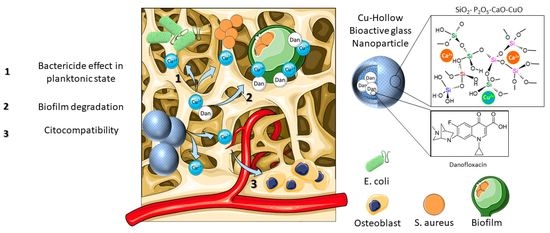
Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Nanoparticles
2.2. Physicochemical Characterization
2.3. Ion Release Assay
2.4. Loading and Release of Danofloxacin
2.5. In Vitro Assays in Pre-Osteoblastic Mammalian Cells
2.5.1. Cytotoxicity Assays in Pre-Osteoblastic Mammalian Cells
2.5.2. Internalization of HBGN and Morphology Studies
2.5.3. Flow Cytometry Assays
2.5.4. Cell Size and Complexity Analysis
2.5.5. Cell Viability, Intracellular Reactive Oxygen Species (ROS) Content
2.6. In Vitro Microbiology Assays
2.6.1. Bacterial Growth Inhibition Tests in the Planktonic State
2.6.2. Halo Inhibition Assays
2.6.3. Biofilm Degradation Assay
2.7. Statistical Analysis
3. Results and Discussion
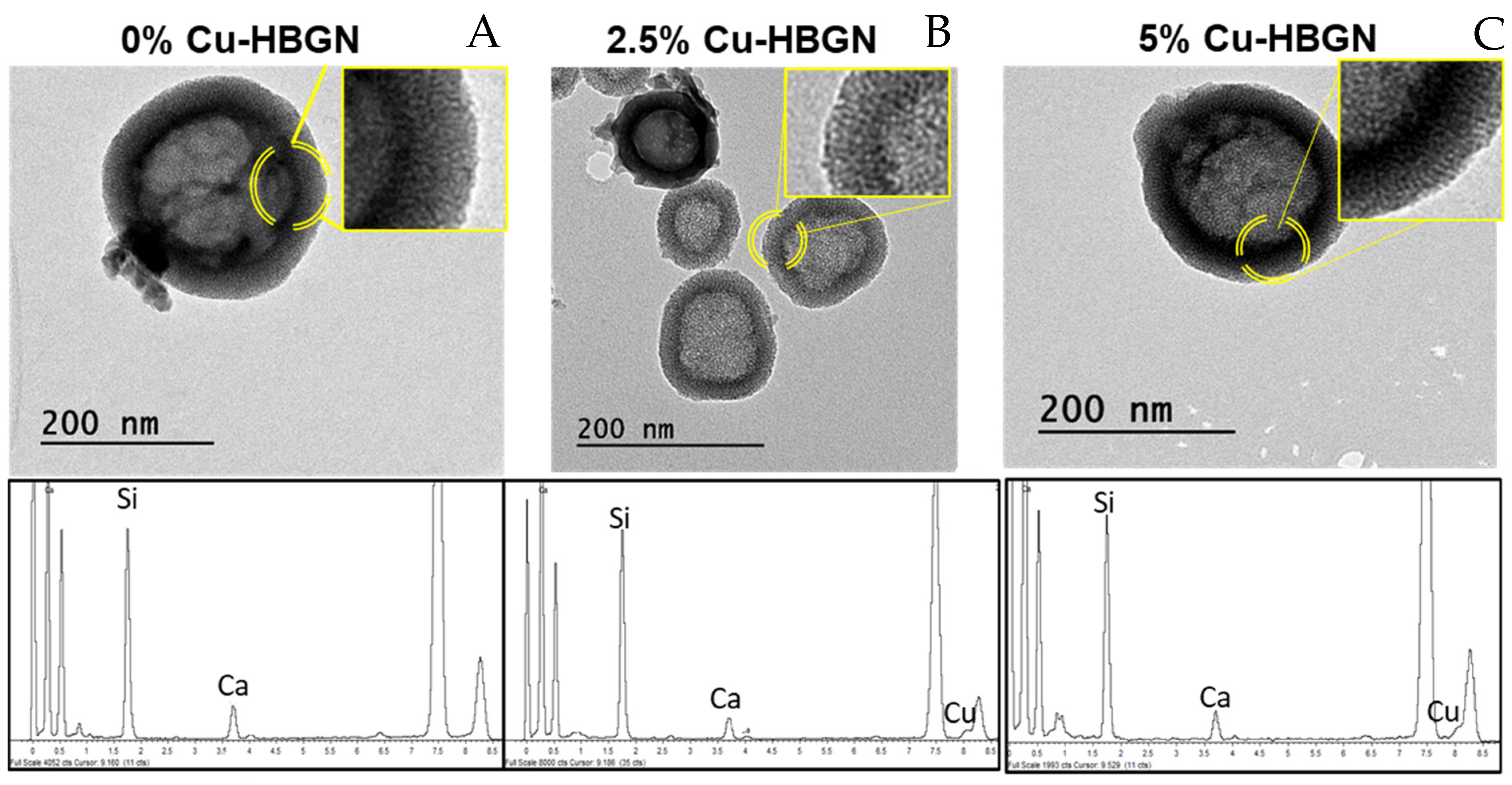
3.1. Characterization of the Microstructure of the Nanoparticles
3.2. Ions Release Assay
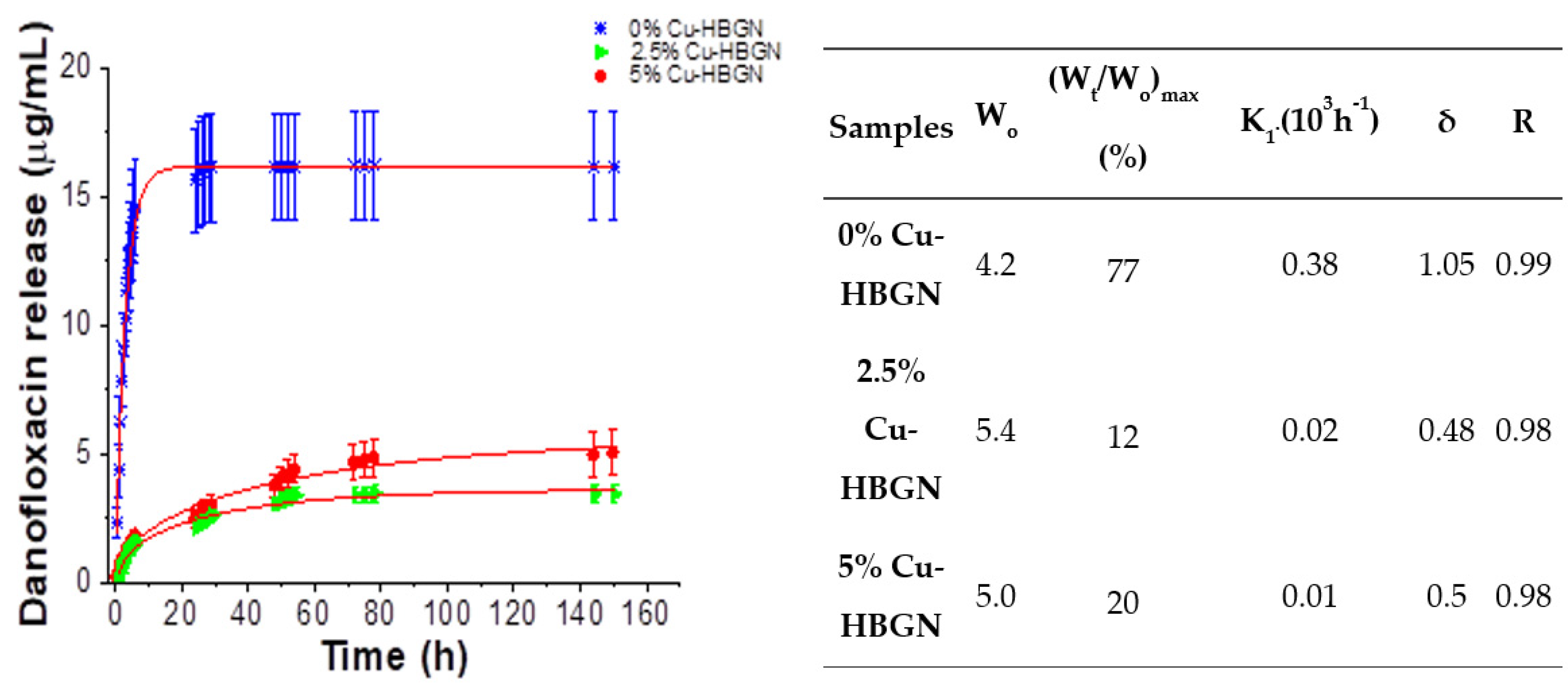
3.3. Danofloxacin Release Assay
3.4. Cells Assays
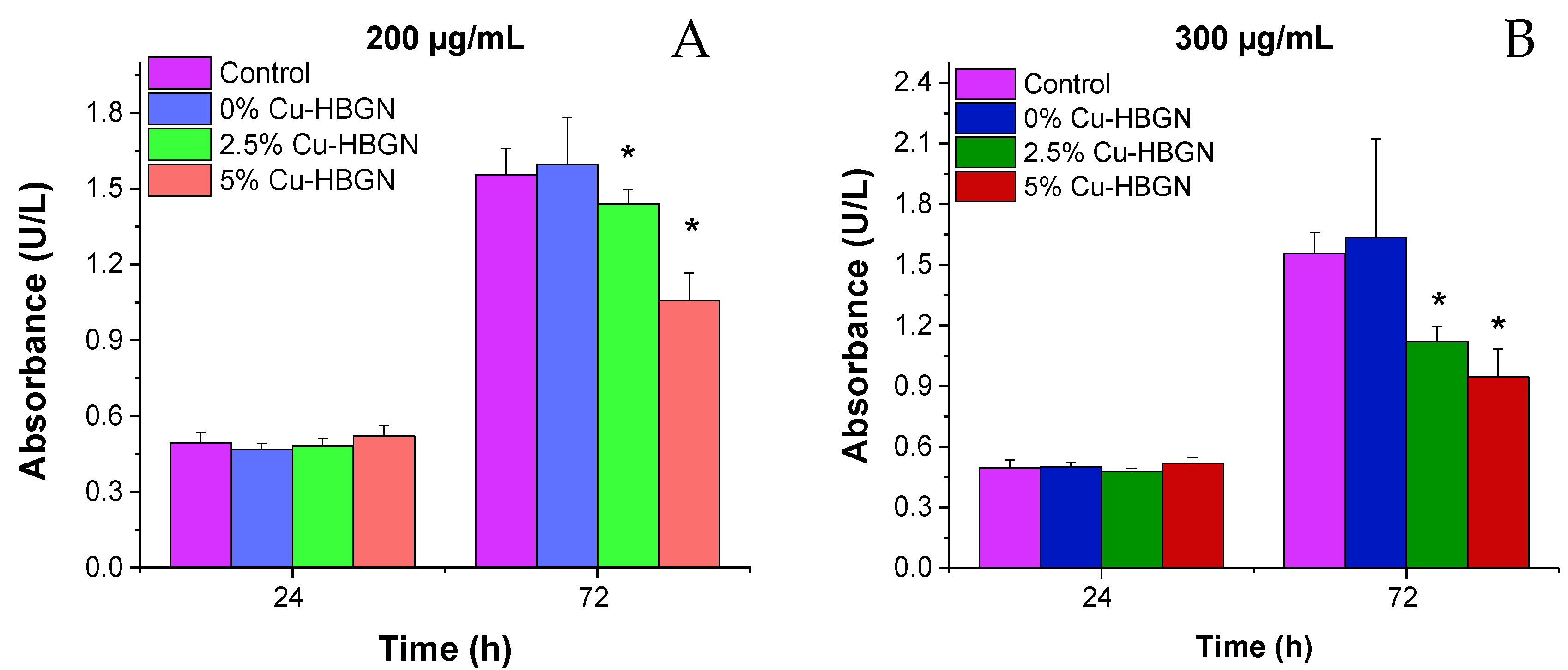
3.4.1. Cytotoxicity Assays in Pre-Osteoblastic Mammalian Cells
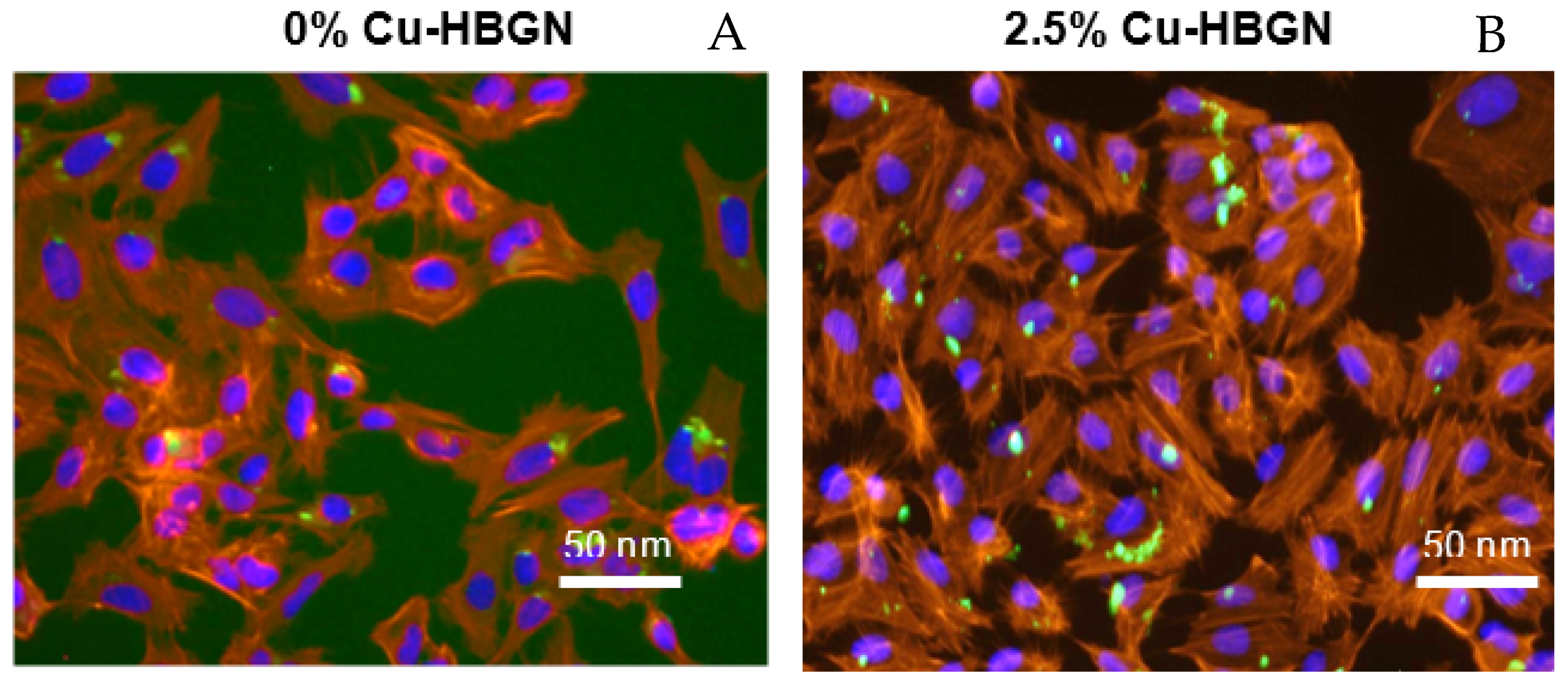
3.4.2. Internalization of HBGN and Morphology Results in MC3T3-E1 Cells
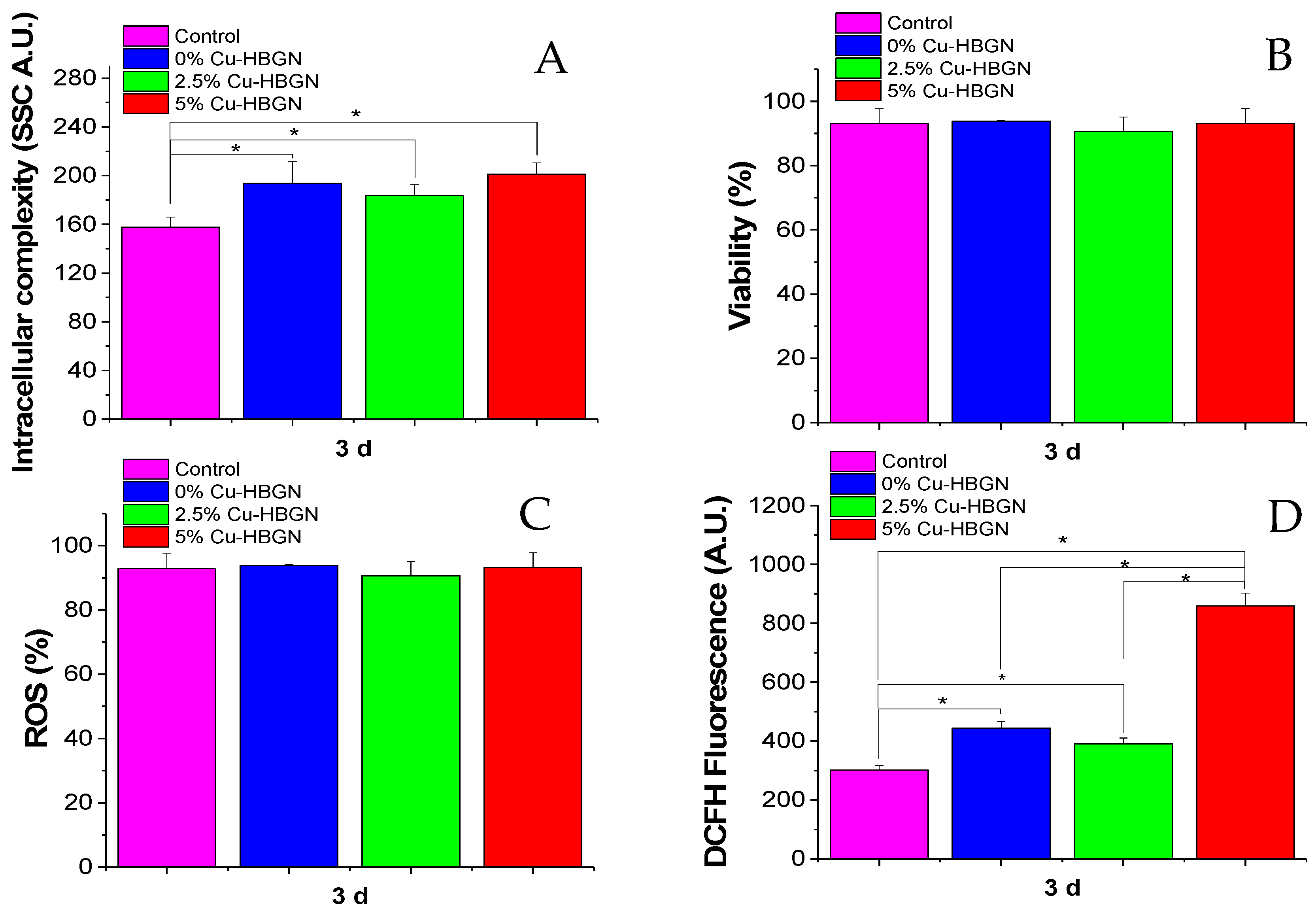
3.4.3. Flow Cytometry Studies
3.5. Bacterial Assays
3.5.1. Bacterial Growth Inhibition Tests in the Planktonic State
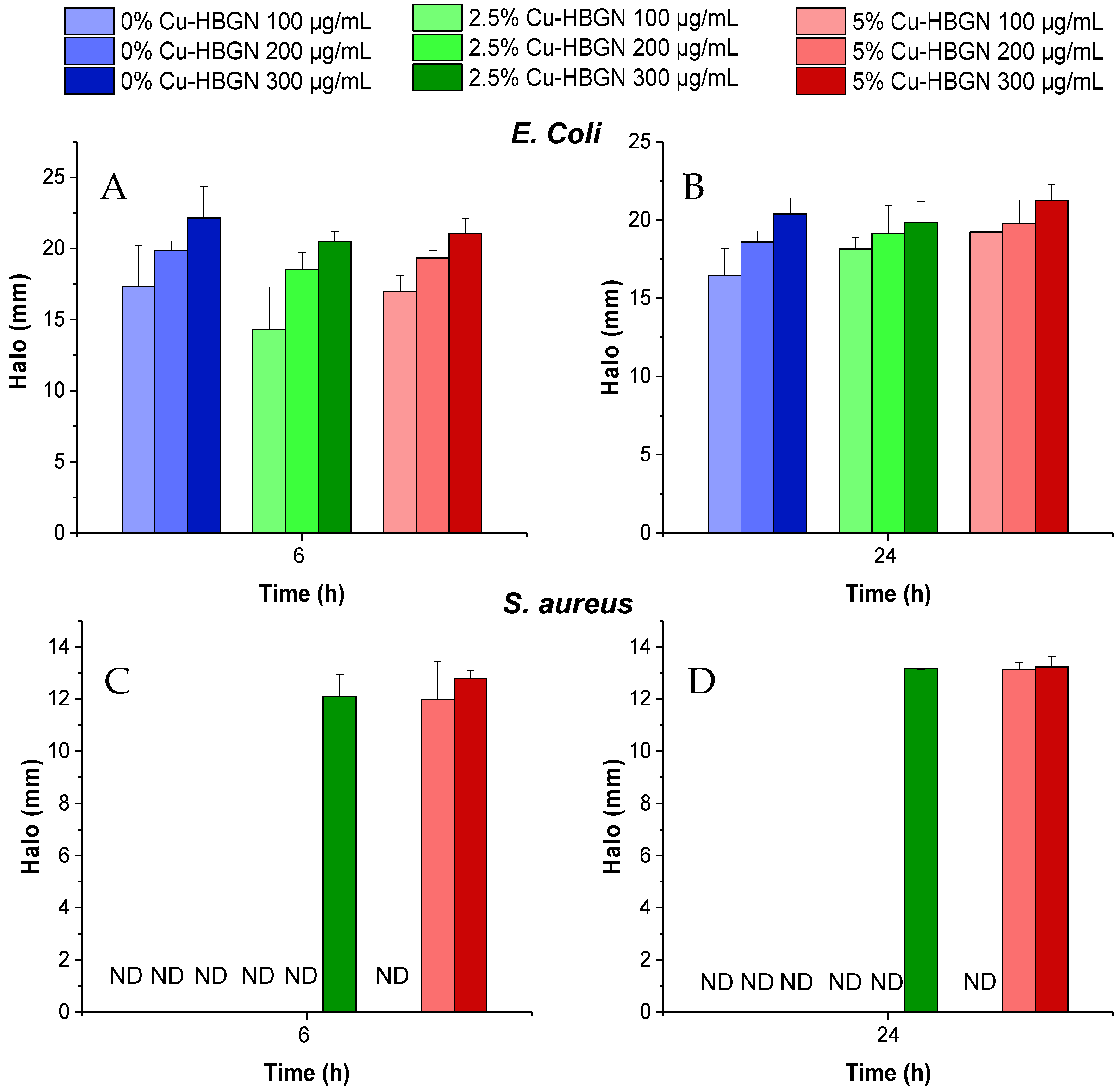
3.5.2. Halo Inhibition Assays
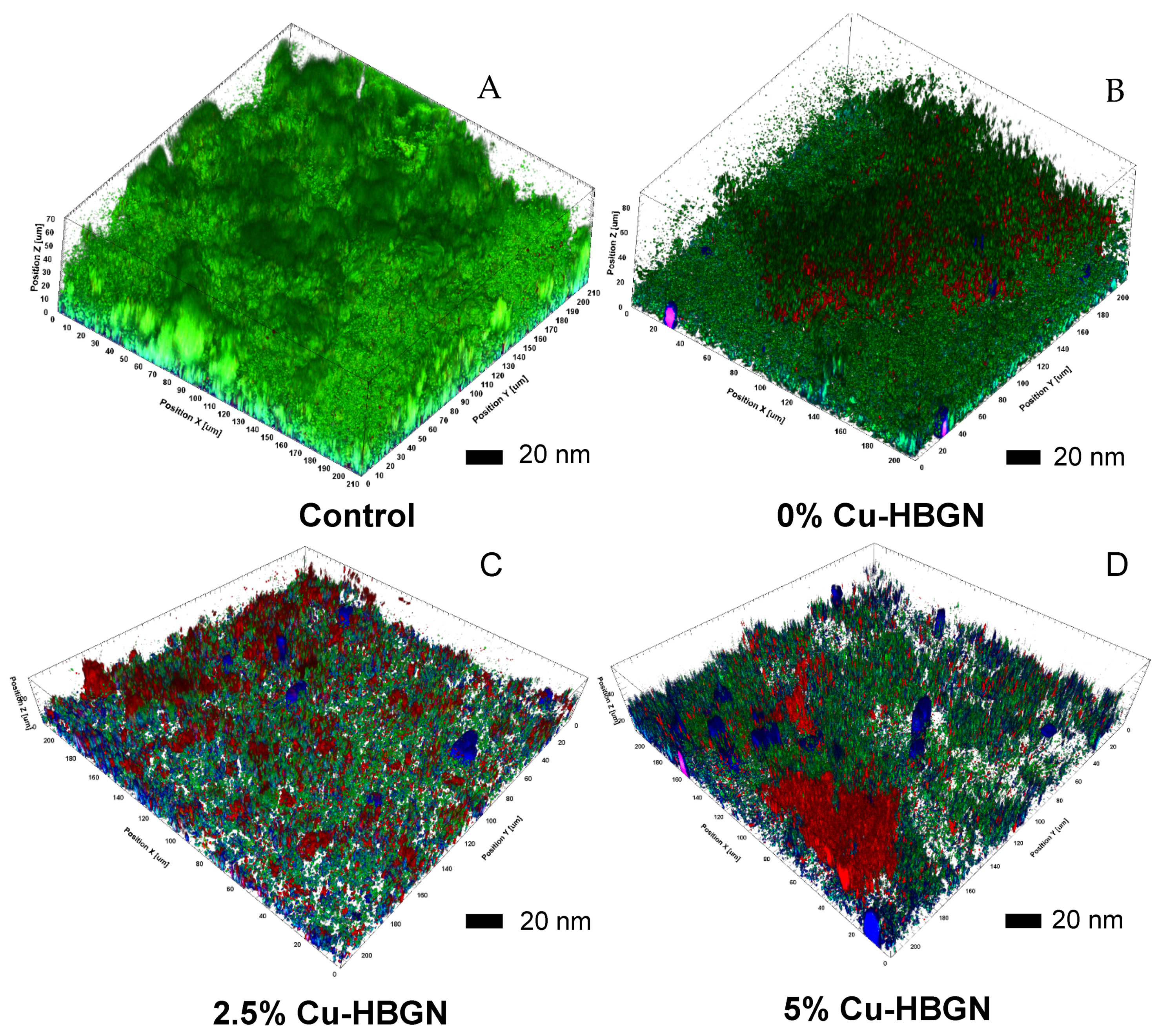
3.5.3. Biofilm Degradation Assay
4. Conclusions
- The three compositions investigated containing 0, 2.5 or 5% of CuO showed high values of specific surface area and pore volume and two different sizes of pores which made them versatile for loading with diverse biomolecules and drugs.
- The nanosystem was able to release biologically effective amounts of therapeutic inorganic ions and danofloxacin. The Cu-loading of the HBGN produced more gradual release of danofloxacin that was extended for over one week.
- Assays with MC3T3-E1 pre-osteoblasts showed a biocompatible behavior after 3 d of contact of the nanoparticles with cells.
- Microbiological assays showed that copper ions enhanced the bactericidal effect of danofloxacin against E. coli and S. aureus in both planktonic and sessile state. Moreover, the nanosystem was able to degrade a biofilm preformed of S. aureus at minimal concentration, suggesting its suitability as a bactericide agent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keating, J.F.; Simpson, A.H.R.W.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. 2005, 87, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F.; et al. Current State of Bone Adhesives—Necessities and Hurdles. Materials 2019, 12, 3975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, W. Bone and Joint Infections: From Microbiology to Diagnostics and Treatment; John Wiley & Sons: Chichester, UK, 2015; pp. 1–393. [Google Scholar] [CrossRef]
- Brady, R.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis: Clinical overview and mechanisms of infection persistence. Clin. Microbiol. Newsl. 2006, 28, 65–72. [Google Scholar] [CrossRef]
- Spellberg, B.; Lipsky, B.A. Systemic Antibiotic Therapy for Chronic Osteomyelitis in Adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Waldvogel, F.A.; Medoff, G.; Swartz, M.N. Osteomyelitis: A review of clinical features, therapeutic considerations and unusual aspects. N. Engl. J. Med. 1970, 282, 198–206. [Google Scholar] [CrossRef]
- Waldvogel, F.A.; Medoff, G.; Swartz, M.N. Osteomyelitis: A review of clinical features, therapeutic considerations and unusual aspects (second of three parts). N. Engl. J. Med. 1970, 282, 260–266. [Google Scholar] [CrossRef]
- Waldvogel, F.A.; Medoff, G.; Swartz, M.N. Osteomyelitis: A review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N. Engl. J. Med. 1970, 282, 316–322. [Google Scholar] [CrossRef]
- Conterno, L.O.; Turchi, M.D. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst. Rev. 2013, 6, CD004439. [Google Scholar] [CrossRef]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466. [Google Scholar] [CrossRef] [Green Version]
- Smeltzer, M.S.; Gillaspy, A.F. Molecular Pathogenesis of Staphylcoccal Osteomyelitis. Poult. Sci. 2000, 79, 1042–1049. [Google Scholar] [CrossRef]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; Van Bambeke, F. Antibiotic activity against small-colony variants of Staphylococcus aureus: Review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 2013, 68, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial Persistence as a Phenotypic Switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M. Evolution of Biomaterials. Front. Mater. 2022, 9, 864016. [Google Scholar] [CrossRef]
- Lew, P.D.P.; Waldvogel, P.F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Salinas, A.J.; Vallet-Regí, M. Bioactive Glasses: From Macro to Nano. Int. J. Appl. Glas. Sci. 2013, 4, 149–161. [Google Scholar] [CrossRef]
- Sui, B.; Zhong, G.; Sun, J. Drug-loadable Mesoporous Bioactive Glass Nanospheres: Biodistribution, Clearance, BRL Cellular Location and Systemic Risk Assessment via 45Ca Labelling and Histological Analysis. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bastakoti, B.P.; Yamauchi, Y. Smart soft-templating synthesis of hollow mesoporous bioactive glass spheres. Chem. Eur. J. 2015, 21, 8038–8042. [Google Scholar] [CrossRef]
- Zhu, Y.; Kaskel, S. Microporous and Mesoporous Materials Comparison of the in vitro bioactivity and drug release property of mesoporous bioactive glasses (MBGs) and bioactive glasses (BGs) scaffolds. Micropor. Mesopor. Mater. 2009, 118, 176–182. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous bioactive glasses: Relevance of their porous structure compared to that of classical bioglasses. Biomed. Glasses 2015, 1, 140–150. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, A.; Cabañas, M.V.; Peña, J.; Sánchez-Salcedo, S. Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials. Pharmaceutics 2021, 13, 1981. [Google Scholar] [CrossRef]
- Salinas, A.J.; Esbrit, P. Mesoporous Bioglasses Enriched with Bioactive Agents for Bone Repair, with a Special Highlight of María Vallet-Regí’s Contribution. Pharmaceutics 2022, 14, 202. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. Mesoporous bioactive glasses for regenerative medicine. Mater. Today Bio. 2021, 11, 100121. [Google Scholar] [CrossRef]
- Colilla, M.; Martínez-Carmona, M.; Sánchez-Salcedo, S.; Ruiz-González, M.L.; González-Calbet, J.M.; Vallet-Regí, M. A novel zwitterionic bioceramic with dual antibacterial capability. J. Mater. Chem. B 2014, 2, 5639–5651. [Google Scholar] [CrossRef]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz-Dosque, M.; Perez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol–gel method. Mater. Sci. Eng. C 2013, 33, 3795–3801. [Google Scholar] [CrossRef]
- Heras, C.; Sanchez-Salcedo, S.; Lozano, D.; Peña, J.; Esbrit, P.; Vallet-Regi, M.; Salinas, A.J. Osteostatin potentiates the bioactivity of mesoporous glass scaffolds containing Zn2+ ions in human mesenchymal stem cells. Acta Biomater. 2019, 89, 359–371. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Holguín, J.; Sánchez-Salcedo, S.; Vallet-Regí, M.; Salinas, A.J. Development and evaluation of copper-containing mesoporous bioactive glasses for bone defects therapy. Micropor. Mesopor. Mater. 2020, 308, 110454. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Bordeleau, L.J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Christopher Ellison, E.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol.-Hear. Circ. Physiol. 2002, 282, 1821–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998, 69, 326–335. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, J.; Xu, S.; Wang, K.; Yu, S. Effects of Cu2+ and pH on osteoclastic bone resorption in vitro. Prog. Nat. Sci. 2003, 13, 266–270. [Google Scholar] [CrossRef]
- Nojiri, H.; Saita, Y.; Morikawa, D.; Kobayashi, K.; Tsuda, C.; Miyazaki, T.; Saito, M.; Marumo, K.; Yonezawa, I.; Kaneko, K.; et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J. Bone Miner. Res. 2011, 26, 2682–2694. [Google Scholar] [CrossRef]
- Opsahl, W.; Zeronian, H.; Ellison, M.; Lewis, D.; Rucker, R.B.; Riggins, R.S. Role of Copper in Collagen Cross-linking and Its Influence on Selected Mechanical Properties of Chick Bone and Tendon. J. Nutr. 1982, 112, 708–716. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Hoon Byeon, J.; Park, J.H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Koch, A.L. Growth and form of the bacterial cell wall. Am. Sci. 1990, 78, 327–341. Available online: https://www.jstor.org/stable/29774119 (accessed on 9 April 2022).
- Ohsumi, Y.; Kitamoto, K.; Anraku, Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 1988, 170, 2676–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, Z.G.; Ni, H.W.; Xu, B.F.; Xiong, J.; Xiong, P.Y. Microstructure and antibacterial properties of AISI 420 stainless steel implanted by copper ions. Thin Solid Films 2005, 492, 93–100. [Google Scholar] [CrossRef]
- Yoshida, Y.; Furuta, S.; Niki, E. Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim. Biophys. Acta 1993, 1210, 81–88. [Google Scholar] [CrossRef]
- Ural, M.N.; Uney, K. Pharmacokinetic Behavior and Pharmacokinetic/Pharmacodynamic Integration of Danofloxacin Following Single or Co-Administration with Meloxicam in Healthy Lambs and Lambs with Respiratory Infections. Antibiotics 2021, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Grobbel, M.; Lübke-Becker, A.; Wieler, L.H.; Froyman, R.; Friederichs, S.; Filios, S. Comparative quantification of the in vitro activity of veterinary fluoroquinolones. Vet. Microbiol. 2007, 124, 73–81. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, C., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Science: Bethesda, MD, USA, 2004. [Google Scholar]
- Zheng, K.; Taccardi, N.; Beltrán, A.M.; Sui, B.; Zhou, T.; Marthala, V.R.R.; Hartmann, M.; Boccaccini, A.R. Timing of calcium nitrate addition affects morphology, dispersity and composition of bioactive glass nanoparticles. RSC Adv. 2016, 6, 95101–95111. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Chen, X.; Li, Y.; Miao, G.; Wang, G.; Tian, T.; Zeng, L.; Chen, X. Facile synthesis and in vitro bioactivity of radial mesoporous bioactive glass with high phosphorus and calcium content. Adv. Powder Technol. 2020, 31, 3307–3317. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Incorporation and effects of mesoporous SiO2-CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ALOthman, Z.A. Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, J.; Dong, Y. Effect of mesoporous bioactive glass on odontogenic differentiation of human dental pulp stem cells. PeerJ 2021, 9, e12421. [Google Scholar] [CrossRef]
- Liang, Q.; Hu, Q.; Miao, G.; Yuan, B.; Chen, X. A facile synthesis of novel mesoporous bioactive glass nanoparticles with various morphologies and tunable mesostructure by sacrificial liquid template method. Mater. Lett. 2015, 148, 45–49. [Google Scholar] [CrossRef]
- Min, Z.; Huixue, W.; Yujie, Z.; Lixin, J.; Hai, H.; Yufang, Z. Synthesis of monodispersed mesoporous bioactive glass nanospheres for bone repair. Mater. Lett. 2016, 171, 259–262. [Google Scholar] [CrossRef]
- Tabia, Z.; El Mabrouk, K.; Bricha, M.; Nouneh, K. Mesoporous bioactive glass nanoparticles doped with magnesium: Drug delivery and acellular in vitro bioactivity. RSC Adv. 2019, 9, 12232–12246. [Google Scholar] [CrossRef] [Green Version]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol—Gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Kurtuldu, F.; Mutlu, N.; Michálek, M.; Zheng, K.; Masar, M.; Liverani, L.; Chen, S.; Galusek, D.; Boccaccini, A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: Bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2021, 124, 112050. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.F.; Rizk, R.A.; Ahmed, M.M. Synthesis, characterization and microbiological response of silver doped bioactive glass nanoparticles. Ceram. Int. 2012, 38, 177–188. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Song, Z.; Gao, L.; Hong, H.; Liu, C. Hierarchically macroporous/mesoporous POC composite scaffolds with IBU-loaded hollow SiO2 microspheres for repairing infected bone defects. J. Mater. Chem. B 2016, 4, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.; Beltrán, A.M.; Nawaz, Q.; Michálek, M.; Boccaccini, A.R.; Zheng, K. Combination of Selective Etching and Impregnation Toward Hollow Mesoporous Bioactive Glass Nanoparticles. Nanomaterials 2021, 11, 1846. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; He, J.; Wang, C.; Yao, K.; Gou, Z. Copper-containing mesoporous bioactive glass coatings on orbital implants for improving drug delivery capacity and antibacterial activity. Biotechnol. Lett. 2014, 36, 961–968. [Google Scholar] [CrossRef]
- Jiménez-Holguín, J.; López-Hidalgo, A.; Sánchez-Salcedo, S.; Peña, J.; Vallet-Regí, M.; Salinas, A.J. Strontium-Modified Scaffolds Based on Mesoporous Bioactive Glasses/Polyvinyl Alcohol Composites for Bone Regeneration. Materials 2020, 13, 5526. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Fathi, M.H.; Braissant, O. A comparative physico-chemical study of bioactive glass and bone-derived hydroxyapatite. Ceram. Int. 2011, 37, 1601–1607. [Google Scholar] [CrossRef]
- Smeets, R.; Kolk, A.; Gerressen, M.; Driemel, O.; Maciejewski, O.; Hermanns-Sachweh, B.; Riediger, D.; Stein, J.M. A new biphasic osteoinductive calcium composite material with a negative Zeta potential for bone augmentation. Head Face Med. 2009, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Colilla, M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef]
- Sharifi, E.; Bigham, A.; Yousefiasl, S.; Trovato, M.; Ghomi, M.; Esmaeili, Y.; Samadi, P.; Zarrabi, A.; Ashrafizadeh, M.; Sharifi, S.; et al. Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation. Adv. Sci. 2021, 9, 2102678. [Google Scholar] [CrossRef]
- Seedher, N.; Agarwal, P. Effect of metal ions on some pharmacologically relevant interactions involving fluoroquinolone antibiotics. Drug Metab. Drug Interact. 2010, 25, 17–24. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Balas, F.; Colilla, M.; Manzano, M.; Vallet-Regí, M. Functionalization degree of SBA-15 as key factor to modulate sodium alendronate dosage. Micropor. Mesopor. Mater. 2008, 116, 4–13. [Google Scholar] [CrossRef]
- Ekkapongpisit, M.; Giovia, A.; Follo, C.; Caputo, G.; Isidoro, C. Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: Effects of size and surface charge groups. Int. J. Nanomed. 2012, 7, 4147. [Google Scholar] [CrossRef] [Green Version]
- Popara, J.; Accomasso, L.; Vitale, E.; Gallina, C.; Roggio, D.; Iannuzzi, A.; Raimondo, S.; Rastaldo, R.; Alberto, G.; Catalano, F.; et al. Silica nanoparticles actively engage with mesenchymal stem cells in improving acute functional cardiac integration. Nanomedicine 2018, 13, 1121–1138. [Google Scholar] [CrossRef] [Green Version]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Udall, J.N.; Moscicki, R.A.; Preffer, F.I.; Ariniello, P.D.; Carter, E.A.; Bhan, A.K.; Bloch, K.J. Flow Cytometry: A New Approach to the Isolation and Characterization of Kupffer Cells. Adv. Exp. Med. Biol. 1987, 216 A, 821–827. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.K.; Dansby, K.; El-Sayed, M.A. Improving the Flow Cytometry-based Detection of the Cellular Uptake of Gold Nanoparticles. Anal. Chem. 2019, 91, 14261–14267. [Google Scholar] [CrossRef]
- Shin, H.R.; Kwak, M.; Lee, T.G.; Lee, J.Y. Quantifying the level of nanoparticle uptake in mammalian cells using flow cytometry. Nanoscale 2020, 12, 15743–15751. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Schieke, S.M.; McCoy, J.P.; Finkel, T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell Cycle 2008, 7, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhalima, L.; Amri, S.; Bensouilah, M.; Ouzrout, R. Antibacterial effect of copper sulfate against multi-drug resistant nosocomial pathogens isolated from clinical samples. Pakistan J. Med. Sci. 2019, 35, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Prosser, B.L.; Taylor, D.; Dix, B.A.; Cleeland, R. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob. Agents Chemother. 1987, 31, 1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 2003, 49, 711–745. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]




| Samples | H2O (mL) | CTAB (mg) | NH3 (mL) | THF (mL) | PS-b-PAA (mg) | TEP (µL) | Ca(NO3)2 · 4H2O (mg) | Cu(NO3)2· 2.5H2O (mg) | TEOS (µL) |
|---|---|---|---|---|---|---|---|---|---|
| 0% Cu-HBGN | 64.75 | 140 | 2.1 | 14 | 70 | 22 | 109 | - | 455 |
| 2.5% Cu-HBGN | 64.75 | 140 | 2.1 | 14 | 70 | 22 | 93 | 14 | 455 |
| 5% Cu-HBGN | 64.75 | 140 | 2.1 | 14 | 70 | 22 | 77 | 29 | 455 |
| Samples | TEM (nm) | ζ Potential (mV) | SBET (m2 g−1) | VP (cm3 g−1) | DP (nm) | Danofloxacin Loaded (wt-%) | Atomic Composition (EDS) (wt)% | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | P | Ca | Cu | |||||||
| 0% Cu- HBGN | 193 ± 65 | −22.1 ± 4.8 | 826 | 1.22 | 2.4 | 4.2 ± 2.9 | 87.5 (75.5) | 0.3 (2.5) | 12.1 (18) | 0 |
| 2.5% Cu- HBGN | 188 ± 24 | −23.5 ± 4.9 | 731 | 0.85 | 2.6 | 5.4 ± 2.6 | 90.5 (79.5) | 0 (2.5) | 7.1 (15.5) | 2.6 (2.5) |
| 5% Cu- HBGN | 204 ± 48 | −22.8 ± 4.8 | 739 | 0.75 | 2.6 | 5.0 ± 0.5 | 86.3 (79.5) | 0.1 (2.5) | 8.8 (13) | 4.8 (5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Holguín, J.; Sánchez-Salcedo, S.; Cicuéndez, M.; Vallet-Regí, M.; Salinas, A.J. Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment. Pharmaceutics 2022, 14, 845. https://doi.org/10.3390/pharmaceutics14040845
Jiménez-Holguín J, Sánchez-Salcedo S, Cicuéndez M, Vallet-Regí M, Salinas AJ. Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment. Pharmaceutics. 2022; 14(4):845. https://doi.org/10.3390/pharmaceutics14040845
Chicago/Turabian StyleJiménez-Holguín, Javier, Sandra Sánchez-Salcedo, Mónica Cicuéndez, María Vallet-Regí, and Antonio J. Salinas. 2022. "Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment" Pharmaceutics 14, no. 4: 845. https://doi.org/10.3390/pharmaceutics14040845
APA StyleJiménez-Holguín, J., Sánchez-Salcedo, S., Cicuéndez, M., Vallet-Regí, M., & Salinas, A. J. (2022). Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment. Pharmaceutics, 14(4), 845. https://doi.org/10.3390/pharmaceutics14040845