Optimizing Antimicrobial Dosing for Critically Ill Patients with MRSA Infections: A New Paradigm for Improving Efficacy during Continuous Renal Replacement Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Parameters Collection
Clearance Calculation
- CVVH modality clearance:CLpre-filter = Quf × SC × Qplasma/(Qplasma + Quf)CLpost-filter = Quf × SC
- CVVHD modality clearance:CLCVVHD = Qd × SA
- CVVHDF modality clearance:CLpre-filter = (Qf × SC + Qd × SA) × Qplasma/(Qplasma + Qf)where CLpre-filter and CLpost-filter represents the transmembrane clearance during prefilter hemofiltration and prefilter hemofiltration, respectively. SC represents the sieving coefficient which was defined as the ratio of drug concentration in the ultrafiltrate to plasma, SA represents the saturation coefficient which was used to describe the ability of a drug to diffuse through the filter membrane, Quf represents the ultrafiltration flow rate, Qplasma represents the plasma flow rate, Qf represents the replacement fluid rate used in CVVH, and Qd represents the dialysate fluid rate used in CVVHD.CLpost-filter = Qf × SC + Qd × SAwhere Qplasma represents the plasma flow rate and Qb represents the blood flow rate.Qplasma = Qb × (1 − hematocrit)
2.2. PD Data
2.3. Monte Carlo Simulation (MCS)
3. Results
3.1. Antimicrobial Agent Loading Dose
3.2. Antimicrobial Agent Maintenance Dose
3.3. The Impact of CRRT Dose on Maintenance Dose of Antimicrobial Agent
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA J. Am. Med. Assoc. 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S344–S349. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.A.; de Kraker, M.; Scicluna, E.; van de Sande-Bruinsma, N.; Tiemersma, E.; Monen, J.; Grundmann, H. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in invasive isolates from southern and eastern Mediterranean countries. J. Antimicrob. Chemother. 2007, 60, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Hanberger, H.; Walther, S.; Leone, M.; Barie, P.S.; Rello, J.; Lipman, J.; Marshall, J.C.; Anzueto, A.; Sakr, Y.; Pickkers, P.; et al. Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: Results from the EPIC II study. Int. J. Antimicrob. Agents 2011, 38, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, C.G.; Edwards, D.I.; Fraise, A.P.; Gould, F.K.; Ridgway, G.L.; Warren, R.E. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrob. Chemother. 2006, 57, 589–608. [Google Scholar] [CrossRef]
- Claisse, G.; Zufferey, P.J.; Trone, J.C.; Maillard, N.; Delavenne, X.; Laporte, S.; Ollier, E. Predicting the dose of vancomycin in ICU patients receiving different types of RRT therapy: A model-based meta-analytic approach. Br. J. Clin. Pharmacol. 2019, 85, 1215–1226. [Google Scholar] [CrossRef]
- Tandukar, S.; Palevsky, P.M. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019, 155, 626–638. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensiv. Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Schetz, M.; Ferdinande, P.; Van den Berghe, G.; Verwaest, C.; Lauwers, P. Pharmacokinetics of continuous renal replacement therapy. Intensiv. Care Med. 1995, 21, 612–620. [Google Scholar] [CrossRef]
- Chaijamorn, W.; Jitsurong, A.; Wiwattanawongsa, K.; Wanakamanee, U.; Dandecha, P. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Int. J. Antimicrob. Agents 2011, 38, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Corti, N.; Rudiger, A.; Chiesa, A.; Marti, I.; Jetter, A.; Rentsch, K.; Muller, D.; Bechir, M.; Maggiorini, M. Pharmacokinetics of daily daptomycin in critically ill patients undergoing continuous renal replacement therapy. Chemotherapy 2013, 59, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yasuno, N.; Katada, S.; Hisaka, A.; Hanafusa, N.; Noiri, E.; Yahagi, N.; Fujita, T.; Suzuki, H. Proposal of a phar-macokinetically optimized dosage regimen of antibiotics in patients receiving continuous hemodiafiltration. Antimicrob. Agents. Chemother. 2011, 55, 5804–5812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellmann, R.; Falkensammer, G.; Seger, C.; Weiler, S.; Kountchev, J.; Joannidis, M. Teicoplanin pharmacokinetics in critically ill patients on continuous veno-venous hemofiltration. Int. J. Clin. Pharmacol. Ther. 2010, 48, 243–249. [Google Scholar]
- Trotman, R.L.; Williamson, J.C.; Shoemaker, D.M.; Salzer, W.L. Antibiotic Dosing in Critically Ill Adult Patients Receiving Continuous Renal Replacement Therapy. Clin. Infect. Dis. 2005, 41, 1159–1166. [Google Scholar] [CrossRef]
- DelDot, M.E.; Lipman, J.; Tett, S.E. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous hae-modiafiltration. Br. J. Clin. Pharmacol. 2004, 58, 259–268. [Google Scholar] [CrossRef]
- Carcelero, E.; Soy, D. Antibiotic dose adjustment in the treatment of MRSA infections in patients with acute renal failure undergoing continuous renal replacement therapies. Enferm. Infecc. Microbiol. Clin. 2012, 30, 249–256. [Google Scholar] [CrossRef]
- Covajes, C.; Scolletta, S.; Penaccini, L.; Ocampos-Martinez, E.; Abdelhadii, A.; Beumier, M.; Jacobs, F.; de Backer, D.; Vincent, J.-L.; Taccone, F.S. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int. J. Antimicrob. Agents 2013, 41, 261–266. [Google Scholar] [CrossRef]
- Charoensareerat, T.; Chaijamorn, W.; BoonPeng, A.; Srisawat, N.; Pummangura, C.; Pattharachayakul, S. Optimal vancomycin dosing regimens for critically ill patients with acute kidney injury during continuous renal replacement therapy: A Monte Carlo simulation study. J. Crit. Care 2019, 54, 77–82. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, S.A.; Kim, C.W.; Kang, E.; Choi, Y.H.; Park, I. High variability of teicoplanin concentration in patients with continuous venovenous hemodiafiltration. Hemodial. Int. 2019, 23, 69–76. [Google Scholar] [CrossRef]
- Xu, X.; Khadzhynov, D.; Peters, H.; Chaves, R.L.; Hamed, K.; Levi, M.; Corti, N. Population pharmacokinetics of daptomycin in adult patients undergoing continuous renal replacement therapy. Br. J. Clin. Pharmacol. 2016, 83, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Kirkpatrick, C.M.; Lipman, J. Monte Carlo simulations: Maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J. Antimicrob. Chemother. 2010, 66, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bonati, M.; Traina, G.L.; Villa, G.; Salvadeo, A.; Gentile, M.G.; Fellin, G.; Rosina, R.; Cavenaghi, L.; Buniva, G. Teicoplanin phar-macokinetics in patients with chronic renal failure. Clin. Pharmacokinet. 1987, 12, 292–301. [Google Scholar] [CrossRef]
- Xie, F.; Li, S.; Cheng, Z. Population pharmacokinetics and dosing considerations of daptomycin in critically ill patients undergoing continuous renal replacement therapy. J. Antimicrob. Chemother. 2020, 75, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Churchwell, M.D.; Pasko, D.A.; Mueller, B.A. Daptomycin Clearance during Modeled Continuous Renal Replacement Therapy. Blood Purif. 2006, 24, 548–554. [Google Scholar] [CrossRef]
- Vilay, A.M.; Grio, M.; Depestel, D.D.; Sowinski, K.M.; Gao, L.; Heung, M.; Salama, N.N.; Mueller, B.A. Daptomycin pharmacoki-netics in critically ill patients receiving continuous venovenous hemodialysis. Crit. Care Med. 2011, 39, 19–25. [Google Scholar] [CrossRef]
- Macias, W.L.; Mueller, B.A.; Scarim, S.K. Vancomycin pharmacokinetics in acute renal failure: Preservation of nonrenal clearance. Clin. Pharmacol. Ther. 1991, 50, 688–694. [Google Scholar] [CrossRef]
- VA/NIH Acute Renal Failure Trial Network; Palevsky, P.M.; Zhang, J.H.; O’Connor, T.Z.; Chertow, G.M.; Crowley, S.T.; Choudhury, D.; Finkel, K.W.; Kellum, J.A.; Paganini, E.P.; et al. Intensity of Renal Support in Critically Ill Patients with Acute Kidney Injury. N. Engl. J. Med. 2008, 359, 7–20. [Google Scholar] [CrossRef]
- Choi, G.; Gomersall, C.; Tian, Q.; Joynt, G.; Freebairn, R.; Lipman, J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit. Care Med. 2009, 37, 2268–2282. [Google Scholar] [CrossRef]
- Gashti, C.N.; Rodby, R.A.; Huang, Z.; Gao, D.; Zhang, W. Effects of High Blood Flow and High Pre-Dilution Replacement Fluid Rates on Small Solute Clearances in Hemofiltration. Blood Purif. 2011, 32, 266–270. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Kuti, J.L.; Kiffer, C.R.; Mendes, C.M.F.; Nicolau, D.P. Pharmacodynamic comparison of linezolid, teicoplanin and vancomycin against clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci collected from hospitals in Brazil. Clin. Microbiol. Infect. 2008, 14, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Galar, A.; Muñoz, P.; Valerio, M.; Cercenado, E.; García-González, X.; Burillo, A.; Sánchez-Somolinos, M.; Juárez, M.; Verde, E.; Bouza, E. Current use of daptomycin and systematic therapeutic drug monitoring: Clinical experience in a tertiary care institution. Int. J. Antimicrob. Agents 2019, 53, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gregor, V.E.; Sun, Z.; Ayida, B.K.; Winters, G.C.; Murphy, D.; Simonsen, K.B.; Vourloumis, D.; Fish, S.; Froelich, J.M.; et al. Structure-Guided Discovery of Novel Aminoglycoside Mimetics as Antibacterial Translation Inhibitors. Antimicrob. Agents Chemother. 2005, 49, 4942–4949. [Google Scholar] [CrossRef]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef]
- Zelenitsky, S.A.; Ariano, R.E.; Zhanel, G.G. Pharmacodynamics of empirical antibiotic monotherapies for an intensive care unit (ICU) population based on Canadian surveillance data. J. Antimicrob. Chemother. 2010, 66, 343–349. [Google Scholar] [CrossRef]
- Roberts, J.A.; Pea, F.; Lipman, J. The Clinical Relevance of Plasma Protein Binding Changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.-A. Pharmacokinetics–pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef]
- Van de Vijsel, L.M.; Walker, S.A.; Walker, S.E.; Yamashita, S.; Simor, A.; Hladunewich, M. Initial vancomycin dosing recommen-dations for critically ill patients undergoing continuous venovenous hemodialysis. Can. J. Hosp. Pharm. 2010, 63, 196–206. [Google Scholar]
- Matsumoto, K.; Takesue, Y.; Ohmagari, N.; Mochizuki, T.; Mikamo, H.; Seki, M.; Takakura, S.; Tokimatsu, I.; Takahashi, Y.; Kasahara, K.; et al. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 365–380. [Google Scholar] [CrossRef]
- Wolter, K.; Claus, M.; Fritschka, E. Pharmacokinetics and dosage recommendations of teicoplanin in patients treated by continuous veno-venous haemodialysis (CVVHD). Eur. J. Clin. Pharmacol. 1994, 46, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y.; et al. Recommendation of Antimicrobial Dosing Optimization During Continuous Renal Replacement Therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Mueller, B.A. Antibiotic dosing in critically ill patients receiving CRRT: Under dosing is over prevalent. Semin. Dial. 2014, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.; Li, X.; Zhao, S.; He, N.; Zhai, S.; Ge, Q. Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis. Antibiotics 2021, 10, 1392. [Google Scholar] [CrossRef]
- Li, Q.; Liang, F.; Sang, L.; Li, P.; Lv, B.; Tan, L.; Liu, X.; Chen, W. Pharmacokinetics of and maintenance dose recommendations for vancomycin in severe pneumonia patients undergoing continuous venovenous hemofiltration with the combination of predilution and postdilution. Eur. J. Clin. Pharmacol. 2020, 76, 211–217. [Google Scholar] [CrossRef]
- Yagasaki, K.; Gando, S.; Matsuda, N.; Kameue, T.; Ishitani, T.; Hirano, T.; Iseki, K. Pharmacokinetics of teicoplanin in critically ill patients undergoing continuous hemodiafiltration. Intensive Care Med. 2003, 29, 2094–2095. [Google Scholar] [CrossRef]
- Wenisch, J.M.; Meyer, B.; Fuhrmann, V.; Saria, K.; Zuba, C.; Dittrich, P.; Thalhammer, F. Multiple-dose pharmacokinetics of daptomycin during continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 2012, 67, 977–983. [Google Scholar] [CrossRef]
- Khadzhynov, D.; Slowinski, T.; Lieker, I.; Spies, C.; Puhlmann, B.; König, T.; Uhrig, A.; Eggers, K.; Neumayer, H.H.; Traunmüller, F.; et al. Plasma pharmacokinetics of daptomycin in critically ill patients with renal failure and undergoing CVVHD. Int. J. Clin. Pharmacol. Ther. 2011, 49, 656–665. [Google Scholar] [CrossRef]


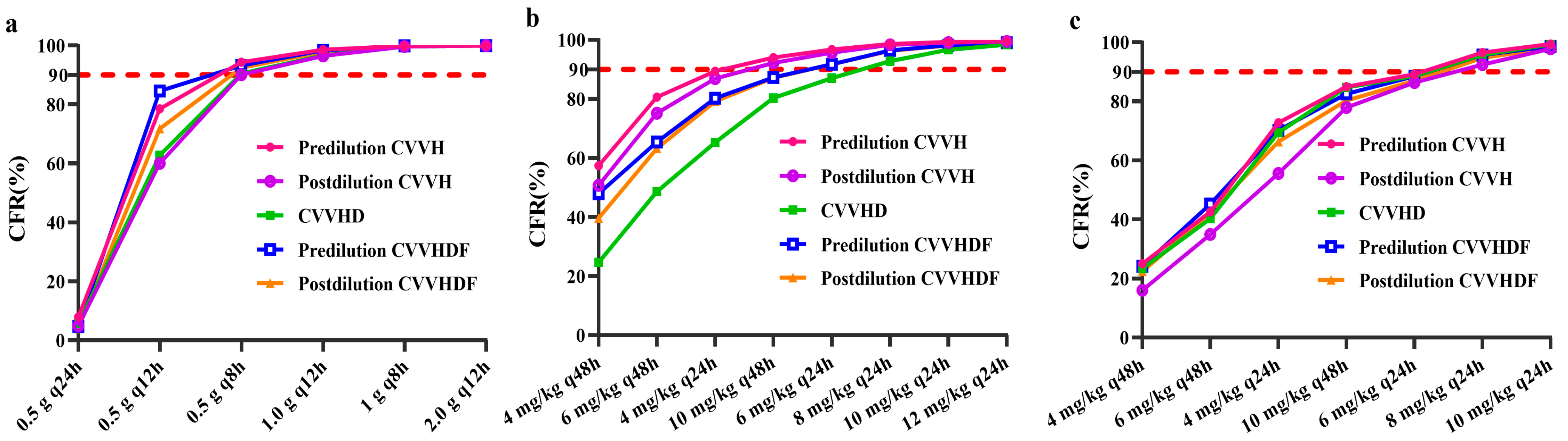
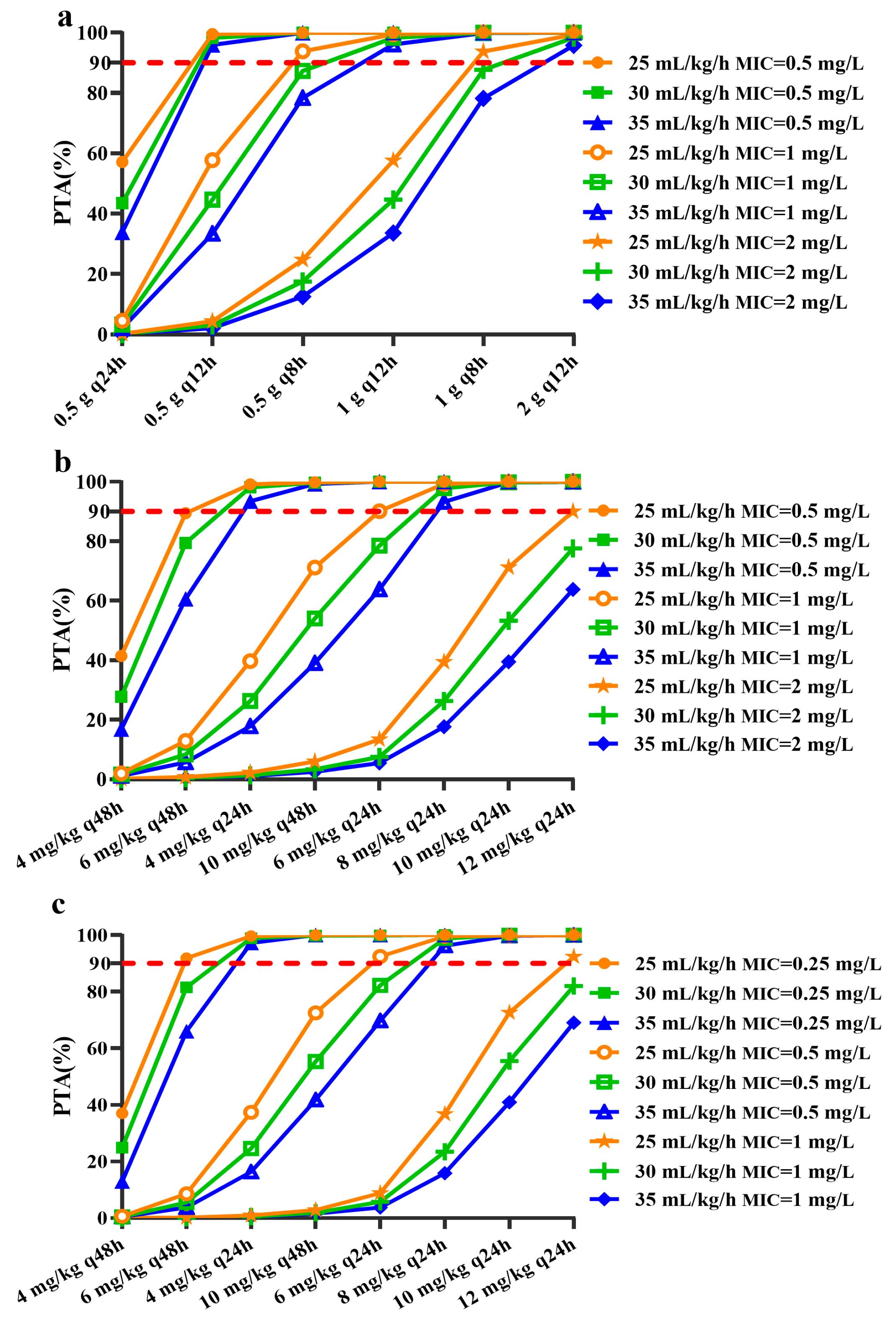
| Antimicrobial Agents | Vancomycin [17,18] | Teicoplanin [12,13,19,21] | Daptomycin [22,23,24] |
|---|---|---|---|
| Vd | 0.57 ± 0.26 L/kg (0.17–1.37) | 1.60 ± 0.70 L/kg (1.10–2.10) | 6.33 ± 1.65 L (5.67–7.00) |
| CLNR (mL/min) | 16.2 ± 7.0 (3.8–23.3) | 6.3 ± 2.2 (0–10.6) | 5.0 |
| SC | 0.73 ± 0.10 (0.43–0.89) | 0.14 ± 0.03 (0–1.0) | 0.19 ± 0.02 (0–1.0) |
| SA | 0.71 ± 0.14 (0–1.0) | 0.33 ± 0.02 (0–1.0) | 0.15 ± 0.01 (0–1.0) |
| Weight (kg) | 84.1 ± 18.9 | ||
| CRRT% delivered | 95.0 ± 35.0 | ||
| CRRT dose (mL/kg/h) | 22.0 ± 6.1 | ||
| Dialysate fluid rate (mL/h) | 820.0 ± 250.0 | ||
| Replacement fluid rate (mL/h) | 830.0 ± 249.0 | ||
| Blood flow (mL/min) | 140.0 ± 40.0 | ||
| Antimicrobial Agents | CRRT Modalities | CRRT Doses (mL/kg/h) | MIC (mg/L) | |||
|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | |||
| Vancomycin | CVVH a | 25–30 | - | 0.5 g q12 h | 0.5 g q8 h | 1 g q 8 h |
| CVVH a | 35 | - | 0.5 g q12 h | 1 g q12 h | 2 g q12 h | |
| Teicoplanin | CVVH | 25 | - | 4 mg/kg q48 h | 4 mg/kg q24 h | 8 mg/kg q24 h |
| CVVHD | 25 | - | 6 mg/kg q48 h | 6 mg/kg q24 h | 12 mg/kg q24 h | |
| CVVHDF | 25 | - | 6 mg/kg q48 h | 10 mg/kg q48 h | 10 mg/kg q24 h | |
| CVVH | 30–35 | - | 6 mg/kg q48 h | 6 mg/kg q24 h | 10 mg/kg q24 h | |
| CVVHD | 30–35 | - | 4 mg/kg q24 h | 8 mg/kg q24 h | >12 mg/kg q24 h | |
| CVVHDF | 30–35 | - | 4 mg/kg q24 h | 6 mg/kg q24 h | 12 mg/kg q24 h | |
| Daptomycin | CVVH a | 25 | 6 mg/kg q48 h | 6 mg/kg q24 h | 12 mg/kg q24 h | - |
| CVVH a | 30–35 | 4 mg/kg q24 h | 8 mg/kg q24 h | >12 mg/kg q24 h | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, S.; Wang, Q.; Wang, C.; Qiu, Y.; Yang, L.; Han, R.; Du, Q.; Chen, L.; Dong, Y.; et al. Optimizing Antimicrobial Dosing for Critically Ill Patients with MRSA Infections: A New Paradigm for Improving Efficacy during Continuous Renal Replacement Therapy. Pharmaceutics 2022, 14, 842. https://doi.org/10.3390/pharmaceutics14040842
Chen J, Li S, Wang Q, Wang C, Qiu Y, Yang L, Han R, Du Q, Chen L, Dong Y, et al. Optimizing Antimicrobial Dosing for Critically Ill Patients with MRSA Infections: A New Paradigm for Improving Efficacy during Continuous Renal Replacement Therapy. Pharmaceutics. 2022; 14(4):842. https://doi.org/10.3390/pharmaceutics14040842
Chicago/Turabian StyleChen, Jiaojiao, Sihan Li, Quanfang Wang, Chuhui Wang, Yulan Qiu, Luting Yang, Ruiying Han, Qian Du, Lei Chen, Yalin Dong, and et al. 2022. "Optimizing Antimicrobial Dosing for Critically Ill Patients with MRSA Infections: A New Paradigm for Improving Efficacy during Continuous Renal Replacement Therapy" Pharmaceutics 14, no. 4: 842. https://doi.org/10.3390/pharmaceutics14040842
APA StyleChen, J., Li, S., Wang, Q., Wang, C., Qiu, Y., Yang, L., Han, R., Du, Q., Chen, L., Dong, Y., & Wang, T. (2022). Optimizing Antimicrobial Dosing for Critically Ill Patients with MRSA Infections: A New Paradigm for Improving Efficacy during Continuous Renal Replacement Therapy. Pharmaceutics, 14(4), 842. https://doi.org/10.3390/pharmaceutics14040842






