeling in Heart Failure with Reduced Ejection Fraction. JACC: Heart Fail. 2019, 7, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Varna, M.; Xuan, H.V.; Fort, E. Gold Nanoparticles in Cardiovascular Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1470. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Leary, J.F. Nanoparticles for Multimodal in Vivo Imaging in Nanomedicine. Int. J. Nanomed. 2014, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.P.A.; Ranjan, S.; Kinnunen, S.; Correia, A.; Talman, V.; Mäkilä, E.; Barrios-Lopez, B.; Kemell, M.; Balasubramanian, V.; Salonen, J.; et al. Drug-Loaded Multifunctional Nanoparticles Targeted to the Endocardial Layer of the Injured Heart Modulate Hypertrophic Signaling. Small 2017, 13, 1701276. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Prada, K.X.; Lam, J.; Kamato, D.; Xu, Z.P.; Little, P.J.; Ta, H.T. Targeted Molecular Imaging of Cardiovascular Diseases by Iron Oxide Nanoparticles. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold Nanoparticles: A New X-Ray Contrast Agent. Br. J. Radiol. 2014, 79, 248–253. [Google Scholar] [CrossRef]
- Ghann, W.E.; Aras, O.; Fleiter, T.; Daniel, M.-C. Synthesis and Biological Studies of Highly Concentrated Lisinopril-Capped Gold Nanoparticles for CT Tracking of Angiotensin Converting Enzyme (ACE). In Proceedings of the SPIE Defense, Security, And Sensing, Orlando, FL, USA, 25–29 April 2011; Volume 8025, pp. 114–125. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talman, V.; Kivelä, R. Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Uludağ, H. Nanoparticulate Systems for Growth Factor Delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Marchenko, Y.Y.; Dobrodumov, A.V.; Mikhrina, A.L.; Martynova, M.G.; Bystrova, O.A.; Yakovenko, I.V.; Ischenko, A.M. Superparamagnetic Iron Oxide Nanoparticles Conjugated with Epidermal Growth Factor (SPION–EGF) for Targeting Brain Tumors. Int. J. Nanomed. 2014, 9, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.L.; Dhalla, N.S. Differences in Concentric Cardiac Hypertrophy and Eccentric Hypertrophy. Card. Adapt. Mol. Mech. 2013, 4, 147–166. [Google Scholar] [CrossRef]
- Carabello, B.A. Concentric versus Eccentric Remodeling. J. Card. Fail. 2002, 8, S258–S263. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of Physiological and Pathological Cardiac Hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.T. Overview of MicroRNAs in Cardiac Hypertrophy, Fibrosis, and Apoptosis. Int. J. Mol. Sci. 2016, 17, 749. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oduk, Y.; Zhu, W.; Kannappan, R.; Zhao, M.; Borovjagin, A.V.; Oparil, S.; Jay Zhang, J. VEGF Nanoparticles Repair the Heart after Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H278–H284. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF Function in Angiogenesis: Signalling Pathways, Biological Responses and Therapeutic Inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Ferrara, N. VEGF-A: A Critical Regulator of Blood Vessel Growth. Eur. Cytokine Netw. 2009, 20, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B Is Dispensable for Blood Vessel Growth but Critical for Their Survival, and VEGF-B Targeting Inhibits Pathological Angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zentilin, L.; Puligadda, U.; Lionetti, V.; Zacchigna, S.; Collesi, C.; Pattarini, L.; Ruozi, G.; Camporesi, S.; Sinagra, G.; Pepe, M.; et al. Cardiomyocyte VEGFR-1 Activation by VEGF-B Induces Compensatory Hypertrophy and Preserves Cardiac Function after Myocardial Infarction. FASEB J. 2010, 24, 1467–1478. [Google Scholar] [CrossRef]
- Kivelä, R.; Hemanthakumar, K.A.; Vaparanta, K.; Robciuc, M.; Izumiya, Y.; Kidoya, H.; Takakura, N.; Peng, X.; Sawyer, D.B.; Elenius, K.; et al. Endothelial Cells Regulate Physiological Cardiomyocyte Growth via VEGFR2-Mediated Paracrine Signaling. Circulation 2019, 139, 2570. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Kim, Y.T.; Duvall, C.L.; Bellamkonda, R.V.; Gupta, D.; Lin, A.S.; Weiss, D.; Taylor, W.R.; Guldberg, R.E. Sustained VEGF Delivery via PLGA Nanoparticles Promotes Vascular Growth. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, 1959–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Dwyer, J.; Murphy, R.; Dolan, E.B.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.P.; Cryan, S.A. Development of a Nanomedicine-Loaded Hydrogel for Sustained Delivery of an Angiogenic Growth Factor to the Ischaemic Myocardium. Drug Deliv. Transl. Res. 2019, 10, 440–454. [Google Scholar] [CrossRef]
- Qiao, B.; Nie, J.J.; Shao, Y.; Li, Y.; Zhang, C.; Hao, W.; Li, S.; Chen, D.; Yu, B.; Li, H.H.; et al. Functional Nanocomplexes with Vascular Endothelial Growth Factor A/C Isoforms Improve Collateral Circulation and Cardiac Function. Small 2020, 16, e1905925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, K.; Nagumo, Y.; Kim, M.; Kim, H.J.; Kyung, H.W.; Chung, H.J.; Sekine, H.; Shimizu, T.; Kanazawa, H.; Okano, T.; et al. Local Release of VEGF Using Fiber Mats Enables Effective Transplantation of Layered Cardiomyocyte Sheets. Macromol. Biosci. 2017, 17, 1700073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, W.; Ou, L.; Wang, W.; Delyagina, E.; Lux, C.; Sorg, H.; Riehemann, K.; Steinhoff, G.; Ma, N. Targeted Delivery of Human VEGF Gene via Complexes of Magnetic Nanoparticle-Adenoviral Vectors Enhanced Cardiac Regeneration. PLoS ONE 2012, 7, e39490. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.S.; Song, J.Y.; Yoon, S.J.; Park, Y.; Kim, D.; Yuk, S.H. Temperature-Induced Gel Formation of Core/Shell Nanoparticles for the Regeneration of Ischemic Heart. J. Control. Release 2010, 146, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Pinski, S.L.; Trohman, R.G. Interference in Implanted Cardiac Devices, Part II. Pacing Clin. Electrophysiol. PACE 2002, 25, 1496–1509. [Google Scholar] [CrossRef] [Green Version]
- Brakenhielm, E.; González, A.; Díez, J. Role of Cardiac Lymphatics in Myocardial Edema and Fibrosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA Based Drug Delivery Systems: Promising Carriers for Wound Healing Activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Flores-Munoz, M.; Work, L.M.; Douglas, K.; Denby, L.; Dominiczak, A.F.; Graham, D.; Nicklin, S.A. Angiotensin-(1-9) Attenuates Cardiac Fibrosis in the Stroke-Prone Spontaneously Hypertensive Rat via the Angiotensin Type 2 Receptor. Hypertension 2012, 59, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepúlveda-Rivas, S.; Leal, M.S.; Pedrozo, Z.; Kogan, M.J.; Ocaranza, M.P.; Morales, J.O. Nanoparticle-Mediated Angiotensin-(1-9) Drug Delivery for the Treatment of Cardiac Hypertrophy. Pharmaceutics 2021, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Lomis, N.; Westfall, S.; Shum-Tim, D.; Prakash, S. Synthesis and Characterization of Peptide Conjugated Human Serum Albumin Nanoparticles for Targeted Cardiac Uptake and Drug Delivery. PLoS ONE 2021, 16, e0254305. [Google Scholar] [CrossRef] [PubMed]
- Lomis, N.; Sarfaraz, Z.K.; Alruwaih, A.; Westfall, S.; Shum-Tim, D.; Prakash, S. Albumin Nanoparticle Formulation for Heart-Targeted Drug Delivery: In Vivo Assessment of Congestive Heart Failure. Pharmaceuticals 2021, 14, 697. [Google Scholar] [CrossRef]
- Bejarano, J.; Rojas, A.; Ramírez-Sagredo, A.; Riveros, A.L.; Morales-Zavala, F.; Flores, Y.; Riquelme, J.A.; Guzman, F.; Araya, E.; Chiong, M.; et al. Light-Induced Release of the Cardioprotective Peptide Angiotensin-(1–9) from Thermosensitive Liposomes with Gold Nanoclusters. J. Control. Release 2020, 328, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Barta, A.; Koneracka, M.; Zavisova, V.; Kubovcikova, M.; Klimentova, J.; Török, J.; Zemancikova, A.; Cebova, M. Protective Effects of Nanoparticle-Loaded Aliskiren on Cardiovascular System in Spontaneously Hypertensive Rats. Molecules 2019, 24, 2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Matoba, T.; Tokutome, M.; Funamoto, D.; Katsuki, S.; Ikeda, G.; Nagaoka, K.; Ishikita, A.; Nakano, K.; Koga, J.I.; et al. Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation. Sci. Rep. 2016, 6, 29601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennig, R.; Pollinger, K.; Tessmar, J.; Goepferich, A. Multivalent Targeting of AT1 Receptors with Angiotensin II-Functionalized Nanoparticles. J. Drug Target. 2015, 23, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, C.; Cabigas, E.B.; Pendergrass, K.D.; Brown, M.E.; Luo, Y.; Davis, M.E. Functionalized Dendrimer-Based Delivery of Angiotensin Type 1 Receptor SiRNA for Preserving Cardiac Function Following Infarction. Biomaterials 2013, 34, 3729–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvir, T.; Bauer, M.; Schroeder, A.; Tsui, J.H.; Anderson, D.G.; Langer, R.; Liao, R.; Kohane, D.S. Nanoparticles Targeting the Infarcted Heart. Nano Lett. 2011, 11, 4411–4414. [Google Scholar] [CrossRef] [Green Version]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 Receptors: Regulation, Signalling and Function. Blood Press. 2009, 12, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Cuffe, M.S.; Califf, R.M.; Adams, K.F.; Benza, R.; Bourge, R.; Colucci, W.S.; Massie, B.M.; O’Connor, C.M.; Pina, I.; Quigg, R.; et al. Short-Term Intravenous Milrinone for Acute Exacerbation of Chronic Failure: A Randomized Controlled Trial. JAMA 2002, 287, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, C.M. Role of Angiotensin II in Cardiovascular Disease—Therapeutic Implications of More than a Century of Research. JRAAS J. Renin-Angiotensin-Aldosterone Syst. 2006, 7, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, I.; Fox, S.; Hollins, A.; Akhtar, S. Delivery Strategies for SiRNA-Mediated Gene Silencing. Curr. Drug Deliv. 2006, 3, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Japp, A.G.; Newby, D.E. The Apelin–APJ System in Heart Failure: Pathophysiologic Relevance and Therapeutic Potential. Biochem. Pharmacol. 2008, 75, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Ceylan-Isik, A.F.; Kandadi, M.R.; Xu, X.; Hua, Y.; Chicco, A.J.; Ren, J.; Nair, S. Apelin Administration Ameliorates High Fat Diet-Induced Cardiac Hypertrophy and Contractile Dysfunction. J. Mol. Cell. Cardiol. 2013, 63, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Serpooshan, V.; Sivanesan, S.; Huang, X.; Mahmoudi, M.; Malkovskiy, A.V.; Zhao, M.; Inayathullah, M.; Wagh, D.; Zhang, X.J.; Metzler, S.; et al. [Pyr1]-Apelin-13 Delivery via Nano-Liposomal Encapsulation Attenuates Pressure Overload-Induced Cardiac Dysfunction. Biomaterials 2015, 37, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Yang, H.N.; Yi, S.W.; Kim, J.H.; Park, K.H. Neoangiogenesis of Human Mesenchymal Stem Cells Transfected with Peptide-Loaded and Gene-Coated PLGA Nanoparticles. Biomaterials 2016, 76, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhong, T.; Guo, T.; Miao, C.; Zhou, C.; Long, H.; Wu, H.; Zheng, S.; Wang, L.; Wang, T. Apelin Promotes Mesenchymal Stem Cells Survival and Vascularization under Hypoxic-Ischemic Condition in Vitro Involving the Upregulation of Vascular Endothelial Growth Factor. Exp. Mol. Pathol. 2017, 102, 203–209. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Q.; Sun, X.; He, H.; Wang, Q.; Wu, Y.; Liu, Y.; Wang, Y.; Li, C. Qishen Granule Improved Cardiac Remodeling via Balancing M1 and M2 Macrophages. Front. Pharmacol. 2019, 10, 1399. [Google Scholar] [CrossRef]
- Ismahil, M.A.; Hamid, T.; Bansal, S.S.; Patel, B.; Kingery, J.R.; Prabhu, S.D. Remodeling of the Mononuclear Phagocyte Network Underlies Chronic Inflammation and Disease Progression in Heart Failure Critical Importance of the Cardiosplenic Axis. Circ. Res. 2014, 114, 266–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Koga, J.I.; Tokutome, M.; Matoba, T.; Ikeda, G.; Nakano, K.; Egashira, K. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Left Ventricular Remodeling After Acute Myocardial Infarction by Inhibiting Monocyte-Mediated Inflammation. Int. Heart J. 2017, 58, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majmudar, M.D.; Yoo, J.; Keliher, E.J.; Truelove, J.J.; Iwamoto, Y.; Sena, B.; Dutta, P.; Borodovsky, A.; Fitzgerald, K.; di Carli, M.F.; et al. Polymeric Nanoparticle PET/MR Imaging Allows Macrophage Detection in Atherosclerotic Plaques. Circ. Res. 2013, 112, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keliher, E.J.; Ye, Y.X.; Wojtkiewicz, G.R.; Aguirre, A.D.; Tricot, B.; Senders, M.L.; Groenen, H.; Fay, F.; Perez-Medina, C.; Calcagno, C.; et al. Polyglucose Nanoparticles with Renal Elimination and Macrophage Avidity Facilitate PET Imaging in Ischaemic Heart Disease. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Ueno, T.; Dutta, P.; Keliher, E.; Leuschner, F.; Majmudar, M.; Marinelli, B.; Iwamoto, Y.; Figueiredo, J.L.; Christen, T.; Swirski, F.K.; et al. Nanoparticle PET-CT Detects Rejection and Immunomodulation in Cardiac Allografts. Circ. Cardiovasc. Imaging 2013, 6, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahrendorf, M.; Keliher, E.; Marinelli, B.; Leuschner, F.; Robbins, C.S.; Gerszten, R.E.; Pittet, M.J.; Swirski, F.K.; Weissleder, R. Detection of Macrophages in Aortic Aneurysms by Nanoparticle Positron Emission Tomography-Computed Tomography. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 750–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishige, K.; Kacher, D.F.; Libby, P.; Josephson, L.; Ganz, P.; Weissleder, R.; Aikawa, M. High-Resolution Magnetic Resonance Imaging Enhanced with Superparamagnetic Nanoparticles Measures Macrophage Burden in Atherosclerosis. Circulation 2010, 122, 1707–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, M.J.; Frias, J.C.; Amirbekian, V.; Briley-Saebo, K.C.; Mani, V.; Samber, D.; Abbate, A.; Aguinaldo, J.G.S.; Massey, D.; Fuster, V.; et al. Macrophage-Specific Lipid-Based Nanoparticles Improve Cardiac Magnetic Resonance Detection and Characterization of Human Atherosclerosis. JACC Cardiovasc. Imaging 2009, 2, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and Functionalization of Iron Oxide Nanoparticles for Biomedical Applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging: Evaluation of Size-Dependent Imaging Properties, Storage Stability and Safety. Int. J. Nanomed. 2018, 13, 1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bietenbeck, M.; Florian, A.; Faber, C.; Sechtem, U.; Yilmaz, A. Remote Magnetic Targeting of Iron Oxide Nanoparticles for Cardiovascular Diagnosis and Therapeutic Drug Delivery: Where Are We Now? Int. J. Nanomed. 2016, 11, 3191. [Google Scholar] [CrossRef] [Green Version]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage Endocytosis of Superparamagnetic Iron Oxide Nanoparticles: Mechanisms and Comparison of Ferumoxides and Ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.S.; Wilson, L.B.; Ashari, P.; Jordan, E.K.; Lewis, B.K.; Frank, J.A. A Model of Lysosomal Metabolism of Dextran Coated Superparamagnetic Iron Oxide (SPIO) Nanoparticles: Implications for Cellular Magnetic Resonance Imaging. NMR Biomed. 2005, 18, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kingery, J.R.; Hamid, T.; Lewis, R.K.; Ismahil, M.A.; Bansal, S.S.; Rokosh, G.; Townes, T.M.; Ildstad, S.T.; Jones, S.P.; Prabhu, S.D. Leukocyte INOS Is Required for Inflammation and Pathological Remodeling in Ischemic Heart Failure. Basic Res. Cardiol. 2017, 112, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Behera, M.; Mahapatra, C.; Sundaresan, N.R.; Chatterjee, K. Nanostructured Polymer Scaffold Decorated with Cerium Oxide Nanoparticles toward Engineering an Antioxidant and Anti-Hypertrophic Cardiac Patch. Mater. Sci. Eng. C 2021, 118, 111416. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.; Li, M.; Jia, H.; Han, X.; Zhang, J.; Zou, Y.; Tan, B.; Liang, W.; Shang, Y.; et al. Rebuilding Postinfarcted Cardiac Functions by Injecting TIIA@PDA Nanoparticle-Cross-Linked ROS-Sensitive Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 2880–2890. [Google Scholar] [CrossRef]
- Nabofa, W.E.E.; Alashe, O.O.; Oyeyemi, O.T.; Attah, A.F.; Oyagbemi, A.A.; Omobowale, T.O.; Adedapo, A.A.; Alada, A.R.A. Cardioprotective Effects of Curcumin-Nisin Based Poly Lactic Acid Nanoparticle on Myocardial Infarction in Guinea Pigs. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Somasuntharam, I.; Yehl, K.; Carroll, S.L.; Maxwell, J.T.; Martinez, M.D.; Che, P.L.; Brown, M.E.; Salaita, K.; Davis, M.E. Knockdown of TNF-α by DNAzyme Gold Nanoparticles as an Anti-Inflammatory Therapy for Myocardial Infarction. Biomaterials 2016, 83, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium Oxide Nanoparticles Inhibits Oxidative Stress and Nuclear Factor-ΚB Activation in H9c2 Cardiomyocytes Exposed to Cigarette Smoke Extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Azfer, A.; Rogers, L.M.; Wang, X.; Kolattukudy, P.E. Cardioprotective Effects of Cerium Oxide Nanoparticles in a Transgenic Murine Model of Cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Xia, H.; Chen, X.; Li, J. Curcumin Nanoparticles Attenuate Lipotoxic Injury in Cardiomyocytes Through Autophagy and Endoplasmic Reticulum Stress Signaling Pathways. Front. Pharmacol. 2021, 12, 85. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heide, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for Cardiomyocyte Renewal in Humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.-Y.; Silberstein, L.E.; dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte Proliferation Contributes to Heart Growth in Young Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadota, S.; Shiba, Y. Pluripotent Stem Cell-Derived Cardiomyocyte Transplantation for Heart Disease Treatment. Curr. Cardiol. Rep. 2019, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Fu, X.; Yang, P.C. Exosomes Generated from IPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases: Exosomes from IPSC-Derivatives for Heart Disease. Circ. Res. 2017, 120, 407. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Makkar, R.R. Stem-Cell Transplantation in Myocardial Infarction: A Status Report. Ann. Intern. Med. 2004, 140, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yang, J.; Huang, X.; Guo, W.; Li, S.; Zhou, H.; Li, J.; Cao, F.; Chen, Y. Poly(Lactide-Co-Glycolide)-Monomethoxy-Poly-(Polyethylene Glycol) Nanoparticles Loaded with Melatonin Protect Adipose-Derived Stem Cells Transplanted in Infarcted Heart Tissue. Stem Cells 2018, 36, 540–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- das Ghosh, L.; Ravi, V.; Jain, A.; Panicker, A.G.; Sundaresan, N.R.; Chatterjee, K. Sirtuin 6 Mediated Stem Cell Cardiomyogenesis on Protein Coated Nanofibrous Scaffolds. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Chen, H.; Yang, H.; Wu, H.; Zhao, X.; Wang, H.; Chour, T.; Neofytou, E.; Ding, D.; Daldrup-Link, H.; et al. Photoacoustic Imaging of Embryonic Stem Cell-Derived Cardiomyocytes in Living Hearts with Ultrasensitive Semiconducting Polymer Nanoparticles. Adv. Funct. Mater. 2018, 28, 1704939. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, E.R.; Hableel, G.; Hu, T.; Kim, T.; Li, J.; Gonzalez-Pech, N.I.; Cheng, D.J.; Lemaster, J.E.; Xie, Y.; et al. Increasing the Efficacy of Stem Cell Therapy via Triple-Function Inorganic Nanoparticles. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Kü, E.; Roell, W.; Breitbach, M.; Wecker, S.; Wiedermann, D.; Buehrle, C.; Welz, A.; Hescheler, J.; Fleischmann, B.K.; Hoehn, M. Stem Cell Implantation in Ischemic Mouse Heart: A High-Resolution Magnetic Resonance Imaging Investigation. NMR Biomed. 2005, 18, 362–370. [Google Scholar] [CrossRef]
- Dai, W.; Hale, S.L.; Kay, G.L.; Jyrala, A.J.; Kloner, R.A. Delivering Stem Cells to the Heart in a Collagen Matrix Reduces Relocation of Cells to Other Organs as Assessed by Nanoparticle Technology. Regen. Med. 2009, 4, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct Cell Reprogramming: Approaches, Mechanisms and Progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; Chen, J.; Li, Q.; Gao, J.; Tan, H.; Zhu, Y.; Wang, Z.; Li, M.; Yang, H.; et al. Direct in Vivo Reprogramming with Non-Viral Sequential Targeting Nanoparticles Promotes Cardiac Regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Alan Payne, J.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-Mediated in Vitro and in Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Lee, E.; Kim, J.; Kwon, Y.W.; Kwon, Y.; Kim, J. Efficient in Vivo Direct Conversion of Fibroblasts into Cardiomyocytes Using a Nanoparticle-Based Gene Carrier. Biomaterials 2019, 192, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.A.; Talman, V.; Torrieri, G.; Liu, D.; Marques, G.; Moslova, K.; Liu, Z.; Pinto, J.F.; Hirvonen, J.; Ruskoaho, H.; et al. Dual-Drug Delivery Using Dextran-Functionalized Nanoparticles Targeting Cardiac Fibroblasts for Cellular Reprogramming. Adv. Funct. Mater. 2018, 28, 1705134. [Google Scholar] [CrossRef]
- Giacca, M. Cardiac Regeneration After Myocardial Infarction: An Approachable Goal. Curr. Cardiol. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Fang, R.; Qiao, S.; Liu, Y.; Meng, Q.; Chen, X.; Song, B.; Hou, X.; Tian, W. Sustained Co-Delivery of BIO and IGF-1 by a Novel Hybrid Hydrogel System to Stimulate Endogenous Cardiac Repair in Myocardial Infarcted Rat Hearts. Int. J. Nanomed. 2015, 10, 4691. [Google Scholar] [CrossRef] [PubMed]
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ Functions and Their Regulation at a Glance. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pretorius, D.; Zhou, Y.; Nakada, Y.; Yang, J.; Zhang, J. TT-10–Loaded Nanoparticles Promote Cardiomyocyte Proliferation and Cardiac Repair in a Mouse Model of Myocardial Infarction. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. The ErbB/HER Family of Protein-Tyrosine Kinases and Cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Makki, N.; Thiel, K.W.; Miller, F.J. The Epidermal Growth Factor Receptor and Its Ligands in Cardiovascular Disease. Int. J. Mol. Sci. 2013, 14, 20597–20613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 Triggers Mammalian Heart Regeneration by Promoting Cardiomyocyte Dedifferentiation and Proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, E.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; de Brito VieiraNeto, J.; da Silva, I.J., Jr.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR Targeting for Cancer Therapy: Pharmacology and Immunoconjugates with Drugs and Nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.R.; Pelacho, B.; Garbayo, E.; Imbuluzqueta, I.; Díaz-Herráez, P.; Abizanda, G.; Gavira, J.J.; Simón-Yarza, T.; Albiasu, E.; Tamayo, E.; et al. Controlled Delivery of Fibroblast Growth Factor-1 and Neuregulin-1 from Biodegradable Microparticles Promotes Cardiac Repair in a Rat Myocardial Infarction Model through Activation of Endogenous Regeneration. J. Control. Release 2014, 173, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbayo, E.; Gavira, J.J.; de Yebenes, M.G.; Pelacho, B.; Abizanda, G.; Lana, H.; Blanco-Prieto, M.J.; Prosper, F. Catheter-Based Intramyocardial Injection of FGF1 or NRG1-Loaded MPs Improves Cardiac Function in a Preclinical Model of Ischemia-Reperfusion. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Gil, S.; Abizanda, G.; Iglesias, E.; Garbayo, E.; Prósper, F.; Blanco-Prieto, M.J. NRG1 PLGA MP Locally Induce Macrophage Polarisation toward a Regenerative Phenotype in the Heart after Acute Myocardial Infarction. J. Drug Target. 2018, 27, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Lavin, A.C.; Balkan, W.; Hare, J.M.; White, I.A. Neuregulin-1, in a Conducive Milieu with Wnt/BMP/Retinoic Acid, Prolongs the Epicardial-Mediated Cardiac Regeneration Capacity of Neonatal Heart Explants. J. Stem Cells Regen. Med. 2021, 17, 18. [Google Scholar] [CrossRef]
- Kohane, D.S. Microparticles and Nanoparticles for Drug Delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors Relating to the Biodistribution & Clearance of Nanoparticles & Their Effects on in Vivo Application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; You, J.; Yao, X.; Lu, Q.; Guo, W.; Shen, Y. Superparamagnetic Iron Oxide Nanoparticles Promote Ferroptosis of Ischemic Cardiomyocytes. J. Cell. Mol. Med. 2020, 24, 11030. [Google Scholar] [CrossRef]
- Nagarajan, M.; Maadurshni, G.B.; Tharani, G.K.; Udhayakumar, I.; Kumar, G.; Mani, K.P.; Sivasubramanian, J.; Manivannan, J. Exposure to Zinc Oxide Nanoparticles (ZnO-NPs) Induces Cardiovascular Toxicity and Exacerbates Pathogenesis—Role of Oxidative Stress and MAPK Signaling. Chem. Biol. Interact. 2021, 351, 109719. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.R.; Yue, X.; Zou, B.; Shi, H.; Yu, H.; Liu, K.; Lin, X.; Xu, J.; Yang, C.; Wu, A.; et al. Acute Toxicity of Nickel Nanoparticles in Rats after Intravenous Injection. Int. J. Nanomed. 2014, 9, 1393. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated Liposomes: Immunological Responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [Green Version]
| Study | Year | Nanomaterial | Administration Route | Study Design | Outcome |
|---|---|---|---|---|---|
| O’Dwyer et al. [46] | 2020 | Hyaluronic acid hydrogel embedded with star-shaped polyglutamic acid polypeptides complexed with VEGF (400 nm measured by DLS and 200 by NTA) | N/A | In vitro drug release study | 35 days of sustained VEGF release; |
| Qiao et al. [47] | 2020 | Crosslinked negatively charged heparin polysaccharide nanoparticle loaded with VEGF-A or VEGF-C (155 nm and 150 nm, respectively) | Intravenous injection | Acute myocardial infarction by left anterior descending artery ligation in mice | Delivery of VEGF-A enhanced angiogenesis, while delivery of VEGF-C triggered lymphangiogenesis and diminished local edema; Sequential administration resulted in improved heart function and reduced scar tissue; |
| Oduk et al. [38] | 2018 | PLGA nanoparticles loaded with VEGF (113 nm); | Injection into the peri-necrotic area | Immunocompromised NOD/SCID mice with left anterior descending coronary artery ligation | Improved vascular density, myocardial thickness, and decreased size of the necrotic area, independent on the dose; 31 days of sustained VEGF release; |
| Nagase et al. [48] | 2017 | PLGA nanoparticles loaded with VEGF (110.9 ± 12.0 nm) | N/A | Poly(vinyl alcohol) fiber mat incorporating nanoparticles loaded with VEGF for the transplantation of multilayered cardiomyocytes, implanted in the subcutaneous tissue of an athymic rat | After two weeks, the cardiomyocytes were still viable; The thickness of the sheet was preserved; Enhanced maturation of the blood vessels; |
| Zhang et al. [49] | 2012 | Magnetic nanobeads/adenoviral vectors-encoded hVEGF (300–600 nm) | Intravenous injection | Magnetic field guided treatment of experimentally induced acute myocardial infarction by left anterior descending artery ligation in rats | Increased hVEGF expression in heart at the luminal pole of the endothelial cells; Increased thickness of myocardium, the density of capillary vessels and decreased collagen deposition; |
| Oh et al. [50] | 2010 | 270 nm nanoparticles with a core composed of lecithin and VEGF and a shell represented by poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer | Injection into the peri-necrotic area | The aqueous solution of nanoparticles was mixed with propylene glycol monocaprylate and, thus, a gel was formed at the site of myocardial infarction Acute myocardial infarction by left anterior descending artery ligation in rats | Significantly increased capillary density in the necrotic area with or without the gel formulation; The gel formulation did not change the arterial elastance (an indicator of heart compliance); |
| Study | Year | Nanomaterial | Administration Route | Study Design | Outcome |
|---|---|---|---|---|---|
| Sepúlveda-Rivas et al. [55] | 2021 | Hybrid nanoparticles based on polymeric nanoparticles and gold nanospheres encapsulating angiotensin-(1–9) (sizes between 86.3 ± 1.3 and 108.1 ± 2.4) | N/A | In vitro norepinephrine-induced hypertrophy of CMs | No cytotoxic effect; The release of angiotensin-(1-9) peaks at 15 min; Prevented the hypertrophy of CMs; |
| Lomis et al. [56] | 2021 | 215.2 ± 4.7 nm human serum albumin nanoparticles conjugated with angiotensin II peptide delivering milrinone | N/A | In vitro experiments on embryonic rat CMs (H9C2 line) | The nanomaterial has improved safety profile and reduced cytotoxicity compared to milrinone lactate; Significantly increased uptake by hypoxic and hypertrophic cells; |
| Lomis et al. [57] | 2021 | Human serum albumin nanoparticles conjugated with angiotensin II peptide delivering milrinone (sizes between 190.2 ± 5.7 and 245.6 ± 3.5 nm) | Intravenous or subcutaneous injection | Congestive heart failure induced by left anterior descending artery ligation in rat | Significantly improved the left ventricle ejection fraction at 24 h compared to nanoparticle-free formulation; After one hour the difference between these two approaches was not evident; The effect declined with no significant difference after 1 week of treatment; Intravenous administration was more effective than subcutaneous injections; |
| Bejarano et al. [58] | 2020 | 190 nm thermosensitive liposomes encapsulating angiotensin-(1-9), coated with gold nanoclusters | Retrograde perfusion model | Ex vivo rat heart perfused with angiotensin-(1-9) after near infrared laser irradiation | No cytotoxic effect induced by the nanoplatform or by increased temperature; The biological effect of angiotensin-(1-9) was not affected; Improved heart function; |
| Pechanova et al. [59] | 2019 | 279 nm polylactide acid nanoparticles loaded with aliskiren | Gavage | Spontaneously hypertensive rats | 25% blood pressure reduction compared to 10% in the case of nanoparticle-free formulation; Increased NOS activity in the heart; Decreased the amount of collagen in the aorta; |
| Nakano et al. [60] | 2016 | 200 nm PLGA nanoparticles incorporating irbesartan | Intravenous injection | Ischemia-reperfusion mouse model | The accumulation of the nanoparticles was observed only in the diseased tissue; The nanoparticle accumulated into CMs, monocytes and neutrophils; The concentration of irbesartan was 17-fold higher by nanovehicle delivery; Ameliorated the functions of the heart; |
| Hennig et al. [61] | 2015 | * Fluorescent core-shell quantum dots conjugated with angiotensin II | N/A | Rat mesangial cells, human adrenal gland carcinoma cells (NCI-H295R) and HeLa cells | Specific uptake of the nanoparticles by endocytosis in cell lines expressing AT1 (rat mesangial cells and NCI-H295R) was observed, with localization especially in the perinuclear region; Compared to pristine quantum dots, the proposed nanoparticles triggered a calcium influx into cytosol; |
| Liu et al. [62] | 2013 | Tadpole dendrimers conjugated with oligo-arginine for siRNA delivery to silence AT1 (sizes between 143 ± 29 nm and 247 ± 76 nm) | Intramyocardial injection | Ischemia-reperfusion rat model | At day 3, the expression level of AT1 mRNA was significantly reduced compared to control, with a slight tendency of AT2 level increment; The function of the heart was ameliorated and the necrotic area was reduced; |
| Ghann et al. [27] | 2011 | 14.3 nm gold nanoparticles coated with lisinopril | # | Transmission electron microscopy evaluation of mice lung tissue | The nanosystem targeted the angiotensin-converting enzyme in the lung; The tendency to form large aggregates was observed; |
| Dvir et al. [63] | 2011 | 142 ± 8 nm PEGylated liposome decorated with an aminoacidic sequence that targets the AT1 receptors (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) | Right jugular vein injection | Acute myocardial infarction induced by left anterior descending artery ligation in mice | About 50% of the CMs were targeted by the nanomaterial and around 80% under hypoxic conditions; The nanoparticles accumulate mainly in the left ventricle, while the healthy tissue exhibited only negligible accumulation; |
| Study | Year | Nanomaterial | Imaging | Purpose |
|---|---|---|---|---|
| Keliher et al. [77] | 2017 | 5 nm polyglucose-based nanoparticles labeled with 18F; | PET | Imaging myocardial infarction and atherosclerosis; |
| Ueno et al. [78] | 2013 | 20 nm iron oxide nanoparticles cross-linked with the PET isotope copper-64 and labeled with a fluorophore; | PET/CT | In vivo heart allograft imaging; |
| Majmudar et al. [76] | 2013 | 13 nm dextran nanoparticles modified with desferoxamine and radiolabeled with zirconium-89; | Hybrid PET/MRI | Evaluation of inflammation in atherosclerosis; |
| Nahrendorf et al. [79] | 2011 | * Dextran-coated iron oxide nanoparticles labeled with fluorine-18 and a fluorophore; | PET/CT | Evaluation of inflammation in aortic aneurysm; |
| Morishige et al. [80] | 2010 | 5 nm core composed by superparamagnetic iron oxide with a 10 nm dextran coat; | MRI | Evaluation of inflammation in atherosclerosis; |
| Lipinski et al. [81] | 2009 | 125 nm lipid-based nanoparticles, loaded with gadolinium, targeting the macrophage scavenger receptor-B (CD36); | MRI | Atherosclerosis evaluation; |
| Study | Year | Nanomaterial | Study Design | Outcome |
|---|---|---|---|---|
| Jain et al. [89] | 2021 | Polycaprolactone blended with gelatin nanofibers decorated with 43 ± 5 nm cerium oxide nanoparticles | Phenylephrine-induced hypertrophy on neonatal primary cardiomyocytes | Reduced ROS; Prevent CMs hypertrophy; |
| Wang et al. [90] | 2019 | 300 nm tanshinone IIA nanoparticles entrapped in hydrogel, ROS-sensitive | In vivo myocardial infarction induced by left anterior descending coronary artery ligation | Increased ejection fraction; Decreased necrotic area; Blocked inflammatory gene expression (IL-1β, IL-6, TNF-α) |
| Nabofa et al. [91] | 2018 | 284.0 ± 17.9 nm poly (lactic acid) nanoparticles encapsulating curcumin and nisin | Isoproterenol induced myocardial infarction in guinea pigs | Prevented CMs necrosis by reduced ROS production; Reduced MPO activity; |
| Somasuntharam et al. [92] | 2016 | Gold nanoparticles functionalized with deoxyribozyme (14 ± 3 nm measured by TEM and 80 nm determined by dynamic light scattering) | In vivo myocardial infarction induced by left anterior descending coronary artery ligation | In vivo TNF-α silencing; Reduced iNOS, IL-12b, IL-1β and IL-6; |
| Niu et al. [93] | 2011 | * Cerium oxide nanoparticles | Oxidative stress induced in H9c2 cardiomyocytes by cigarette smoke extract | The nanoparticles prevented the oxidative damage by reduced ROS production with concomitant anti-inflammatory effect (inhibition of NF-κB activation and of inflammatory gene expression); |
| Niu et al. [94] | 2007 | 7 nm cerium oxide nanoparticles | Transgenic mice with heart-specific expression of MCP-1 | Reduced macrophage infiltration and pro-inflammatory cytokines; Reduced peroxynitrite formation; Ameliorated left ventricle dysfunction and heart remodeling; |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonciar, D.; Mocan, T.; Agoston-Coldea, L. Nanoparticles Targeting the Molecular Pathways of Heart Remodeling and Regeneration. Pharmaceutics 2022, 14, 711. https://doi.org/10.3390/pharmaceutics14040711
Gonciar D, Mocan T, Agoston-Coldea L. Nanoparticles Targeting the Molecular Pathways of Heart Remodeling and Regeneration. Pharmaceutics. 2022; 14(4):711. https://doi.org/10.3390/pharmaceutics14040711
Chicago/Turabian StyleGonciar, Diana, Teodora Mocan, and Lucia Agoston-Coldea. 2022. "Nanoparticles Targeting the Molecular Pathways of Heart Remodeling and Regeneration" Pharmaceutics 14, no. 4: 711. https://doi.org/10.3390/pharmaceutics14040711
APA StyleGonciar, D., Mocan, T., & Agoston-Coldea, L. (2022). Nanoparticles Targeting the Molecular Pathways of Heart Remodeling and Regeneration. Pharmaceutics, 14(4), 711. https://doi.org/10.3390/pharmaceutics14040711





