Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Helenalin 3,4-Dimethoxycinnamate (H-DMCA)
2.3. Dermal Absorption Experiments
2.4. UHPLC-HRMS Analysis
2.5. UHPLC-HRMS Method Validation
2.6. UHPLC-FLD Analysis
2.7. Microscopy
3. Results and Discussion
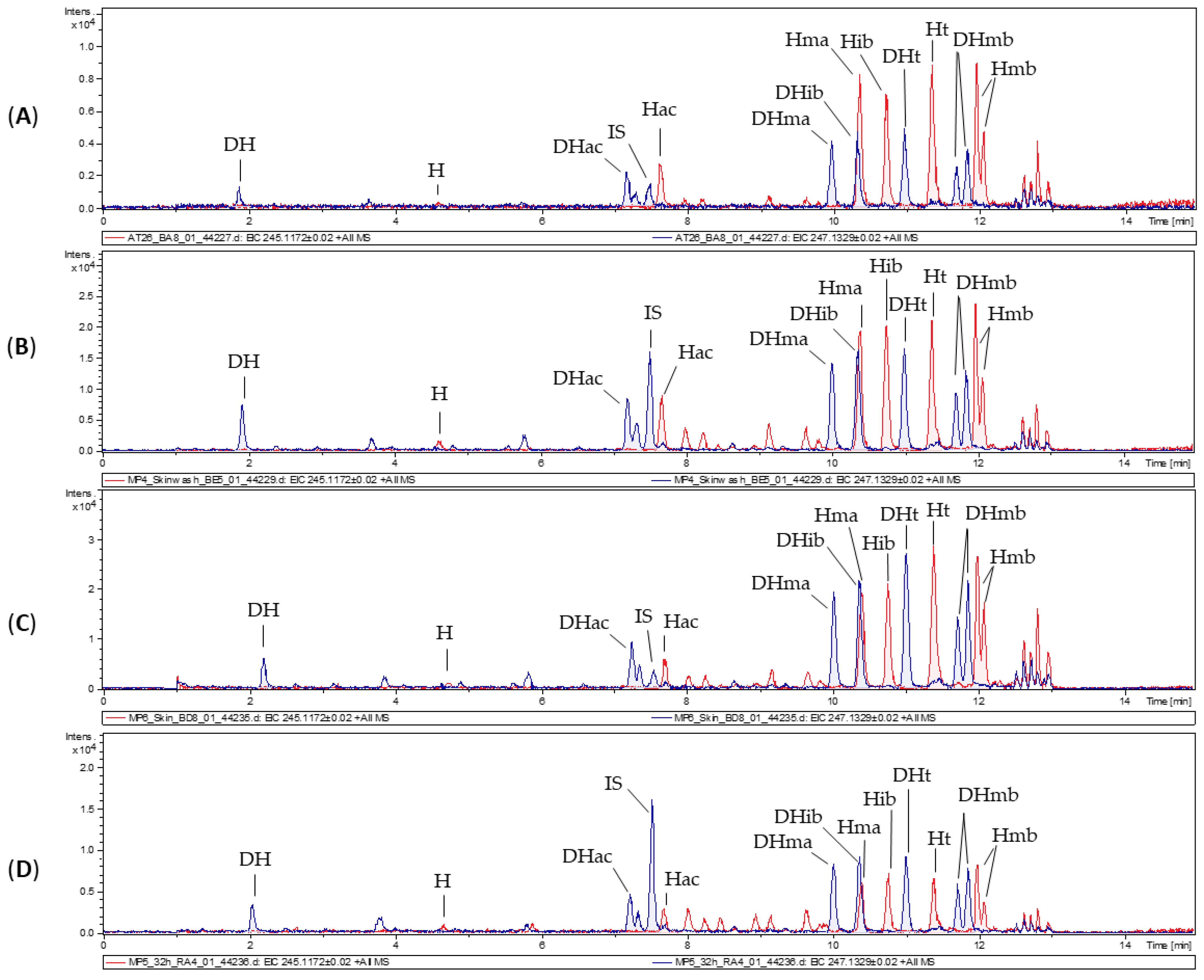
3.1. Characterization of Arnica Tincture
3.2. Dermal Absorption of Arnica Tincture
3.3. Metabolic Activity of Skin Samples
3.4. Synthesis and Characterization of a Fluorescence-Labeled Sesquiterpene Lactone
3.5. Dermal Absorption Experiments with the Fluorescence-Labeled Sesquiterpene Lactone
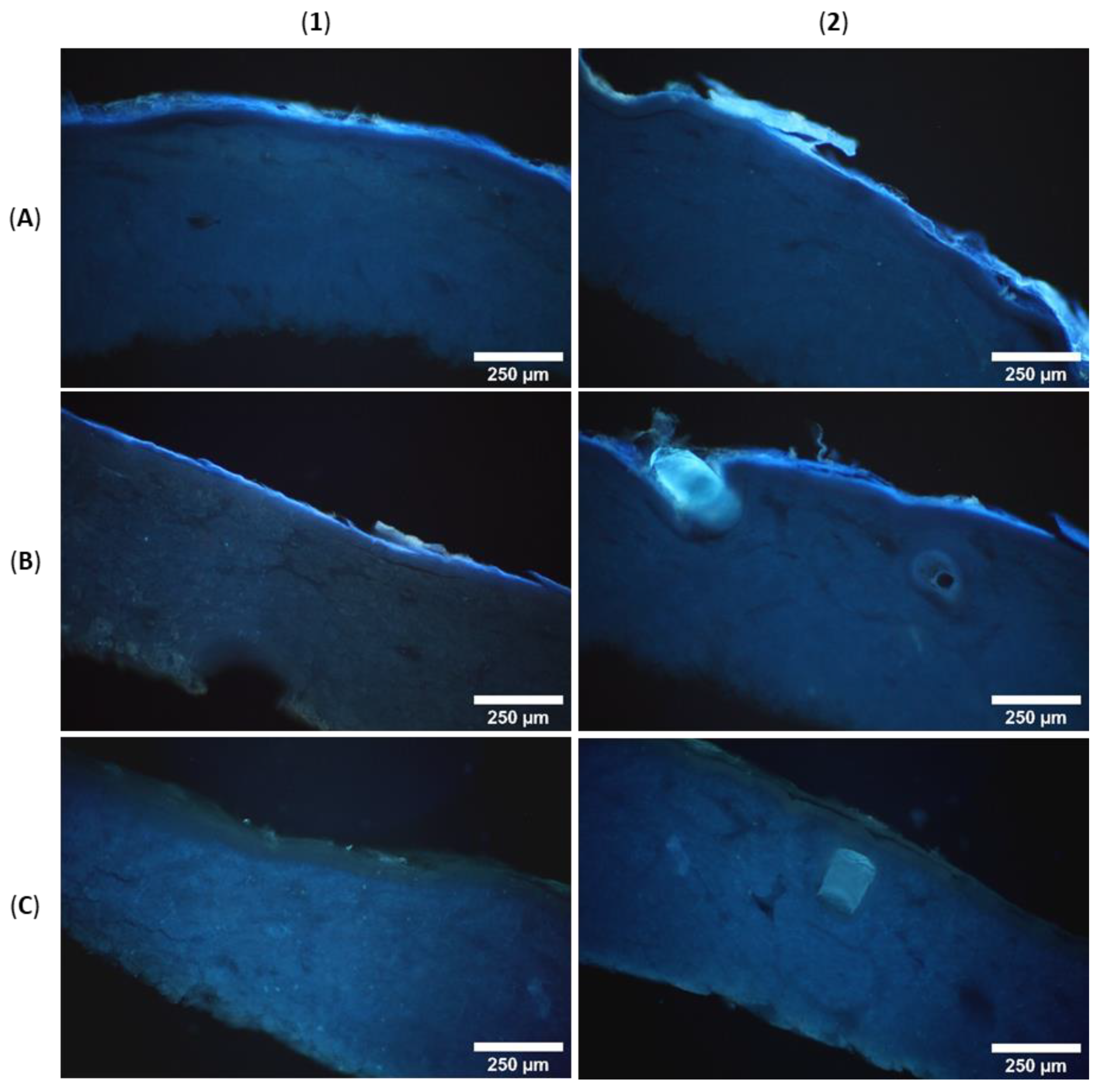
3.6. Microscopic Analysis of Skin Penetration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willuhn, G. Arnica flowers, pharmacology, toxicology and analysis of sesquiterpene lactones, their main active substances. In Phytomedicines of Europe; Lawson, L.D., Bauer, R., Eds.; American Chemical Society: Washington, DC, USA, 1998; Volume 691. [Google Scholar]
- Montesino, N.L.; Kaiser, M.; Brun, R.; Schmidt, T.J. Search for Antiprotozoal Activity in Herbal Medicinal Preparations; New Natural Leads against Neglected Tropical Diseases. Molecules 2015, 20, 14118–14138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulsten, I.F.; Costa-Silva, T.A.; Mesquita, J.T.; Lima, M.L.; Galuppo, M.K.; Taniwaki, N.N.; Borborema, S.E.T.; Da Costa, F.B.; Schmidt, T.J.; Tempone, A.G. Investigation of the Anti-Leishmania (Leishmania) infantum Activity of Some Natural Sesquiterpene Lactones. Molecules 2017, 22, 685. [Google Scholar] [CrossRef] [PubMed]
- Robledo, S.M.; Vélez, I.D.; Schmidt, T.J. Arnica Tincture Cures Cutaneous Leishmaniasis in Golden Hamsters. Molecules 2018, 23, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamante, V.; Ceschel, G.C.; Marazzita, S.; Ronchi, C.; Fini, A. Effect of Vehicles on Topical Application of Aloe Vera and Arnica Montana Components. Drug Deliv. 2007, 14, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Tekko, I.A.; Bonner, M.C.; Bowen, R.D.; Williams, A.C. Permeation of bioactive constituents from Arnica montana preparations through human skin in-vitro. J. Pharm. Pharmacol. 2006, 58, 1167–1176. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Bilia, A.R.; Casiraghi, A.; Cilurzo, F.; Minghetti, P.; Montanari, L.; Vincieri, F.F. Evaluation of skin permeability of sesquiterpenes of an innovative supercritical carbon dioxide Arnica extract by HPLC/DAD/MS. Pharmazie 2005, 60, 36–38. [Google Scholar]
- Wagner, S.; Suter, A.; Merfort, I. Skin penetration studies of Arnica preparations and of their sesquiterpene lactones. Planta Med. 2004, 70, 897–903. [Google Scholar] [CrossRef] [Green Version]
- OECD. OECD Guideline for the Testing of Chemicals. In Dermatotoxicology; Test No. 428: Skin Absorption: In Vitro Method; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Qvist, M.H.; Hoeck, U.; Kreilgaard, B.; Madsen, F.; Frokjaer, S. Evaluation of Göttingen minipig skin for transdermal in vitro permeation studies. Eur. J. Pharm. Sci. 2000, 11, 59–68. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies. Env/Jm/Mono 2004, 2, 1–31. [Google Scholar] [CrossRef]
- Stricker-Krongrad, A.; Shoemake, C.R.; Liu, J.; Brocksmith, D.; Bouchard, G. The importance of minipigs in dermal safety assessment: An overview. Cutan. Ocul. Toxicol. 2017, 36, 105–113. [Google Scholar] [CrossRef]
- Reimer, C.; Ha, N.T.; Sharifi, A.R.; Geibel, J.; Mikkelsen, L.F.; Schlather, M.; Weigend, S.; Simianer, H. Assessing breed integrity of Göttingen Minipigs. BMC Genom. 2020, 21, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojumdar, E.H.; Madsen, L.B.; Hansson, H.; Taavoniku, I.; Kristensen, K.; Persson, C.; Morén, A.K.; Mokso, R.; Schmidtchen, A.; Ruzgas, T.; et al. Probing Skin Barrier Recovery on Molecular Level Following Acute Wounds: An In Vivo/Ex Vivo Study on Pigs. Biomedicines 2021, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol. In Vitro 2015, 29, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Riezk, A.; Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of Amphotericin B-Loaded Chitosan Nanoparticles against Experimental Cutaneous Leishmaniasis. Molecules 2020, 25, 4002. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, F.M.; Behrens, M.; Humpf, H.-U.; Robledo, S.M.; Schmidt, T.J. In Vitro Metabolism of Helenalin Acetate and 11α,13-Dihydrohelenalin Acetate: Natural Sesquiterpene Lactones from Arnica. Metabolites 2022, 12, 88. [Google Scholar] [CrossRef]
- EMA. ICH Guideline M10 on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation (accessed on 17 January 2022).
- Choy, Y.B.; Prausnitz, M.R. The rule of five for non-oral routes of drug delivery: Ophthalmic, inhalation and transdermal. Pharm. Res. 2011, 28, 943–948. [Google Scholar] [CrossRef] [Green Version]
- EDQM. Arnicae flos. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Willuhn, G.; Leven, W.; Luley, C. Arnikabluten DAB 10. Untersuchungen zur qualitativen und quantitativen Variabilität des Sesquiterpenlactonegehaltes der offizinellen Arnikadrogen. Dtsch. Apoth. Ztg. 1994, 134, 4077–4085. [Google Scholar]
- Schmidt, T.J. Helenanolide-type sesquiterpene lactones—III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg. Med. Chem. 1997, 5, 645–653. [Google Scholar] [CrossRef]
- Wagner, S.; Kratz, F.; Merfort, I. In vitro behaviour of sesquiterpene lactones and sesquiterpene lactone-containing plant preparations in human blood, plasma and human serum albumin solutions. Planta Med. 2004, 70, 227–233. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Lyss, G.; Pahl, H.L.; Merfort, I. Helenanolide type sesquiterpene lactones. Part 5: The role of glutathione addition under physiological conditions. Bioorg. Med. Chem. 1999, 7, 2849–2855. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Merk, H.F. Drug metabolism in the skin. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Rudolph, T.; Pietzsch, J.; Meurer, M. Conversion of vitamin D3 to 1α,25-dihydroxyvitamin D3 in human skin equivalents. Exp. Dermatol. 2000, 9, 97–103. [Google Scholar] [CrossRef]
- Kawakubo, Y.; Merk, H.F.; Masaoudi, T.A.; Sieben, S.; Blömeke, B. N-Acetylation of paraphenylenediamine in human skin and keratinocytes. J. Pharmacol. Exp. Ther. 2000, 292, 150–155. [Google Scholar]
- Lutjen, A.B.; Quirk, M.A.; Barbera, A.M.; Kolonko, E.M. Synthesis of (E)-cinnamyl ester derivatives via a greener Steglich esterification. Bioorg. Med. Chem. 2018, 26, 5291–5298. [Google Scholar] [CrossRef]
- Moniz, T.; Lima, S.A.C.; Reis, S. Human skin models: From healthy to disease-mimetic systems; characteristics and applications. Br. J. Pharmacol. 2020, 177, 4314–4329. [Google Scholar] [CrossRef]
- Liebich, H.-G. Funktionelle Histologie der Haussäugetiere; Schattauer: Stuttgart, Germany, 2004; Volume 4. [Google Scholar]
- Wu, D.D.; Irwin, D.M.; Zhang, Y.P. Molecular evolution of the keratin associated protein gene family in mammals, role in the evolution of mammalian hair. BMC Evol. Biol. 2008, 8, 241. [Google Scholar] [CrossRef] [Green Version]
- Hering, A.; Ochocka, J.R.; Baranska, H.; Cal, K.; Stefanowicz-Hajduk, J. Mangiferin and Hesperidin Transdermal Distribution and Permeability through the Skin from Solutions and Honeybush Extracts (Cyclopia sp.)-A Comparison Ex Vivo Study. Molecules 2021, 26, 6547. [Google Scholar] [CrossRef]
- Schwarz, R.; Le Roux, J.M.; Schaller, R.; Neurand, K. Micromorphology of the skin (epidermis, dermis, subcutis) of the dog. Onderstepoort J. Vet. Res. 1979, 46, 105–109. [Google Scholar]




| STL | Formula | MW [g/mol] | HBA | HBD | clogP 1 | c [µg/mL] |
|---|---|---|---|---|---|---|
| H | C15H18O4 | 262.3050 | 4 | 1 | 0.6513 | 15.26 ± 1.09 |
| Hac | C17H20O5 | 304.3420 | 5 | 0 | 1.5038 | 24.79 ± 1.45 |
| Hma | C19H22O5 | 330.3800 | 5 | 0 | 2.1470 | 69.22 ± 0.93 |
| Hib | C19H24O5 | 332.3960 | 5 | 0 | 2.3418 | 51.50 ± 2.54 |
| Ht | C20H24O5 | 344.4070 | 5 | 0 | 2.6760 | 76.68 ± 4.09 |
| Hmb | C20H26O5 | 346.4230 | 5 | 0 | 2.8708 | 108.06 ± 4.23 |
| DH | C15H20O4 | 264.3210 | 4 | 1 | 0.6781 | 65.50 ± 3.12 |
| DHac | C17H22O5 | 306.3580 | 5 | 0 | 1.5305 | 11.24 ± 0.51 |
| DHma | C19H24O5 | 332.3960 | 5 | 0 | 2.1738 | 20.19 ± 1.22 |
| DHib | C19H26O5 | 334.4120 | 5 | 0 | 2.3685 | 46.66 ± 4.80 |
| DHt | C20H26O5 | 346.4230 | 5 | 0 | 2.7028 | 12.31 ± 0.67 |
| DHmb | C20H28O5 | 348.4390 | 5 | 0 | 2.8975 | 22.60 ± 0.59 |
| STL Percentage | Porcine Skin | Human Skin A | Human Skin B |
|---|---|---|---|
| receptor fluid | 8.4 ± 4.0% | 14.6 ± 3.8% | 36.4 ± 3.6% |
| skin extract | 6.9 ± 1.5% | 7.8 ± 1.6% | 6.0 ± 1.2% |
| skin wash solution | 2.4 ± 0.7% | 2.2 ± 0.7% | 0.7 ± 0.3% |
| remaining in skin | 82.3 ± 4.7% | 75.4 ± 4.2% | 56.8 ± 3.9% |
| Metabolite | Rt (min) | Formula | [M+H]+ | RHA | RHB | RP | SHA | SHB | SP |
|---|---|---|---|---|---|---|---|---|---|
| Hac-H2O | 4.9 | C15H20O5 | 323.1490 | ++ | ++ | ++ | ++ | ++ | ++ |
| Hma-H2O | 8.0 | C19H24O6 | 349.1646 | ++ | ++ | ++ | ++ | ++ | ++ |
| Hib-H2O | 8.2 | C19H26O6 | 351.1803 | ++ | ++ | ++ | ++ | ++ | ++ |
| Ht-H2O | 9.1 | C20H26O6 | 363.1803 | ++ | ++ | ++ | ++ | +++ | +++ |
| Hmb-H2O | 9.9 | C20H28O6 | 365.1959 | ++ | +++ | ++ | ++ | ++ | ++ |
| DHac-H2O | 4.3 | C15H22O5 | 325.1646 | + | ++ | + | ++ | ++ | ++ |
| DHma-H2O | 7.0 | C19H26O6 | 351.1803 | ++ | ++ | ++ | ++ | ++ | ++ |
| DHib-H2O | 7.4 | C19H28O6 | 353.1959 | ++ | ++ | ++ | ++ | ++ | ++ |
| DHt-H2O | 8.7 | C20H28O6 | 365.1959 | ++ | ++ | ++ | ++ | ++ | ++ |
| DHmb-H2O | 9.3 | C20H30O6 | 367.2116 | ++ | ++ | ++ | ++ | ++ | ++ |
| Hac-NAC | 6.0 | C22H29NO8S | 468.1687 | + | + | + | - | - | - |
| Hma-NAC | 8.2 | C24H31NO8S | 494.1843 | + | + | + | - | - | - |
| Hib-NAC | 8.4 | C24H33NO8S | 496.1200 | + | + | + | - | - | - |
| Ht-NAC | 8.9 | C25H37NO8S | 508.2000 | + | + | + | - | - | - |
| Hmb-NAC | 9.4 | C25H35NO8S | 510.2156 | + | + | + | - | - | - |
| DHma-NAC | 7.8 | C24H33NO8S | 496.2000 | + | + | + | ++ | ++ | ++ |
| DHib-NAC | 8.1 | C24H35NO8S | 498.2156 | + | + | + | ++ | ++ | ++ |
| DHt-NAC | 8.6 | C25H39NO8S | 510.2156 | + | + | + | ++ | ++ | ++ |
| DHmb-NAC | 9.1 | C25H37NO8S | 512.2313 | + | + | + | ++ | ++ | ++ |
| Hac-Cys | 3.3 | C20H27NO7S | 426.1581 | + | - | + | - | - | + |
| Hma-Cys | 5.5 | C22H29NO7S | 452.1738 | + | + | + | + | - | + |
| Hib-Cys | 5.8 | C22H31NO7S | 454.1894 | + | + | + | + | - | + |
| Ht-Cys | 6.4 | C23H31NO7S | 466.1894 | + | + | + | + | - | + |
| Hmb-Cys | 6.8 | C23H33NO7S | 468.2051 | + | + | + | + | - | + |
| DHac-OH | 6.0 | C17H22O6 | 323.1490 | +++ | +++ | +++ | ++ | ++ | ++ |
| DHma-OH | 8.1 | C19H24O6 | 349.1647 | +++ | +++ | +++ | +++ | +++ | +++ |
| DHib-OH | 8.5 | C19H26O6 | 351.1803 | +++ | +++ | +++ | +++ | +++ | +++ |
| DHt-OH | 9.0 | C20H26O6 | 363.1803 | +++ | +++ | +++ | +++ | +++ | +++ |
| DHmb-OH | 9.6 | C20H28O6 | 365.1960 | +++ | +++ | +++ | +++ | +++ | +++ |
| DHac-OH-H2O | 3.0 | C17H24O7 | 341.1595 | ++ | ++ | ++ | + | + | + |
| DHma-OH-H2O | 5.4 | C19H26O7 | 367.1752 | ++ | ++ | ++ | + | + | + |
| DHib-OH-H2O | 5.8 | C19H28O7 | 369.1908 | ++ | ++ | ++ | + | + | + |
| DHt-OH-H2O | 6.5 | C20H29O7 | 381.1908 | ++ | ++ | ++ | + | + | + |
| DHmb-OH-H2O | 7.0 | C20H30O7 | 383.2065 | ++ | ++ | ++ | + | + | + |
| STL Percentage | Porcine Skin |
|---|---|
| receptor fluid | 0.9 ± 0.2% |
| skin extract | 70.1 ± 5.8% |
| skin wash | 14.7 ± 6.5% |
| remaining in skin | 14.4 ± 11.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jürgens, F.M.; Herrmann, F.C.; Robledo, S.M.; Schmidt, T.J. Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture. Pharmaceutics 2022, 14, 742. https://doi.org/10.3390/pharmaceutics14040742
Jürgens FM, Herrmann FC, Robledo SM, Schmidt TJ. Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture. Pharmaceutics. 2022; 14(4):742. https://doi.org/10.3390/pharmaceutics14040742
Chicago/Turabian StyleJürgens, Franziska M., Fabian C. Herrmann, Sara M. Robledo, and Thomas J. Schmidt. 2022. "Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture" Pharmaceutics 14, no. 4: 742. https://doi.org/10.3390/pharmaceutics14040742







