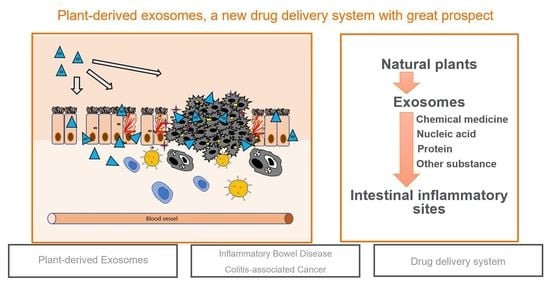
Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer
Abstract
:1. Introduction
- (1)
- Good biocompatibility;
- (2)
- No toxicity and low immunogenicity;
- (3)
- Specifically targeting ability;
- (4)
- Extending the cycle period and prolonging the action time of the drugs;
- (5)
- Production capability in large quantities;
- (6)
- Crossing the Blood–Brain Barrier (BBB) but not the placenta.
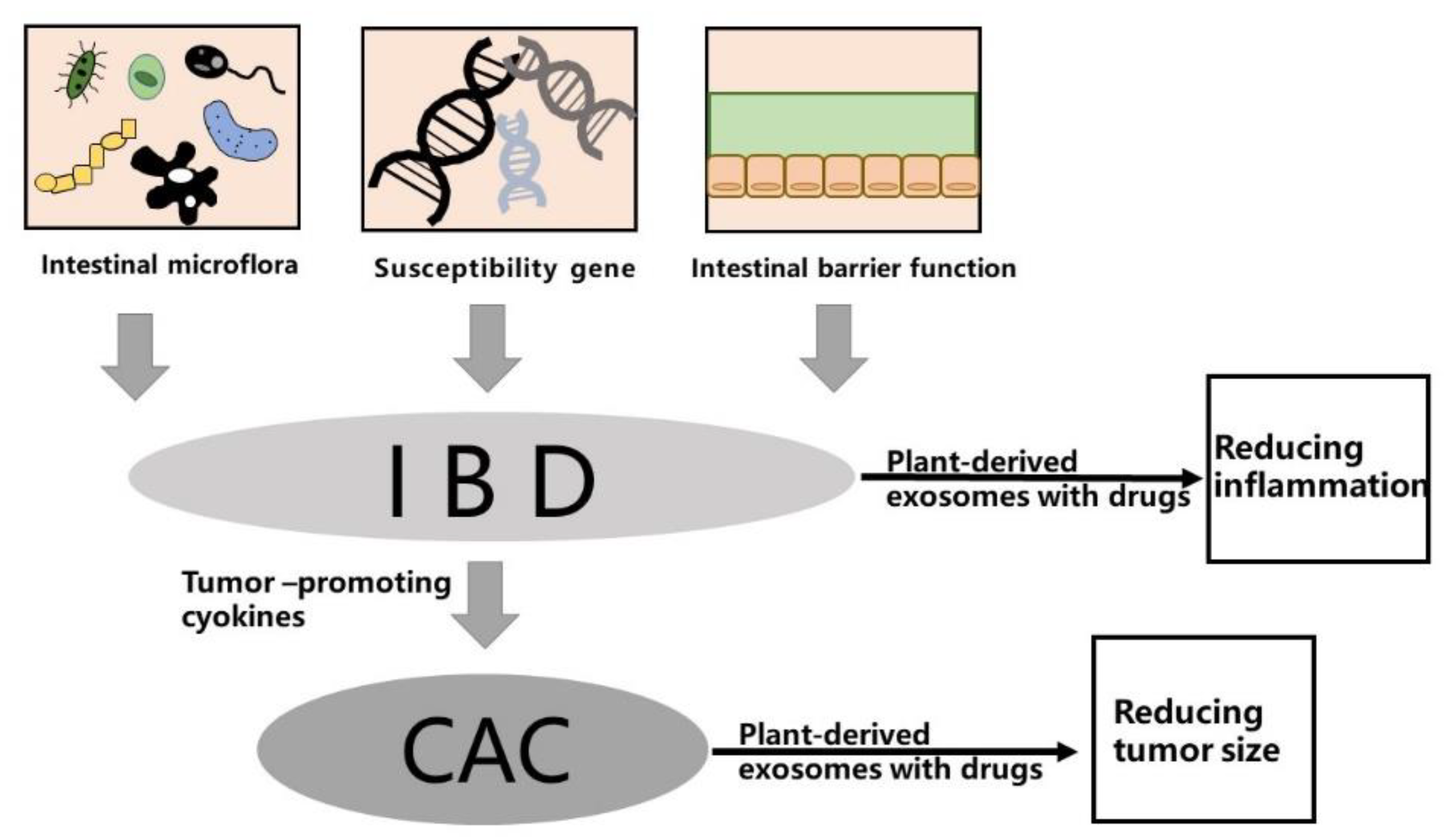
2. Pathogenesis of IBD and CAC
2.1. Gut Microbiota
2.2. Genetic Factors
2.3. Intestinal Microbial Barrier
3. Existing Nanocarriers and Limitations
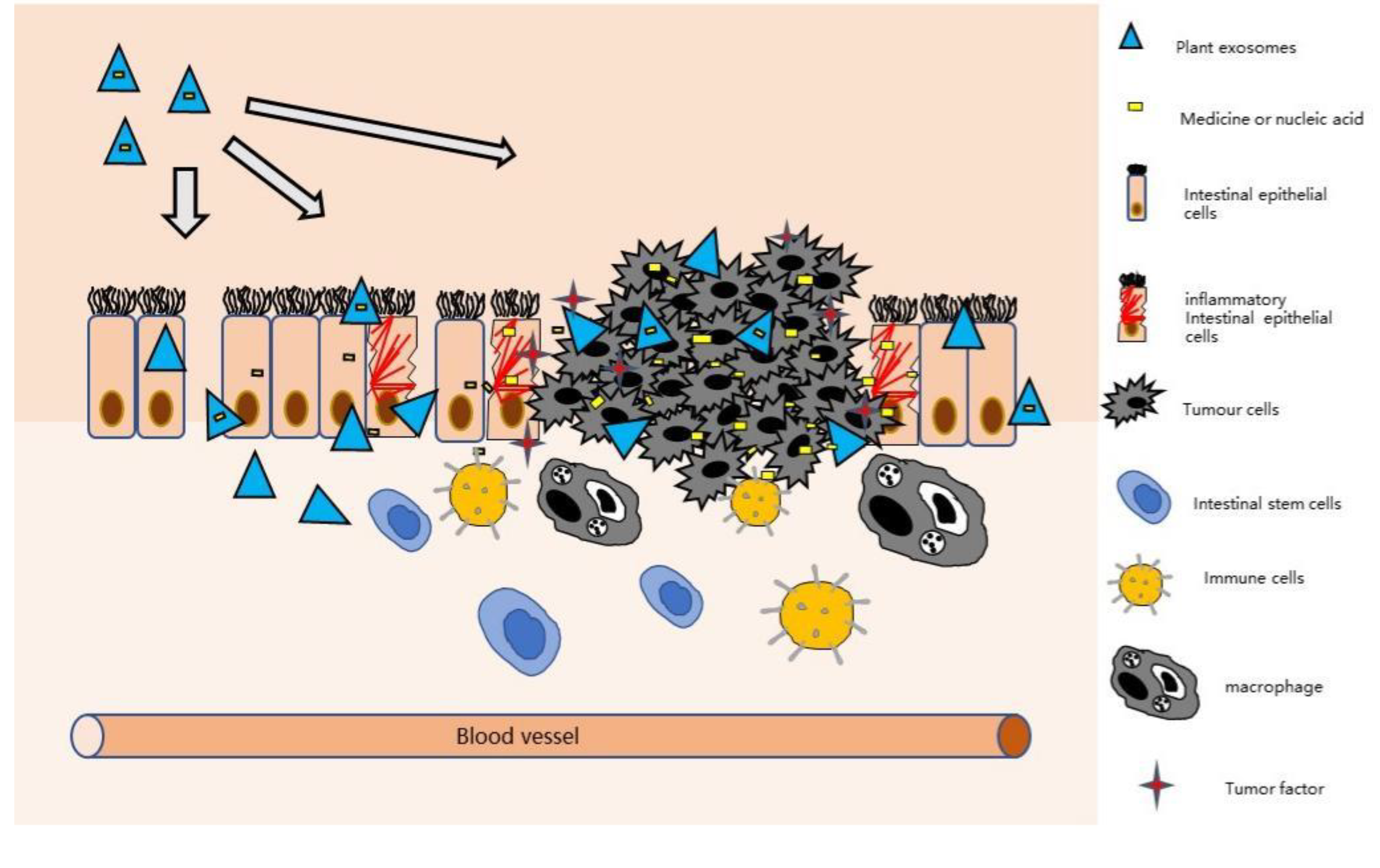
4. Plant-Derived Exosomes Have the Ability to Treat IBD and CAC
4.1. Isolation and Purification of Plant-Derived Exosomes
4.2. Characterization and Constituents of Plant-Derived Exosomes
4.3. Plant-Derived Exosomes Can Relieve Intestinal Inflammation
5. Colon Targeting Mechanism of Plant-Derived Exosomes
5.1. Electrostatic Interaction
5.2. Ligand-Modified NPs
6. Plant-Derived Exosomes as Drug Carriers for the Treatment of IBD and CAC
6.1. Chemical Medicine Loading by Plant-Derived Exosomes
6.2. Nucleic Acid Loading by Plant-Derived Exosomes
7. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sykora, J.; Pomahacova, R.; Kreslova, M.; Cvalinova, D.; Stych, P.; Schwarz, J. Current Global Trends in the Incidence of Pediatric-Onset Inflammatory Bowel Disease. World J. Gastroenterol. 2018, 24, 2741–2763. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory Bowel Disease: An Expanding Global Health Problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, F.; Yang, C.H.; Wang, L.X.; Sung, J.; Garg, P.; Zhang, M.Z.; Merlin, D. Oral Targeted Delivery by Nanoparticles Enhances Efficacy of an Hsp90 Inhibitor by Reducing Systemic Exposure in Murine Models of Colitis and Colitis-Associated Cancer. J. Crohns. Colitis 2020, 14, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Veloso, P.M.; Machado, R.; Nobre, C. Mesalazine and Inflammatory Bowel Disease-From Well-Established Therapies to Progress Beyond the State of the Art. Eur. J. Pharm. Biopharm. 2021, 167, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and Colitis-Associated Cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in Oral Nano-Delivery Systems for Colon Targeted Drug Delivery in Inflammatory Bowel Disease: Selective Targeting to Diseased Versus Healthy Tissue. Nanomed. Nanotechnol. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [Green Version]
- Radmanesh, F.; Razi, M.; Shalizar-Jalali, A. Curcumin Nano-Micelle Induced Testicular Toxicity in Healthy Rats; Evidence for Oxidative Stress and Failed Homeostatic Response by Heat Shock Proteins 70–2a and 90. Biomed. Pharmacother. 2021, 142, 111945. [Google Scholar] [CrossRef]
- Hu, M.; Palic, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox. Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2021, 9, 811971. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, Opportunity, and Perspective on Exosome Isolation–Efforts for Efficient Exosome-Based Theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Yang, C.H.; Merlin, D. Nanoparticle-Mediated Drug Delivery Systems for The Treatment Of IBD: Current Perspectives. Int. J. Nanomed. 2019, 14, 8875–8889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.M.; Zhuang, X.Y.; Deng, Z.B.; Jiang, H.; Mu, J.Y.; Wang, Q.L.; Xiang, X.Y.; Guo, H.X.; Zhang, L.F.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic Exosome-Like Vesicles: A New Way of Protein Secretion in Plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peswani Sajnani, S.L.; Zhang, Y.; Vllasaliu, D. Exosome-Based Therapies for Mucosal Delivery. Int. J. Pharm. 2021, 608, 121087. [Google Scholar] [CrossRef]
- Zu, M.; Xie, D.; Canup, B.S.B.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ Nanotherapeutics from Tea Leaves for Orally Targeted Prevention and Alleviation of Colon Diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural Exosome-Like Nanovesicles from Edible Tea Flowers Suppress Metastatic Breast Cancer Via ROS Generation and Microbiota Modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Rome, S. Biological Properties of Plant-Derived Extracellular Vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.W.; Uchiyama, K. Uptake of MicroRNAs from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Feng, S.Y.; Wang, X.; Long, K.R.; Luo, Y.; Wang, Y.H.; Ma, J.D.; Tang, Q.Z.; Jin, L.; Li, X.W.; et al. Identification of Exosome-Like Nanoparticle-Derived Micrornas from 11 Edible Fruits and Vegetables. Peerj. 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Trentini, M.; Zanotti, F.; Tiengo, E.; Camponogara, F.; Degasperi, M.; Licastro, D.; Lovatti, L.; Zavan, B. An Apple a Day Keeps the Doctor Away: Potential Role of miRNA 146 on Macrophages Treated with Exosomes Derived from Apples. Biomedicines 2022, 10, 415. [Google Scholar] [CrossRef]
- Mu, J.Y.; Zhuang, X.Y.; Wang, Q.L.; Jiang, H.; Deng, Z.B.; Wang, B.M.; Zhang, L.F.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S. Orally administered liposomal formulations for colon targeted drug delivery. Front. Pharmacol. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Bermudez, P.; Blesa, J.; Soriano, J.M.; Marcilla, A. Extracellular Vesicles in food: Experimental Evidence of Their Secretion in Grape Fruits. Eur. J. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-alpha-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- Yang, C.H.; Zhang, M.Z.; Merlin, D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef]
- Sarvarian, P.; Samadi, P.; Gholipour, E.; Asenjan, K.S.; Hojjat-Farsangi, M.; Motavalli, R.; Khiavi, F.M.; Yousefi, M. Application of Emerging Plant-Derived Nanoparticles as a Novel Approach for Nano-Drug Delivery Systems. Immunol. Investig. 2021, 25, 1–21. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Lu, L.; Chen, G.X.; Qiu, Y.Y.; Li, M.W.; Liu, D.H.; Hu, D.H.; Gu, X.J.; Xiao, Z.Y. Nanoparticle-based oral delivery systems for colon targeting: Principles and design strategies. Sci. Bull. 2016, 61, 670–681. [Google Scholar] [CrossRef]
- Man, F.L.; Meng, C.; Liu, Y.; Wang, Y.C.; Zhou, Y.; Ma, J.Q.; Lu, R. The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. Aaps. Pharmscitech. 2021, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-Like Nanoparticles from Mulberry Bark Prevent DSS-Induced Colitis Via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Lautenschlager, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.W.; Mu, J.Y.; Dokland, T.; Zhuang, X.Y.; Wang, Q.L.; Jiang, H.; Xiang, X.Y.; Deng, Z.B.; Wang, B.M.; Zhang, L.F.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talley, N.J.; Abreu, M.T.; Achkar, J.P.; Bernstein, C.N.; Dubinsky, M.C.; Hanauer, S.B.; Kane, S.V.; Sandborn, W.J.; Ullman, T.A.; Moayyedi, P.; et al. An Evidence-Based Systematic Review on Medical Therapies for Inflammatory Bowel Disease. Am. J. Gastroenterol. 2011, 106, S2–S25. [Google Scholar] [CrossRef]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jerome, C.; Brayden, D.J.; Schneider, Y.J.; Preat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharmaceut. 2013, 440, 3–12. [Google Scholar] [CrossRef]
- Holmberg, F.E.O.; Pedersen, J.; Jorgensen, P.; Soendergaard, C.; Jensen, K.B.; Nielsen, O.H. Intestinal barrier integrity and inflammatory bowel disease: Stem cell-based approaches to regenerate the barrier. J. Tissue Eng. Regen. M 2018, 12, 923–935. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.W.; Xia, Y.; Li, W.; Wang, K.H.; Wang, L.; Miao, Y.L.; Ma, S. The gut microbiota heterogeneity and assembly changes associated with the IBD. Sci. Rep. 2019, 9, 440. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.J.; Bai, Y.R.; Khan, A.; Zhao, T.; Che, T.J.; Zhang, C.J. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, O.H.; Steenholdt, C.; Juhl, C.B.; Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: A systematic review and meta-analysis of randomized, controlled trials. Eclinicalmedicine 2020, 20, 100271. [Google Scholar] [CrossRef]
- Haag, L.M.; Siegmund, B. Intestinal microbiota and the innate immune system–a crosstalk in Crohn’s disease pathogenesis. Front. Immunol. 2015, 6, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Ther. Adv. Gastroenter. 2016, 9, 606–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.J.; Lloyd-Price, J.; Vatanen, T.; Seksik, P.; Beaugerie, L.; Simon, T.; Vlamakis, H.; Sokol, H.; Xavier, R.J. Linking Strain Engraftment in Fecal Microbiota Transplantation with Maintenance of Remission in Crohn’s Disease. Gastroenterology 2020, 159, 2193–2202.e5. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Denman, S.E.; Morrison, M.; Yu, Z.; Dore, J.; Leclerc, M.; McSweeney, C.S. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010, 16, 2034–2042. [Google Scholar] [CrossRef]
- Richard, A.C.; Peters, J.E.; Savinykh, N.; Lee, J.C.; Hawley, E.T.; Meylan, F.; Siegel, R.M.; Lyons, P.A.; Smith, K.G.C. Reduced monocyte and macrophage TNFSF15/TL1A expression is associated with susceptibility to inflammatory bowel disease. PLoS Genet. 2018, 14, e1007458. [Google Scholar] [CrossRef]
- Mohanan, V.; Nakata, T.; Desch, A.N.; Levesque, C.; Boroughs, A.; Guzman, G.; Cao, Z.; Creasey, E.; Yao, J.; Boucher, G.; et al. C1orf106 is a colitis risk gene that regulates stability of epithelial adherens junctions. Science 2018, 359, 1161–1166. [Google Scholar] [CrossRef] [Green Version]
- Diez-Sainz, E.; Lorente-Cebrian, S.; Aranaz, P.; Riezu-Boj, J.I.; Martinez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef]
- Marsh, V.; Winton, D.J.; Williams, G.T.; Dubois, N.; Trumpp, A.; Sansom, O.J.; Clarke, A.R. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat. Genet. 2008, 40, 1436–1444. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Fukata, M.; Shang, L.M.; Santaolalla, R.; Sotolongo, J.; Pastorini, C.; Espana, C.; Ungaro, R.; Harpaz, N.; Cooper, H.S.; Elson, G.; et al. Constitutive Activation of Epithelial TLR4 Augments Inflammatory Responses to Mucosal Injury and Drives Colitis-associated Tumorigenesis. Inflamm. Bowel Dis. 2011, 17, 1464–1473. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Ermann, J.; Succi, M.D.; Zhou, A.; Hamilton, M.J.; Cao, B.; Korzenik, J.R.; Glickman, J.N.; Vemula, P.K.; Glimcher, L.H.; et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 2015, 7, 300ra128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampfer, A.A.M.; Busch, M.; Schins, R.P.F. Advanced In Vitro Testing Strategies and Models of the Intestine for Nanosafety Research. Chem. Res. Toxicol. 2020, 33, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Q.; Xiong, Y.; Xu, L. Current Strategies and Potential Prospects of Nanomedicine-Mediated Therapy in Inflammatory Bowel Disease. Int. J. Nanomed. 2021, 16, 4225–4237. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10-to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Siontorou, C.G.; Nikoleli, G.P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes Basel 2017, 7, 38. [Google Scholar] [CrossRef]
- Yamasaki, M.; Muraki, Y.; Nishimoto, Y.; Murakawa, Y.; Matsuo, T. Fluorescence-labeled liposome accumulation in injured colon of a mouse model of T-cell transfer-mediated inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2017, 494, 188–193. [Google Scholar] [CrossRef]
- Arranz, A.; Reinsch, C.; Papadakis, K.A.; Dieckmann, A.; Rauchhaus, U.; Androulidaki, A.; Zacharioudaki, V.; Margioris, A.N.; Tsatsanis, C.; Panzner, S. Treatment of experimental murine colitis with CD40 antisense oligonucleotides delivered in amphoteric liposomes. J. Control Release 2013, 165, 163–172. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Zhang, M.; Lama, S.; Wang, L.; Merlin, D. Natural-lipid nanoparticle-based therapeutic approach to deliver 6-shogaol and its metabolites M2 and M13 to the colon to treat ulcerative colitis. J. Control. Release 2020, 323, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Ghoroghchian, P.P.; Zhang, Y.; Hammer, D.A. Adhesion of antibody-functionalized polymersomes. Langmuir 2006, 22, 3975–3979. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhang, N. Micelle-like nanoassemblies based on polymer-drug conjugates as an emerging platform for drug delivery. Expert Opin. Drug Deliv. 2012, 9, 805–822. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 763–771. [Google Scholar] [CrossRef]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 2018, 8, 23–33. [Google Scholar] [CrossRef]
- Kolishetti, N.; Dhar, S.; Valencia, P.M.; Lin, L.Q.; Karnik, R.; Lippard, S.J.; Langer, R.; Farokhzad, O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar] [CrossRef] [Green Version]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.J.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B.; et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, S.; Hossain, M.N.; Conese, M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. Wars 2020, 15, 1096–1122. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plant-Derived Edible Nanoparticles and miRNAs: Emerging Frontier for Therapeutics and Targeted Drug-Delivery. ACS Sustain. Chem. Eng. 2019, 7, 8055–8069. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomed. Nanotechnol. 2020, 29, 102271. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of Exosome-Like Vesicles from Plants by Ultracentrifugation on Sucrose/Deuterium Oxide (D2O) Density Cushions. Methods Mol. Biol. 2016, 1459, 259–269. [Google Scholar] [CrossRef]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.J.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B.; et al. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef]
- Li, X.F.; Bao, H.; Wang, Z.; Wang, M.X.; Fan, B.F.; Zhu, C.; Chen, Z.X. Biogenesis and Function of Multivesicular Bodies in Plant Immunity. Front. Plant Sci. 2018, 9, 979. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.Y.; Deng, Z.B.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiak, L.; Pocsfalvi, G. Membrane Transporters in Citrus clementina Fruit Juice-Derived Nanovesicles. Int. J. Mol. Sci. 2019, 20, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocsfalvi, G.; Turiak, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vekey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant. Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.L.; Wang, M.; He, B.Y.; Lin, F.M.; Palmquist, J.; Huang, S.N.D.; Jin, H.L. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parasramka, M.A.; Ho, E.; Williams, D.E.; Dashwood, R.H. MicroRNAs, diet, and cancer: New mechanistic insights on the epigenetic actions of phytochemicals. Mol. Carcinog. 2012, 51, 213–230. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Xu, F.Y.; Zhang, X.C.; Mu, J.Y.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant. Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hou, D.X.; Chen, X.; Li, D.H.; Zhu, L.Y.; Zhang, Y.J.; Li, J.; Bian, Z.; Liang, X.Y.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 273–274. [Google Scholar] [CrossRef]
- Zhu, W.J.; Liu, Y.; Cao, Y.N.; Peng, L.X.; Yan, Z.Y.; Zhao, G. Insights into Health-Promoting Effects of Plant MicroRNAs: A Review. J. Agric. Food Chem. 2021, 69, 14372–14386. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhou, Y.; Yu, J.J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Viennois, E.; Prasad, M.; Zhang, Y.C.; Wang, L.X.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.L.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the MicroRNA Profile of Ginger Exosome-like Nanoparticles and Their Anti-Inflammatory Effects in Intestinal Caco-2 Cells. J. Agric. Food Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, C.; Teng, Y.; He, L.; Sayed, M.; Mu, J.; Xu, F.; Zhang, X.; Kumar, A.; Sundaram, K.; Sriwastva, M.K.; et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. iScience 2021, 24, 102511. [Google Scholar] [CrossRef]
- Zeki, S.S.; Graham, T.A.; Wright, N.A. Stem cells and their implications for colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 90–100. [Google Scholar] [CrossRef]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 2014. 20, 1165–1179. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.Y.; Yu, C.H.; Rao, Y.F. Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed. Pharm. 2020, 129, 2798–2818. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhuang, X.Y.; Mu, J.Y.; Deng, Z.B.; Jiang, H.; Zhang, L.F.; Xiang, X.Y.; Wang, B.M.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun 2016, 4, 1867. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Choi, J.H.; Deng, Z.J.; Cho, N.J.; Hammond, P.T. Bimodal Tumor-Targeting from Microenvironment Responsive Hyaluronan Layer-by-Layer (LbL) Nanoparticles. Acs. Nano. 2014, 8, 8374–8382. [Google Scholar] [CrossRef] [Green Version]
- Mohan, L.J.; Daly, J.S.; Ryan, B.M.; Ramtoola, Z. The future of nanomedicine in optimising the treatment of inflammatory bowel disease. Scand. J. Gastroenterol. 2019, 54, 18–26. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; De La Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Yang, C.H.; Merlin, D. Oral Delivery of Nanoparticles Loaded with Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. Gastroenterology 2018, 154, S44. [Google Scholar] [CrossRef]
- Takedatsu, H.; Mitsuyama, K.; Torimura, T. Nanomedicine and drug delivery strategies for treatment of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11343–11352. [Google Scholar] [CrossRef]
- Xiao, B.; Ma, L.J.; Merlin, D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin. Drug Deliv. 2017, 14, 65–73. [Google Scholar] [CrossRef]
- Dulai, P.S.; Siegel, C.A.; Colombel, J.F.; Sandborn, W.J.; Peyrin-Biroulet, L. Systematic review: Monotherapy with antitumour necrosis factor alpha agents versus combination therapy with an immunosuppressive for IBD. Gut 2014, 63, 1843–1853. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.L.; Merlin, D. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Sun, M.H.; Wang, D.; Li, G.Y.; Huang, J.G.; Tan, S.W.; Bao, L.; Li, Q.; Li, G.; Si, L.Q. A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis. Biomater. Sci. 2019, 7, 4299–4309. [Google Scholar] [CrossRef]
- Zhang, W.N.; Michalowski, C.B.; Beloqui, A. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Han, M.; Chen, C.; Wang, X.; Han, J.; Gao, Y.; Wang, S. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale 2021, 13, 20157–20169. [Google Scholar] [CrossRef]
- Raimondo, S.; Giavaresi, G.; Lorico, A.; Alessandro, R. Extracellular Vesicles as Biological Shuttles for Targeted Therapies. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles-Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Si, X.Y.; Han, M.K.; Viennois, E.; Zhang, M.Z.; Merlin, D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B 2015, 3, 7724–7733. [Google Scholar] [CrossRef] [PubMed]
- Record, M. Exosome-like Nanoparticles from Food: Protective Nanoshuttles for Bioactive Cargo. Mol. Ther. 2013, 21, 1294–1296. [Google Scholar] [CrossRef] [Green Version]
- Han, W.D.; Xie, B.B.; Li, Y.R.; Shi, L.L.; Wan, J.Q.; Chen, X.N.; Wang, H.X. Orally Deliverable Nanotherapeutics for the Synergistic Treatment of Colitis-Associated Colorectal Cancer. Theranostics 2019, 9, 7458–7473. [Google Scholar] [CrossRef]
- Zu, M.; Ma, Y.; Cannup, B.; Xie, D.; Jung, Y.; Zhang, J.; Yang, C.; Gao, F.; Merlin, D.; Xiao, B. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv. Drug Deliv. Rev. 2021, 176, 113887. [Google Scholar] [CrossRef]
- Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M.A.; Zhang, Y.C.; Zhang, Z.; Baker, M.T.; Zhang, B.Y.; Gewirtz, A.T.; et al. Nanoparticles with Surface Antibody Against CD98 and Carrying CD98 Small Interfering RNA Reduce Colitis in Mice. Gastroenterology 2014, 146, 1289–1300. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Gan, J.J.; Jia, L.X.; Guo, G.X.; Wang, C.M.; Zang, Y.H.; Ding, Z.; Chen, J.N.; Zhang, J.F.; Dong, L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials 2015, 48, 26–36. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, H.; Shi, W.; Chen, L.; Chen, T.; Chen, G.; Wang, W.; Lan, J.; Huang, Z.; Zhang, J.; et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnol. 2021, 19, 439. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Kim, G.; Lee, Y.; Ha, J.; Han, S.; Lee, M. Engineering exosomes for pulmonary delivery of peptides and drugs to inflammatory lung cells by inhalation. J. Control Release 2021, 330, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Wang, H.Z.; Yin, H.R.; Bennett, C.; Zhang, H.G.; Guo, P.X. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Loftus, E.V.; Talwalkar, J.A. Inflammatory Bowel Disease after Liver Transplantation for Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2013, 108, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, T.; Chang, Y.N.; Duan, C.G. Small RNA Functions as a Trafficking Effector in Plant Immunity. Int. J. Mol. Sci. 2019, 20, 2816. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.B.; Rong, Y.; Teng, Y.; Mu, J.Y.; Zhuang, X.Y.; Tseng, M.; Samykutty, A.; Zhang, L.F.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Z.; Wang, X.Y.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef]
- Lange, H.; Gagliardi, D. Catalytic activities, molecular connections, and biological functions of plant RNA exosome complexes. Plant Cell 2021, 34, 967–988. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Kameli, N.; Dragojlovic-Kerkache, A.; Savelkoul, P.; Stassen, F.R. Plant-Derived Extracellular Vesicles: Current Findings, Challenges, and Future Applications. Membranes 2021, 11, 411. [Google Scholar] [CrossRef]


| Drug | Delivery System | Target | Disease | Reference |
|---|---|---|---|---|
| 17-AAG | PLGA | Heat shock protein 90(Hsp90) | UC/CAC | [3] |
| 6-gingerol/ 6-shogaol | Ginger-derived exosomes | TNF-α/IL-6/IL-1β/IL-10/IL-12 | IBD | [91] |
| PLGA/PLA-PEG-FA | [103] | |||
| MTX | Grapefruit-derived exosomes | Macrophage | IBD | [88] |
| Dox | Ginger-derived exosomes/ Ginger-derived exosomes combined with FA | Cellular DNA | CAC | [109] |
| siRNA-CD98 | Ginger-derived exosomes | Epithelial/Macrophage | UC | [117] |
| JSI-124 | Grapefruit-derived exosomes/Grapefruit-derived exosomes combined with FA | Stat3 | Brain tumor | [70] |
| Dtxl | PLGA | Microtubule | Tumor | [105] |
| CPT | Synthetic nanoparticles | Epithelial | CAC | [113] |
| Cy3-siRNA | Termed thioketal nanoparticles (TKNs) | TNF-α | IBD | [118] |
| ASO | Amphoteric liposomes | CD40 | IBD | [58] |
| Indocyanine green (ICG) | Aloe-derived exosomes | Melanoma cell | Melanoma | [119] |
| MiR-21 inhibitor and chemotherapeutics | Engineered exosomes | Cellular DNA | CAC | [120] |
| RAGE-binding peptide (RBP) | Engineered exosomes | Cytokine assays in macrophage cells | Lung inflammation | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics 2022, 14, 822. https://doi.org/10.3390/pharmaceutics14040822
Cai Y, Zhang L, Zhang Y, Lu R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics. 2022; 14(4):822. https://doi.org/10.3390/pharmaceutics14040822
Chicago/Turabian StyleCai, Ying, Luoxin Zhang, Youjian Zhang, and Rong Lu. 2022. "Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer" Pharmaceutics 14, no. 4: 822. https://doi.org/10.3390/pharmaceutics14040822
APA StyleCai, Y., Zhang, L., Zhang, Y., & Lu, R. (2022). Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics, 14(4), 822. https://doi.org/10.3390/pharmaceutics14040822