Pharmacokinetic Variability Drives Palbociclib-Induced Neutropenia in Metastatic Breast Cancer Patients: Drug–Drug Interactions Are the Usual Suspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design and Patients
2.2. Endpoint Analysis (Palbociclib Exposure–Toxicity Relationship)
2.3. Pharmacokinetics
2.4. Exposure–Toxicity Analysis
2.5. Exposure-DDI Relationship Analysis
2.6. Statistical Analysis
3. Results
3.1. Patients
3.2. Clinical–Biological Data and Palbociclib-Induced Toxicity
3.3. Palbociclib Pharmacokinetics and Clinicopathological Features
3.4. Palbociclib Exposure and Co-Medication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, W.; O’Dwyer, P.J.; Finn, R.S.; Ruiz-Garcia, A.; Shapiro, G.I.; Schwartz, G.K.; DeMichele, A.; Wang, D. Characterization of Neutropenia in Advanced Cancer Patients Following Palbociclib Treatment Using a Population Pharmacokinetic-Pharmacodynamic Modeling and Simulation Approach. J. Clin. Pharmacol. 2017, 57, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Matsuo, K.; Takahari, D.; Yokota, T.; Shibata, T.; Ura, T.; Ito, S.; Tajika, M.; Kawai, H.; Muro, K. Neutropenia as a prognostic factor in advanced gastric cancer patients undergoing second-line chemotherapy with weekly paclitaxel. Ann. Oncol. 2010, 21, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhong, X.; Ma, J.; Sun, W.; Han, H.S.; Soliman, H.H.; Loftus, L.S.; Costa, R.L.B.; Armaghani, A.J.; Soyano-Muller, A.E.; et al. Real-world benefit of combination palbociclib and endocrine therapy for metastatic breast cancer and correlation with neutropenia. Cancer Med. 2021, 10, 7665–7672. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Schmidt, M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 2018, 10, 175883591879332. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, D.D. A Population Pharmacokinetic (Pk) Analysis of Palbociclib (Pd-0332991) in Patients (Pts) with Advanced Solid Tumors. Ann. Oncol. 2014, 25, iv154. [Google Scholar] [CrossRef]
- Yu, Y.; Loi, C.-M.; Hoffman, J.; Wang, D. Physiologically Based Pharmacokinetic Modeling of Palbociclib: PBPK Modeling of Palbociclib. J. Clin. Pharmacol. 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Hu, W.; Sung, T.; Jessen, B.A.; Thibault, S.; Finkelstein, M.B.; Khan, N.K.; Sacaan, A.I. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin. Cancer Res. 2016, 22, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- DeMichele, A.; Clark, A.S.; Tan, K.S.; Heitjan, D.F.; Gramlich, K.; Gallagher, M.; Lal, P.; Feldman, M.; Zhang, P.; Colameco, C.; et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: Phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, M.E.; Danesi, R.; Girmenia, C.; Invernizzi, P.; Elvevi, A.; Uguccioni, M.; on behalf of NetworkER+. Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: A multidisciplinary approach is the key to success. Breast Cancer Res. Treat. 2019, 176, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Levêque, D.; Veal, G.; Astier, A. Review of therapeutic drug monitoring of anticancer drugs part two—Targeted therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef]
- Bellet, M.; Ahmad, F.; Villanueva, R.; Valdivia, C.; Palomino-Doza, J.; Ruiz, A.; Gonzalez, X.; Adrover, E.; Azaro, A.; Valls-Margarit, M.; et al. Palbociclib and ribociclib in breast cancer: Consensus workshop on the management of concomitant medication. Ther. Adv. Med. Oncol. 2019, 11, 175883591983386. [Google Scholar] [CrossRef]
- Sun, W.; Klamerus, K.J.; Yuhas, L.M.; Pawlak, S.; Plotka, A.; O’Gorman, M.; Kirkovsky, L.; Kosa, M.; Wang, D. Impact of Acid-Reducing Agents on the Pharmacokinetics of Palbociclib, a Weak Base with pH-Dependent Solubility, with Different Food Intake Conditions. Clin. Pharmacol. Drug Dev. 2017, 6, 614–626. [Google Scholar] [CrossRef]
- Momper, J.D.; Yang, J.; Kerr, J.; Saunders, I.; Smith, J.; Shah, M.M. Interaction Between Cyclosporine and Palbociclib in a Renal Transplant Patient: Case Report and Pharmacokinetic Perspective. J. Pharm. Pract. 2020, 33, 912–914. [Google Scholar] [CrossRef]
- Gowarty, J.L.; Herrington, J.D. Verapamil as a culprit of palbociclib toxicity. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2019, 25, 743–746. [Google Scholar] [CrossRef]
- Roncato, R.; Angelini, J.; Pani, A.; Cecchin, E.; Sartore-Bianchi, A.; Siena, S.; De Mattia, E.; Scaglione, F.; Toffoli, G. CDK4/6 Inhibitors in Breast Cancer Treatment: Potential Interactions with Drug, Gene, and Pathophysiological Conditions. Int. J. Mol. Sci. 2020, 21, 76350. [Google Scholar] [CrossRef]
- Leenhardt, F.; Gracia, M.; Perrin, C.; Muracciole-Bich, C.; Marion, B.; Roques, C.; Alexandre, M.; Firmin, N.; Pouderoux, S.; Mbatchi, L.; et al. Liquid chromatography–tandem mass spectrometric assay for the quantification of CDK4/6 inhibitors in human plasma in a clinical context of drug-drug interaction. J. Pharm. Biomed. Anal. 2020, 188, 113438. [Google Scholar] [CrossRef]
- Diéras, V.; Rugo, H.S.; Schnell, P.; Gelmon, K.; Cristofanilli, M.; Loi, S.; Colleoni, M.; Lu, D.R.; Mori, A.; Gauthier, E.; et al. Long-term Pooled Safety Analysis of Palbociclib in Combination with Endocrine Therapy for HR+/HER2- Advanced Breast Cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Diéras, V.; Harbeck, N.; Joy, A.A.; Gelmon, K.; Ettl, J.; Verma, S.; Lu, D.R.; Gauthier, E.; Schnell, P.; Mori, A.; et al. Palbociclib with Letrozole in Postmenopausal Women with ER+/HER2− Advanced Breast Cancer: Hematologic Safety Analysis of the Randomized PALOMA-2 Trial. Oncologist 2019, 24, 1514–1525. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yu, Y.; Durairaj, C.; Diéras, V.; Finn, R.S.; Wang, D.D. Impact of Dose Reduction on Efficacy: Implications of Exposure-Response Analysis of Palbociclib. Target Oncol. 2020, 16, 69–76. [Google Scholar] [CrossRef]
- Mukai, H.; Shimizu, C.; Masuda, N.; Ohtani, S.; Ohno, S.; Takahashi, M.; Yamamoto, Y.; Nishimura, R.; Sato, N.; Ohsumi, S.; et al. Palbociclib in combination with letrozole in patients with estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int. J. Clin. Oncol. 2019, 24, 274–287. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Rugo, H.S.; Gelmon, K.A.; Cristofanilli, M.; Colleoni, M.; Loi, S.; Colleoni, M.; Lu, D.R.; Mori, A.; Gauthier, E.; et al. Long-Term Pooled Safety Analysis of Palbociclib in Combination with Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Updated Analysis with up to 5 Years of Follow-Up. Oncologist 2021, 26, e749–e755. [Google Scholar] [CrossRef] [PubMed]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Takayama, K.; Taguchi, T. Predictors for development of palbociclib-induced neutropenia in breast cancer patients as determined by ordered logistic regression analysis. Sci. Rep. 2021, 11, 20055. [Google Scholar] [CrossRef] [PubMed]
- Marouille, A.L.; Petit, E.; Kaderbhaï, C.; Desmoulins, I.; Hennequin, A.; Mayeur, D.; Fumet, J.-D.; Ladoire, S.; Tharin, Z.; Ayati, S.; et al. Pharmacokinetic/Pharmacodynamic Model of Neutropenia in Real-Life Palbociclib-Treated Patients. Pharmaceutics 2021, 13, 1708. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hoffman, J.; Plotka, A.; O’Gorman, M.; Shi, H.; Wang, D. Palbociclib (PD-0332991) pharmacokinetics in subjects with impaired renal function. Cancer Chemother. Pharmacol. 2020, 86, 701–710. [Google Scholar] [CrossRef]
- Molenaar-Kuijsten, L.; Braal, C.L.; Groenland, S.L.; Vries, N.; Rosing, H.; Beijnen, J.H.; Koolen, S.L.W.; Vulink, A.J.E.; van Dongen, M.G.J.; Mathijssen, R.H.J.; et al. Effects of the Moderate CYP3A4 Inhibitor Erythromycin on the Pharmacokinetics of Palbociclib: A Randomized Crossover Trial in Patients with Breast Cancer. Clin. Pharmacol. Ther. 2021, 111, 477–484. [Google Scholar] [CrossRef]
- Del Re, M.; Omarini, C.; Diodati, L.; Palleschi, M.; Meattini, I.; Crucitta, S.; Lorenzini, G.; Isca, C.; Fontana, A.; Livi, L.; et al. Drug–drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of metastatic breast cancer patients. ESMO Open 2021, 6, 100231. [Google Scholar] [CrossRef]
- Mir, O.; Touati, N.; Lia, M.; Litière, S.; Le Cesne, A.; Sleijfer, S.; Blay, J.-Y.; Leahy, M.; Young, R.; Mathijssen, R.H.J.; et al. Impact of Concomitant Administration of Gastric Acid-Suppressive Agents and Pazopanib on Outcomes in Soft-Tissue Sarcoma Patients Treated within the EORTC 62043/62072 Trials. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1479–1485. [Google Scholar] [CrossRef] [Green Version]


| Number of Palbociclib Cycles (n) | |
|---|---|
| n | 58 |
| Mean (SD) | 8.9 (3.6) |
| Median (Q1;Q3) | 10.5 (6.0; 12.0) |
| Duration of treatment (months) | |
| n | 58 |
| Mean (SD) | 8.9 (3.8) |
| Median (Q1;Q3) | 11.0 (6.4; 11.3) |
| Dose reduction (n) | |
| At least one dose reduction | |
| No | 39 (67.2%) |
| Yes | 19 (32.8%) |
| If yes: | |
| For hematologic toxicity | 17 (89.5%) |
| For other toxicity | 2 (10.5%) |
| Treatment interruption (n) | |
| At least one treatment interruption | |
| No | 46 (79.3%) |
| Yes | 12 (20.7%) |
| If yes: | |
| For hematologic toxicity | 3 (25.0%) |
| For other toxicity | 9 (75.0%) |
| Neutropenia during the first two cycles (n) | |
| Grade during the first two cycles | |
| Grade 0 | 17 (29.3%) |
| Grade 1 | 1 (1.7%) |
| Grade 2 | 1 (1.7%) |
| Grade 3 | 34 (58.7%) |
| Grade 4 | 5 (8.6%) |
| Incidence of grade 3+ neutropenia during the first two cycles | |
| No | 19 (32.8%) |
| Yes | 39 (67.2%) |
| Variable | Nb Evt/N | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% IC | p Value ‡ | OR | 95% IC | p Value ‡ | ||
| Clinical variables | |||||||
| Age | p = 0.928 | ||||||
| 5 years increase | 39/58 | 0.99 | (0.80; 1.22) | ||||
| BMI (kg/m²) | p = 0.038 | ||||||
| 1-unit increase | 37/56 | 1.14 | (1.00; 1.31) | ||||
| Missing | 2 | ||||||
| Previous treatment | p = 0.461 | ||||||
| No | 14/19 | 1.00 | Ref | ||||
| Yes | 25/39 | 0.64 | (0.19; 2.14) | ||||
| Laboratory data | |||||||
| Lymphocytes (109/L) | p = 0.101 | ||||||
| 1-unit increase | 39/58 | 0.64 | (0.37; 1.12) | ||||
| Leukocytes (109/L) | p = 0.001 | ||||||
| 1-unit increase | 39/58 | 0.61 | (0.44; 0.84) | ||||
| Neutrophils (109/L) | p = 0.007 | p = 0.002 | |||||
| 1-unit increase | 39/58 | 0.62 | (0.42; 0.92) | 0.56 | (0.36; 0.86) | ||
| Hemoglobin (g/dL) | p = 0.103 | ||||||
| 1-unit increase | 39/57 | 1.43 | (0.92; 2.25) | ||||
| Bilirubin (g/dL) | p = 0.201 | ||||||
| 1-unit increase | 36/55 | 1.11 | (0.94; 1.31) | ||||
| Kidney clearance (mL/min/1.73 m²) | p = 0.538 | ||||||
| 10-unit increase | 39/58 | 0.92 | (0.71; 1.19) | ||||
| Treatment data at D15C1 | |||||||
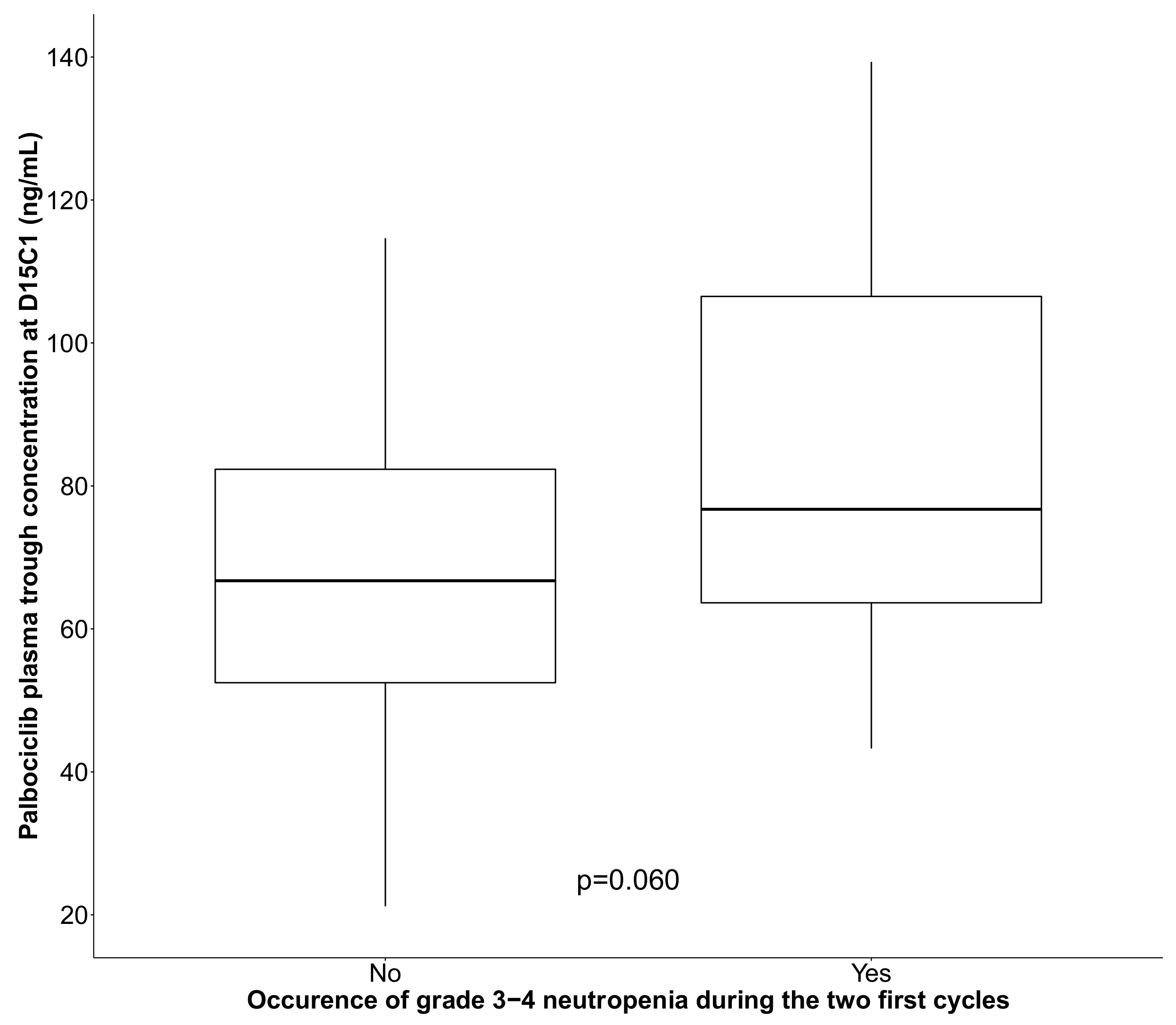
| Palbociclib Ctrough | p = 0.031 | p = 0.008 | |||||
| 10 unit increase | 35/54 | 1.28 | (1.01; 1.64) | 1.42 | (1.06; 1.90) | ||
| CYP3A4 and/or p-gp inhibitor | p = 0.318 | ||||||
| No | 14/19 | 1.00 | Ref | ||||
| Yes | 25/39 | 0.55 | (0.17; 1.77) | ||||
| Antacids | p = 0.183 | ||||||
| No | 28/40 | 1.00 | Ref | ||||
| Yes | 7/14 | 0.43 | (0.12; 1.49) | ||||
| Palbociclib Ctrough | All | Test | ||
|---|---|---|---|---|
| ≤74 ng/mL | >74 ng/mL | |||
| n = 27 | n = 27 | n = 54 | ||
| Sociodemographic and clinical variables at inclusion | ||||
| Age (years) | p = 0.002 | |||
| N | 27 | 27 | 54 | |
| Mean (SD) | 57.1 (12.9) | 67.8 (12.3) | 62.5 (13.6) | |
| Median (Q1;Q3) | 57.0 (48.0; 67.0) | 71.0 (64.0; 76.0) | 65.5 (55.0; 74.0) | |
| Age (Median) | p = 0.003 | |||
| ≤66 years | 20 (74.1%) | 9 (33.3%) | 29 (53.7%) | |
| >66 years | 7 (25.9%) | 18 (66.7%) | 25 (46.3%) | |
| BMI (kg/m²) | p = 0.963 | |||
| N | 25 | 27 | 52 | |
| Mean (SD) | 25.5 (4.9) | 25.5 (4.5) | 25.5 (4.7) | |
| Median (Q1;Q3) | 25.4 (22.1; 29.0) | 24.6 (22.5; 28.1) | 25.0 (22.2; 28.5) | |
| Missing | 2 | 0 | 2 | |
| Alcohol consumption | p = 1.000 | |||
| Non consumer | 23 (85.2%) | 23 (85.2%) | 46 (85.2%) | |
| Former consumer | 0 (0.0%) | 1 (3.7%) | 1 (1.9%) | |
| Consumer | 4 (14.8%) | 3 (11.1%) | 7 (13.0%) | |
| Tobacco consumption | p = 0.322 | |||
| Non-smoker | 17 (63.0%) | 22 (81.5%) | 39 (72.2%) | |
| Former smoker | 5 (18.5%) | 2 (7.4%) | 7 (13.0%) | |
| Smoker | 5 (18.5%) | 3 (11.1%) | 8 (14.8%) | |
| Biological variables at inclusion | ||||
| Creatinine (μmol/L) | p = 0.166 | |||
| N | 27 | 27 | 54 | |
| Mean (SD) | 66.9 (15.9) | 70.1 (13.4) | 68.5 (14.7) | |
| Median (Q1;Q3) | 63.0 (58.0; 70.0) | 66.0 (62.3; 79.0) | 64.5 (59.0; 74.3) | |
| Kidney clearance (ml/min/1.73 m2) | p = 0.017 | |||
| N | 27 | 27 | 54 | |
| Mean (SD) | 93.6 (24.2) | 80.3 (18.2) | 87.0 (22.3) | |
| Median (Q1;Q3) | 96.0 (87.0; 103.0) | 81.0 (67.0; 96.0) | 88.5 (70.0; 100.0) | |
| Albumin (g/L) | p = 0.040 | |||
| N | 21 | 25 | 46 | |
| Mean (SD) | 43.6 (4.5) | 41.3 (4.1) | 42.3 (4.6) | |
| Median (Q1;Q3) | 43.0 (41.7; 47.0) | 42.0 (39.0; 43.5) | 42.0 (40.0; 45.0) | |
| Missing | 6 | 2 | 8 | |
| Variable | n = 52 | |
|---|---|---|
| Coefficient | 95% IC | |
| CYP3A4/P-gp inhibitors | p = 0.035 | |
| No | 1.00 | Ref |
| Yes | 0.22 | (0.01; 0.44) |
| Antacids | p = 0.036 | |
| No | 1.00 | Ref |
| Yes | −0.23 | (−0.46; −0.01) |
| Body surface area at D15C1 | p = 0.787 | |
| 0.5 m² increase | −0.03 | (−0.31; 0.24) |
| Age | p = 0.146 | |
| 5 years increase | 0.03 | (−0.01; 0.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leenhardt, F.; Fiteni, F.; Gauthier, L.; Alexandre, M.; Guiu, S.; Firmin, N.; Pouderoux, S.; Viala, M.; Lossaint, G.; Gautier, C.; et al. Pharmacokinetic Variability Drives Palbociclib-Induced Neutropenia in Metastatic Breast Cancer Patients: Drug–Drug Interactions Are the Usual Suspects. Pharmaceutics 2022, 14, 841. https://doi.org/10.3390/pharmaceutics14040841
Leenhardt F, Fiteni F, Gauthier L, Alexandre M, Guiu S, Firmin N, Pouderoux S, Viala M, Lossaint G, Gautier C, et al. Pharmacokinetic Variability Drives Palbociclib-Induced Neutropenia in Metastatic Breast Cancer Patients: Drug–Drug Interactions Are the Usual Suspects. Pharmaceutics. 2022; 14(4):841. https://doi.org/10.3390/pharmaceutics14040841
Chicago/Turabian StyleLeenhardt, Fanny, Frédéric Fiteni, Ludovic Gauthier, Marie Alexandre, Séverine Guiu, Nelly Firmin, Stéphane Pouderoux, Marie Viala, Gerald Lossaint, Chloé Gautier, and et al. 2022. "Pharmacokinetic Variability Drives Palbociclib-Induced Neutropenia in Metastatic Breast Cancer Patients: Drug–Drug Interactions Are the Usual Suspects" Pharmaceutics 14, no. 4: 841. https://doi.org/10.3390/pharmaceutics14040841
APA StyleLeenhardt, F., Fiteni, F., Gauthier, L., Alexandre, M., Guiu, S., Firmin, N., Pouderoux, S., Viala, M., Lossaint, G., Gautier, C., Mollevi, C., Gracia, M., Gongora, C., Mbatchi, L., Evrard, A., & Jacot, W. (2022). Pharmacokinetic Variability Drives Palbociclib-Induced Neutropenia in Metastatic Breast Cancer Patients: Drug–Drug Interactions Are the Usual Suspects. Pharmaceutics, 14(4), 841. https://doi.org/10.3390/pharmaceutics14040841







